Abstract
Background
The core intrinsic connectivity networks (core-ICNs), encompassing the default-mode network (DMN), salience network (SN) and central executive network (CEN), have been shown to be dysfunctional in individuals with internalizing disorders (IDs, e.g. major depressive disorder, MDD; generalized anxiety disorder, GAD; social anxiety disorder, SOC). As such, source-localized, closed-loop brain training of electrophysiological signals, also known as standardized low-resolution electromagnetic tomography (sLORETA) neurofeedback (NFB), targeting key cortical nodes within these networks has the potential to reduce symptoms associated with IDs and restore normal core ICN function. We intend to conduct a randomized, double-blind (participant and assessor), sham-controlled, parallel-group (3-arm) trial of sLORETA infraslow (<0.1 Hz) fluctuation neurofeedback (sLORETA ISF-NFB) 3 times per week over 4 weeks in participants (n=60) with IDs. Our primary objectives will be to examine patient-reported outcomes (PROs) and neurophysiological measures to (1) compare the potential effects of sham ISF-NFB to either genuine 1-region ISF-NFB or genuine 2-region ISF-NFB, and (2) assess for potential associations between changes in PRO scores and modifications of electroencephalographic (EEG) activity/connectivity within/between the trained regions of interest (ROIs). As part of an exploratory analysis, we will investigate the effects of additional training sessions and the potential for the potentiation of the effects over time.
Methods
We will randomly assign participants who meet the criteria for MDD, GAD, and/or SOC per the MINI (Mini International Neuropsychiatric Interview for DSM-5) to one of three groups: (1) 12 sessions of posterior cingulate cortex (PCC) ISF-NFB up-training (n=15), (2) 12 sessions of concurrent PCC ISF up-training and dorsal anterior cingulate cortex (dACC) ISF-NFB down-training (n=15), or (3) 6 sessions of yoked-sham training followed by 6 sessions genuine ISF-NFB (n=30). Transdiagnostic PROs (Hospital Anxiety and Depression Scale, HADS; Inventory of Depression and Anxiety Symptoms – Second Version, IDAS-II; Multidimensional Emotional Disorder Inventory, MEDI; Intolerance of Uncertainty Scale – Short Form, IUS-12; Repetitive Thinking Questionnaire, RTQ-10) as well as resting-state neurophysiological measures (full-band EEG and ECG) will be collected from all subjects during two baseline sessions (approximately 1 week apart) then at post 6 sessions, post 12 sessions, and follow-up (1 month later). We will employ Bayesian methods in R and advanced source-localisation software (i.e. exact low-resolution brain electromagnetic tomography; eLORETA) in our analysis.
Discussion
This protocol will outline the rationale and research methodology for a clinical pilot trial of sLORETA ISF-NFB targeting key nodes within the core-ICNs in a female ID population with the primary aims being to assess its potential efficacy via transdiagnostic PROs and relevant neurophysiological measures.
Trial registration
Our study was prospectively registered with the Australia New Zealand Clinical Trials Registry (ANZCTR; Trial ID: ACTRN12619001428156). Registered on October 15, 2019.
Similar content being viewed by others
Introduction
Background and rationale
Mental disorders are one of the most common causes of morbidity and mortality worldwide [1] with rates markedly increasing in recent years [2,3,4,5,6]. Here in New Zealand, it is estimated that one in five people is suffering from mental illness at any given time with a majority likely to experience at least one episode at some point in their lifetime [7]. Internalizing disorders (IDs, e.g. generalized anxiety disorder, GAD; social anxiety disorder, SOC; major depressive disorder, MDD; posttraumatic stress disorder, PTSD) are the most prevalent psychopathologies experienced worldwide [1, 8,9,10,11] and can be broadly characterized by a proclivity to direct distress inwardly [12,13,14,15,16]. Notably, IDs are highly comorbid [17,18,19] with females [20,21,22,23] and young people (i.e. <65 years) [8, 10, 17,18,19, 22, 24,25,26,27,28,29] disproportionately affected.
In recent years, neuropsychiatric research is pointing to transdiagnostic, neurobiological aberrations specifically involving the so-called core intrinsic connectivity networks (c-ICNs) which include the default mode network (DMN), central executive network (CEN) and salience network (SN) [30,31,32,33]. Briefly, the DMN is anchored in the posterior cingulate cortex (PCC) and medial prefrontal cortex (mPFC), and putatively subserves internally directed thought [34, 35]. The CEN, anchored in the dorsolateral prefrontal cortex (dlPFC) and posterior parietal cortices (PPC), is associated with executive functioning [36,37,38,39,40,41,42]. Lastly, the SN, anchored in the anterior insula (aINS) and dorsal anterior cingulate cortex (dACC), is believed to be important for the detection of salient stimuli and switching between the other c-ICNs [43, 44]. Additionally, the c-ICNs have also been associated with autonomic nervous system (ANS) modulation [45,46,47,48,49,50] possibly helping to explain the ANS dysfunction consistently reported across psychopathologies [51, 52].
In 2011, the converging neurobiological evidence led Menon and colleagues to propose a unifying theory of psychopathology termed the ‘triple network model’ [31, 53,54,55]. The central tenet of this theory is that the sensorial, cognitive, affective, and behavioural dysfunctions associated with mental illnesses are the result of disruptions within and between the c-ICNs. Since its inception, support for this model has been rapidly mounting within the ID-domain (e.g. [56,57,58,59]). Notably, to our knowledge, our lab was the first to validate this model with (source-space) electroencephalography (EEG) [60].
EEG non-invasively tracks and records electrophysiological signals generated by the brain [34,35,36]. Although traditionally used to assess activity in sensor-space, modern source-space algorithms (e.g. low-resolution brain electromagnetic tomography, LORETA [61, 62]) now allow accurate estimations of the regions (i.e. nodes) responsible for generating the scalp-recorded electrophysiological signals. Further, although standard clinical EEGs typically limit the recording bandwidth to traditional frequency bands (i.e. delta ~1-4 Hz, theta ~4-8 Hz, alpha ~8–12 Hz, beta ~12–30 Hz, gamma >30 Hz), acquisition and analyses of frequencies at the low end of the spectrum, commonly termed electrophysiological infraslow fluctuations (eISFs; <0.1 Hz), are now possible [63]. Russian scientists discovered eISFs over half a century ago, first in rabbits [64, 65] and shortly thereafter in humans [66] but, due in large part to technological challenges, they have received little interest from the scientific and clinicical communities until recently [63, 67,68,69,70]. Putatively engendered by a combination of neuronal and glial currents [63, 68, 71,72,73,74,75,76], eISFs have been shown in both cortical [65, 66, 77] and subcortical [76, 78,79,80,81,82] tissues and are believed to coordinate large-scale ICN organization and long-range information exchange [68, 83,84,85,86,87,88,89,90,91]. As such, treatments specifically targeting eISFs within core nodes of the triple network may address c-ICN dysfunction and offer clinical utility in the treatment of IDs.
Although traditional frontline therapies (i.e. pharmacotherapy and psychotherapy) are effective for many, they offer numerous shortcomings including high failure rates [92,93,94,95,96,97,98], lack of access [22, 99,100,101,102], and marked adverse side-effects [52, 99, 100, 103,104,105]. Closed-loop brain training of electrophysiological (EEG) signals, also known as EEG-neurofeedback (EEG-NFB), is a non-invasive therapy aimed at modulating brain function by teaching individuals, via associative learning (e.g. operant conditioning), to self-regulate their brain function via auditory, visual, and/or tactile feedback [106]. Intriguingly, EEG-NFB’s impact on the brain may intensify following the cessation of therapy putatively due to treatment-induced neuroplasticity; however, a general lack of extended follow-up and failure to assess for the emergence of delayed treatment effects is common in the literature [107]. That said, sceptics assert that comparable clinical improvements in both experimental and control groups in randomized, double-blind, sham-controlled trials suggest that EEG-NFB's efficacy rests entirely on ‘non-specific’ psychosocial factors (i.e. expectations, motivation, demand characteristics, context) [108,109,110,111,112,113,114,115,116,117,118,119]. However, proponents contend that evidence of differential EEG-learning (i.e. greater change in the targeted electrophysiological variable(s) and/or region(s)-of-interest (ROIs) in the genuine versus sham groups), considered by many to be essential for a valid evaluation of EEG-NFB’s specificity [120,121,122,123,124,125,126], was noticeably absent in the trials presented as evidence for wholly non-specific effects [127, 128]. That said, assessments of differential EEG-learning are complicated by a lack of standardized criteria for the determination of learning (or a lack thereof) [129]. In any case, EEG-NFB has shown promising clinical effects in a wide various of conditions [130,131,132,133,134,135] including IDs [102, 136,137,138,139,140,141,142,143,144,145,146,147,148,149,150,151,152,153,154,155]. Further, clinicians have reported success in ID populations using sensor-space EEG-NFB targeting eISFs (ISF-NFB) [156,157,158]. Advanced source-localization (i.e. standardized LORETA, sLORETA [62]) combined with ISF-NFB is a novel introduction to the field that has been shown by our research group in a feasibility trial on obese females to improve sleep and wellbeing with minimal side-effects [159, 160].
To our knowledge, this is the first randomized, double-blind, sham-controlled trial examining the potential effects of source-space ISF-NFB in an ID population. Furthermore, our relatively novel transdiagnostic approach heeds recent calls for a more pragmatic, ecologically valid clinical research [161,162,163,164,165,166,167,168,169,170,171]. As detailed below, our primary objectives will be to examine patient-reported outcomes (PROs) and neurophysiological measures to (1) compare the potential effects of sham ISF-NFB to either genuine 1-region ISF-NFB or genuine 2-region ISF-NFB and (2) assess for potential associations between changes in PRO scores and modifications of EEG activity/connectivity within/between the trained ROIs.
Study objectives
Primary research questions
Objective 1
To compare the potential effectiveness of genuine ISF-NFB versus sham ISF-NFB for treating IDs in a female population. We will assess differences between sham ISF-NFB (sham) and single-region ISF-NFB (ISF1) or multi-region ISF-NFB (ISF2) in PROs and neurophysiological measures (i.e. EEG and HRV) after 6 training sessions (post 6 sessions). We hypothesize that all groups will show clinical improvements via non-specific (e.g. placebo) effects; however, ISF1 and ISF2 groups will demonstrate additional improvements due to specific effects (i.e. effects specific to the modulation of the trained ROIs).
Objective 2
To assess whether there is a potential relationship between changes in PROs and EEG variables post 6 sessions. Specifically, we are interested in whether there is evidence for an association between changes in the primary PRO (i.e. Hospital Anxiety & Depression Scale, HADS) scores and targeted ROI activity and connectivity.
Secondary research questions
Objective 3
To assess the potential clinical effects of an additional 6 treatment sessions (i.e. post-6 to post-12 sessions), we will examine changes in PROs and neurophysiological measures amongst the ISF1 and ISF2 groups. We hypothesize that additional sessions will provide additional benefits for both treatment groups.
Objective 4
To explore the potential for increased medium-term effects from ISF-NFB treatment at 1-month follow-up, we will examine changes in PROs and neurophysiological measures amongst the ISF1 and ISF2 groups.
Objective 5
To explore whether any potential associations observed in Objective 2 can be extended to post 12 sessions and 1-month follow-up amongst the ISF1 and ISF2 groups.
Trial design
This study is a randomized, double-blind (participants and assessors), yoked-sham controlled (playbacks of genuine ISF-NFB sessions from another female ID participant at the same training stage), parallel-group (3-arm = sham, ISF-1, ISF-2), superiority, pilot trial with an allocation ratio of 2:1:1 (sham:ISF1:ISF2).
Methods: participants, interventions, and outcomes
Study population and setting
Our target population is adult females meeting the DSM-5 criteria for one or more IDs of interest (i.e. GAD, SOC and/or MDD). Our trial will recruit all participants from the community in and around Dunedin, New Zealand and be undertaken at the Departments of Surgical Sciences and Psychological Medicine, University of Otago, Dunedin, New Zealand.
Eligibility criteria
Inclusion criteria:
-
1.
Able to give informed consent
-
2.
Adult between 18 and 64 years old
-
3.
Biological female
-
4.
Meets the DSM-5 criteria for one or more of the following current diagnoses:
-
a.
GAD
-
b.
SOC
-
c.
MDD
-
a.
-
5.
Never undergone EEG-NFB therapy
Exclusion criteria:
-
1.
Starting new medications or altering dosages of existing medications <4 weeks prior to their 1st baseline session or at any time during the trial.
-
2.
Currently taking short-acting benzodiazepines (i.e. midazolam, triazolam)
-
3.
Undergoing intensive psychotherapy (e.g. cognitive behavioural therapy)
-
4.
Any externalizing disorder (e.g. antisocial personality disorder, alcohol/substance abuse disorder)
-
5.
Any thought disorder (e.g. mania, bipolar disorder)
-
6.
Any Neurological disorder (e.g. epilepsy)
-
7.
Deemed to be at high-risk of suicide per the Columbia-Suicide Severity Rating Scale (C-SSRS – Screen Version)
-
8.
Pregnant females
-
9.
Pacemaker
-
10.
Post-concussion syndrome
Drop-out criteria:
-
1.
Refusal to participate
-
2.
Misses >1 intervention session
Additional consent provisions for collection and use of participant data and biological specimens
Not applicable: no biological specimens will be collected, and all data is to be used solely in accordance with this trial.
Explanation for the choice of comparators
Our choice of sham-controls allows us to elucidate any potential specific (e.g. non-placebo) effects and addresses widespread concerns of generally weak methodological designs in NFB trials [102, 172,173,174].
Recruitment
To reach the widest possible audience, ID participants will be recruited via both posters placed around the city and targeted Facebook ads with an invitation to participate in a University of Otago mental health study. Advertisements will direct potential participants to a webpage that will describe the trial and invite those interested to complete a short online form which will query basic information including first name, age, date of birth, sex, ethnicity, education level, handedness, mental health history, pregnancy status, presence of electronic implants (i.e. pacemakers), email address, and phone number. Individuals who complete the online form and meet the basic qualifications will be contacted via email and asked to attend an in-person mental health interview at the University of Otago Hospital, Dunedin, New Zealand. Those that agree will be provided directions to the lab and a digital copy of the 7-page participant information sheet (PIS). A reminder text will be sent to potential participants on the day of their interview. Recruitment will continue until our target sample sizes are met and is expected to take 18–24 months. To help foster our recruitment efforts, all participants who complete the study will receive a $40 supermarket voucher as reimbursement for any parking expenses.
Who will take informed consent?
At the initial meeting, a male doctoral/PhD student will (1) provide each potential participant with a paper copy of the participant information sheet written in English, (2) query if they have read and understood the document, (3) ask if they have any questions about the trial, and (4) request written informed consent from individuals willing to participate in the study. Participants will be informed that they may withdraw at any time without giving a reason and that all data collected up to the point of withdrawal may be used in the final analyses.
Screening
Following the attainment of informed consent, a trained male doctoral/PhD student conduct the Mini-International Neuropsychiatric Interview (MINI; English version 7.0.2 for DSM-5) [175]. The MINI is a brief structured diagnostic interview, shown to be both valid and reliable, used to assess the 17 most common psychiatric disorders including MDD, suicidality, bipolar, panic disorder, agoraphobia, SOC, obsessive-compulsive disorder, PTSD, alcohol use disorder, substance use disorder, psychoses, anorexia, bulimia, binge-eating disorder, GAD, and anti-social personality disorder [176, 177]. In the event that the interviewer suspects that the interviewee is at high-risk for suicide per the MINI, he will screen using the Columbia-Suicide Severity Rating Scale (C-SSRS – Screen Version [178]) with affirmative answers to questions 4, 5 and/or 6b initiating immediate referral to Emergency Psychiatric Services. Those meeting the eligibility criteria will be enrolled into the study, have their anthropometric (i.e. height and weight) measurements taken, and be scheduled for their baseline assessments. Participants will also be familiarized with the study equipment, procedures, and personnel.
Baseline assessments
Baseline assessments will take place on two separate occasions approximately 1-week apart with baseline #2 values used as reference. Duplicate baseline assessments will be performed to mitigate the influence of certain non-specific effects (i.e. regression to the mean [179] and elevation bias [180]) that may confound clinical trials. All assessment sessions for a given subject will take place at approximately the same time of day and be led by a female research assistant. Prior to each assessment session, participants will be asked to abstain from (1) food and water for 2 h, (2) smoking/vaping for 8 h, and (3) strenuous exercise, alcohol, caffeine and over-the-counter medication for 24 h. A reminder email and text will be sent to each participant one day prior to and on the day of the assessment sessions, respectively. Adherence to lifestyle restrictions will be queried at the beginning of each session with any breaches recorded. In cases of serious breaches (e.g. consumption of alcohol in the prior 24 h), assessment sessions will be rescheduled. In addition, the subject’s previous night’s sleep duration will be documented, and they will be asked to use the toilet immediately prior to testing to ensure an empty bladder. Together, these standardization procedures are in line with current recommendations for neurophysiological data collection [181,182,183] and will help to control for variability in neurophysiological output stemming from important factors like circadian rhythms [184, 185], gastric distention [182, 186, 187], hydration levels [182, 188], bladder distention [182, 189], caffeine [190, 191], nicotine [192, 193] and alcohol [194, 195].
All PROs (English versions) will be re-created in digital form via Qualtrics [196] which will allow participants to complete them using an iPad during their EEG set-up. To prevent missing data, a visual alert will be generated if any queries on a given form have missing responses. Research has indicated the electronic data collection increases the speed, accuracy, and user acceptability of the process [197,198,199]. The estimated total time to complete the battery of PROs is 20 min. The order of PRO administration will be standardized and based on questionnaire length (i.e. IDAS-II > MEDI > HADS > IUS-12 > RTQ-10).
Following completion of their PROs, neurophysiological data will be collected from each participant using Compumedics Neuroscan SynAmps RT 64-channel amplifier (DC mode, input impedance >10 GΩ, 24-bit analogue-to-digital resolution, common mode rejection >110 dB) using a continuous sampling rate of 1000 Hz. Recordings will take place in a quiet, cool (~15°C), dimly lit room as participants are seated upright in a comfortable chair with their eyes closed. We chose the eyes-closed condition because it has been reported to improve EEG reliability [200, 201]. The 10.5 min resting-state full-band EEGs (fb-EEGs) will use high-density (64-channel) silicone Quik-Cap Hydro Net caps with Ag/AgCl electrode placements corresponding to the international extended 10/20 system. The ground electrode is positioned at AFz with the reference electrode midway between Cz and CPz. Electrooculography (EOG) will track vertical and horizontal eye movements. The cap is soaked in a saline solution at least 30 min prior to application and all electrode impedances will be kept below 10 kΩ. To help reduce impedances, subjects will be asked to arrive with non-braided, dry, clean (i.e. no conditioner, gels, pastes, or sprays) hair. Concurrent with the resting-state EEG, a spontaneous breathing standard limb lead (lead-II) electrocardiogram (ECG) using Ag/AgCl electrodes will be performed. Following, a 10 min metronome paced breathing (12 breaths per minute) ECG with a 1:1 inspiratory/expiratory (I/E) ratio (i.e. 2.5-s inhalation/2.5-s exhalation) was collected. During pacing, participants will be instructed to breathe through their nose at normal depth (i.e. no deep breathing).
Randomization
Following baseline #2 measurements, participants were randomized to one of 3 arms: (1) yoked-sham, (2) ISF1 = PCC up-training, or (3) ISF2 = concurrent PCC up-training and dACC down-training. Sham participants were offered active ISF-NFB upon their completion of the trial, thereby minimizing the potential of sham trial-associated participation barriers [202] as well as addressing any potential ethical concerns of sham-only allocations.
Sequence generation
The randomization scheme will be generated by using the Web site Randomization.com [203] by a lab member from our group with no direct contact with the participants. This tool is a valid randomization program utilized by clinical trial researchers. Block randomization with random block sizes and a 2:1:1 (sham: ISF1: ISF2) allocation will be utilized.
Concealment mechanism
Randomization sequences were kept in the central office in sequentially numbered, sealed, opaque envelopes prepared by the lab member who generated the randomization scheme. To ensure concealment, the block sizes will be known only by this lab member and not be disclosed to any of the researchers who have contact with the participants.
Implementation (enrolment and assignment)
TMP is responsible for participant enrolment and will assign participants to interventions following baseline assessments and upon arrival at their first ISF-NFB session.
Who will be blinded?
This is a double-blind study whereby participants and raters will be unaware of group assignments. The ISF-NFB trainer will not be blinded. To improve participant blinding, all aspects of sham sessions will be identical to active sessions including the live recording of sham participants’ EEGs along with real-time artefact alerts. Blinding integrity will be assessed post 6 sessions via a brief electronic questionnaire whereby participants will be queried as to (1) their perceived group allocation, (2) confidence in their answer to question 1 on a scale of 0–100%, (3) reason for their answer to question 1, and (4) if their group assignment was revealed to them in any way.
Procedure for unblinding if needed
Treatment assignment will be disclosed to trial participants only upon their completion of the study.
Intervention descriptions
Training sessions will commence within 1-week after baseline #2 assessments. To help reduce impedances, subjects were asked to arrive with non-braided, dry, clean (i.e. no conditioner, gels, pastes, or sprays) hair. Participants will attend three 30-min sessions per week, every other day, over 4 consecutive weeks (12 sessions in total). 19-channel sLORETA ISF-NFB training will be performed using a DC coupled amplifier produced by Brainmaster Inc. and the BrainAvatar software (version 4.7.5.844) in a quiet, cool (~15°C), dimly lit room by an unblinded male researcher with >2 years of experience in the administration of NFB. Participants will be seated in a comfortable chair and an appropriately sized Comby EEG cap will be placed on the participant’s head. Using a blunt need and syringe, the scalp will be mildly abraded prior to the application of an electrolyte gel beneath each electrode. It should be noted that the purpose of the cool room and scalp abrasion is to mitigate contamination of the EEG signal by electrodermal (i.e. sweat gland) potentials which are known to mimic brain-derived eISFs [63, 69]. Nineteen-channel EEGs will be recorded with the silver/silver chloride (Ag/AgCl) electrodes positioned according to the International 10–20 system (i.e. Fp1, Fp2, F3, F4, C3, C4, P3, P4, O1, O2, F7, F8, T3, T4, T5, T6, Fz, Cz, Pz) using a linked mastoids reference and a ground electrode positioned centrally between F3, Fp1, Fz and Fpz. The impedances of the active electrodes will be kept below 10 kΩ and a 50 Hz notch filter will be set.
Immediately prior to each training period, a demonstration of motion artefact alerts will be performed with instructions to avoid eye/head/face movements to minimize this non-rewarding feedback. Participants will then be instructed to close their eyes, relax, stay awake, and listen to the sound being played. They will be informed that the sound they hear reflects that they are doing well. Notably, no explicit strategies or instructions were given as, with few exceptions [204], implicit strategies have been shown to produce better outcomes [205,206,207,208,209,210].
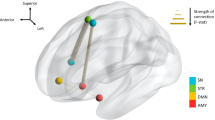
Continuous, real-time auditory feedback will be used for reinforcement and produced within 30 ms of the subject’s eISFs (0.0–0.1 Hz) within the pre-defined ROIs (i.e. dACC and/or PCC; Fig. 1) surpassing the threshold(s). These ROIs were selected because, as outlined in the introduction, they are considered key cortical nodes within the core RSNs which are consistently found to be disrupted in ID populations. sLORETA permits the selection of any cortical region for feedback of the current density using voxels selected based on Montreal Neurological Institute (MNI) coordinates [211]. For a complete list of targeted voxels for this trial, see Additional files 1 and 2.
The reward threshold(s) will be manually adjusted in real-time to maintain a 60% ± 10% success rate. Manual, rather than automated, thresholding was chosen as it has been reported to lead to better EEG-learning [125, 173, 206, 208]. The yoked-sham sessions will be identical to active sessions, including live EEG recordings and real-time motion/EMG artefact alerts, however, the auditory rewards will derive from playbacks of genuine ISF-NFB sessions from another female ID participant at the same training stage recorded via free, open-source Audacity software [212] which uses the computer’s sound card as an audio to digital converter. Importantly, this type of control allows matching of rewards and performance across sham and genuine conditions, thereby controlling as much as possible the learning context and degree of motivation [213] while theoretically severing the operant conditioning aspect of EEG-NFB. Additionally, it has been reported that training effects are more robust when the clinician is present [214], therefore, irrespective of group assignment, the trainer will be present for the duration of all sessions. Further, the trainer will monitor the participants’ protocol adherence. A detailed description of the trial intervention using the Template for Intervention Description and Replication (TIDieR) [215] has been provided in Table 1.
Participant timeline
The trial period for each participant will be approximately 10 weeks and consist of one 30-min screening interview, two 60-min baseline assessments approximately 1 week apart, six 30-min genuine or sham ISF-NFB sessions (3× per week over 2 consecutive weeks) starting within 1 week after baseline #2, a 60-min post 6 sessions assessment, six 30-min genuine ISF-NFB sessions (3× per week over 2 consecutive weeks), a 60-min post 12 sessions assessment, and a 60 min 1-month follow-up assessment (Table 2 and Fig. 2). All post-treatment assessments and procedures will be identical to those performed at baseline.
Criteria for discontinuing or modifying allocated interventions
Participants will be advised that they were able to withdraw at any time without giving a reason or may be withdrawn by the lead investigator if they (1) experience significant adverse effects that were deemed detrimental to their well-being or (2) are unable to adhere to protocol (e.g. missed >1 training session in either 6 session block, started or modified first-line therapies).
Strategies to improve adherence to interventions
We will attempt to mitigate adherence issues via automated email and text message reminders sent on the day of each training session.
Plans to promote participant retention and complete follow-up
Once enrolled, every reasonable effort will be made to follow participants throughout the entirety of the study period via ongoing email and text messaging correspondence. In the event of premature discontinuation of the study for any reason, participants will be made aware that all data collected up to the point of withdrawal may be used for analyses.
Relevant concomitant care permitted or prohibited during the trial
Participants were asked to maintain any current first-line mental health therapies (e.g. pharmacotherapy) for the entire length of the trial period (i.e. baseline through follow-up). Any changes or introductions of first-line therapies (e.g. altered pharmacotherapy dosages, introduction of intensive psychotherapy) will render participants ineligible.
Provisions for post-trial care
In the unlikely event of injury, participants will be eligible to apply for compensation from the Accident Compensation Corporation (ACC) of New Zealand just as they would be if they were injured in an accident at work or at home. Although there are private providers abroad (e.g. Asia, North America, and Europe), should this trial provide evidence of efficacy, there is currently no access to this therapy within New Zealand.
Measurements
Primary outcomes: HADS, fb-EEG
The central importance of PROs in clinical trials has been emphasized by both international health regulatory agencies and patients [216, 217], therefore, the primary outcome of interest will be the HADS [218, 219]. Additionally, the importance targeted EEG-learning assessments in NFB trials has been emphasized by researchers [120,121,122,123,124,125,126], therefore activity (i.e. amplitude of the oscillations) and connectivity (i.e. coordinated amplitude and/or phase of the oscillations) changes within and between the targeted ROIs will also be of primary interest. Primary outcome measures will be collected at baseline, post 6 sessions, post 12 sessions, and 1-month follow-up.
Hospital anxiety depressions scale (HADS)
The HADS is a valid and reliable 14-item, trans-diagnostic PRO measure used to assess anxiety and depression severities [218]. Response options are on a 4-point scale (0–3) based on participants experiences over the past week with anxiety and depression subscale scores graded as follows: 0–7 = ‘normal’, 8–10 = ‘mild’, 11–14 = ‘moderate’, and 15–21 = ‘severe’ [220]. The HADS has been repeatedly shown to be a reliable and valid tool across a variety of settings [219, 221, 222]. There is some debate with respect to whether the HADS is best assessed via the total 14-item score [223,224,225] or two 7-item subscale (anxiety and depression) scores [219, 222, 225, 226]. For our trial, we are considering the anxiety (HADS-A) and depression (HADS-D) subscale scores separately. Importantly, the minimum clinically important difference (MCID) for the HADS subscales is estimated to be a reduction of 1.5 to 2 points [227,228,229].
Full-band EEG (fb-EEG)
The use of full-band EEGs permits the non-invasive examination of the entire spectrum of brain frequencies from infraslow (<0.1 Hz) to gamma (>30 Hz) using scalp-recorded electrical signals acquired from direct current (DC) coupled amplifiers. Further, source-localization software can locate the probable generators (i.e. brain sources) responsible for the acquired electrical signals, thereby permitting us to assess activity (i.e. log-transformed current source density; log-CSD) and connectivity (i.e. lagged linear connectivity; lag-CON). EEG pre-processing will be performed offline using EEGLAB version 14.1.1 [230] and ERPLAB version 6.1.4 [231] running on MATLAB 2021a (The MathWorks, Inc., Natick, MA, USA.). Custom scripts developed in MATLAB utilizing EEGLAB, ERPLAB, and MATLAB functions will be used. The raw EEG will be imported into MATLAB using EEGLAB. Channel locations/coordinates will be determined via EEGLAB’s Montreal Neurological Institute (MNI) coordinate file for BEM dipfit model with the head centre optimized. Non-EEG (i.e. VEOG, HEOG, EKG, EMG, GSR) and four EEG (i.e. F11, FT11, F12, and FT12) channels will then be removed prior to manual co-registration used to match the coordinates of the 60 remaining channels to the realistic Boundary Element Model (MNI) head model. Of note, the four EEG channels selected for removal lack locations in the MNI coordinate file, thereby precluding subsequent pre-processing. The data will then be truncated to retain only the middle 600-s of the time-series, and the PREP pipeline version 0.55.1 [232] will be run to identify and interpolate bad channels, remove line noise, and robust average reference the data. This pipeline has been used previously for evoked potentials and resting-state EEG data [233, 234]. PREPed EEGs with >25% (i.e. >15) bad channels identified will not be considered for further analyses. For identification and marking of artefact-contaminated epochs, continuous PREPed EEGs will be 1 Hz high-pass filtered using a finite impulse response (FIR) filter implemented using EEGLAB’s pop_firwsord function (window = Kaiser 5.653, transition bandwidth = 1.5 Hz, max ripple = 0.001, order = 2416) and segmented into 1-s epochs. Epochs will automatically be marked as artefacts if containing one or more of the following characteristics: (i) absolute voltage exceeds 100 μV, (ii) peak-to-peak voltage exceeds 150 μV in any sliding window of 200 ms width with a step size of 100 ms, (iii) voltage greater than 100 μV resulting from a step-function with a sliding window 200 ms wide with a step size of 50 ms, (iv) sample-to-sample difference exceeding 50 μV, or (v) absolute voltage less than 1 μV for 150 ms (i.e. flat-lined data). Following this, manual verification/correction of epoch classifications (i.e. artefact and non-artefact) will be performed. Manually verified time-series with >50% (i.e. >5 min) artefact-contaminated epochs will be excluded from further analyses. For independent component analysis (ICA), the data will again be 1 Hz high-pass FIR filtered (window = Kaiser 5.653, transition bandwidth = 1.5 Hz, max ripple = 0.001, order = 2416), down-sampled to 500 Hz to reduce computation time, have noisy channels and artefact-contaminated epochs removed, and decomposed into maximally independent components (ICs) which are spatially fixed and temporally discrete [235] using adaptive mixture ICA (AMICA) [236]. AMICA was selected based on its superior performance when compared with other ICA algorithms [237]. The resulting ICA weights will be applied to 1–100 Hz band-pass FIR filtered (window = Kaiser 5.653, transition bandwidth = 1 Hz, max ripple = 0.001, order = 3624) data. We will use ICLabel [238] with manual verification to categorize the ICs as brain or other (i.e. muscle, eye, channel noise, line noise, or other) based on their spatial distribution (scalp topography), time course, spectrograms, event-related potential (ERP) images, and current dipole models using recommendations from Jung et al. [239], Chaumon et al. [240], and the website https://labeling.ucsd.edu/. Finally, bad ICs will be removed, noisy channels interpolated, and the data 0.01–100 Hz bandpass infinite impulse response (IIR) filtered (1st order Butterworth) to give cleaned datasets. This IIR filter has been utilized in previous studies of eISFs [80, 241]. Finally, cleaned datasets with ICA will be downsampled to 128 Hz to reduce computation time and exported to ASCII text files for subsequent analyses. Figure 3 shows an overview of the EEG pre-processing pipeline.
Via LORETA-KEY software (v20210701, freely available at http://www.uzh.ch/keyinst/loreta.htm), ASCII text files will be used as input to compute cross-spectral matrices for each participant for seven frequency bands (infraslow 0.01–0.1 Hz; slow 0.2–1.5 Hz; delta 2–3.5 Hz; theta 4–7.5 Hz; alpha 8–12 Hz; beta 12.5–30 Hz; gamma 30.5–44 Hz) utilizing fast Fourier transform (FFT). To allow for 2 complete cycles of the lowest frequency of interest (i.e. 0.01 Hz) and to obtain smooth power spectral density, EEGs will be segmented into 200-s epochs with Hanning (Hann) tapered windows applied. The cross-spectral matrices will then be averaged for each subject and used as input to exact LORETA (eLORETA) to compute whole-brain current source density (CSD; A/m2) without assuming a pre-defined number of active sources [242, 243]. Using the MNI-152 (Montreal Neurological Institute, Canada) template, eLORETA produces an inverse solution space consisting of 6239 cortical grey matter voxels at 5 mm resolution and has been shown to produce exact, zero-error localizations even in the presence of measurement and structured biological noise. eLORETA performs voxel-by-voxel between-condition comparisons of the CSD distribution. Statistical non-parametric mapping (SnPM) will be performed for each contrast using built-in voxel-wise randomization test (5000 permutations) to calculate the empirical probability distribution for the max-statistic (e.g. the maximum of a t or an F statistic) under the null hypothesis while correcting for multiple testing (i.e. for the collection of tests performed for all electrodes and/or voxels, and for all time samples and/or discrete frequencies). For each contrast, the voxel-level, two-tailed max-statistic was used as input to LORETA-KEY software to identify and visualize differences/changes in log-CSD for each of the seven frequency bands. Furthermore, for each condition, log-CSDs were averaged across all voxels within a 10-mm radius of the centre of mass MNI coordinates derived from previous literature [244] of the targeted ROIs (Fig. 4). This output was exported to Excel (version 2112) and analysed in R (version 4.0.5 [245];) to identify differences/changes in log-CSD between contrasts for each ROI in each frequency band. Next, functional connectivity (FC; i.e. lag-CON [242]) between the targeted ROIs was calculated for each group at each time point in LORETA-Key. Output was then exported to Excel and analysed in R to identify differences/changes between contrasts in each frequency band. Finally, as effective connectivity (EC) reflects directed functional connectivity, significant ROI pairs identified from FC analyses were selected for EC (i.e. Granger causality [246]) analyses. As with FC, EC was calculated in LORETA-Key, exported to Excel, and analysed in R.
Secondary outcomes: MEDI, IDAS-II, IUS-12, RTQ-10, HRV
The Multidimensional Emotional Disorder Inventory (MEDI) [247, 248], Inventory of Depression and Anxiety Symptoms – Second Version (IDAS-II) [249, 250], Intolerance of Uncertainty Scale – Short Form (IUS-12) [251], and Repetitive Thinking Questionnaire (RTQ-10) [252,253,254], and heart rate variability (HRV) will be considered secondary outcomes of interest which are also collected at baseline, post 6 sessions, post 12 sessions, and 1-month follow-up.
Multidimensional emotional disorder inventory (MEDI)
The MEDI, which has been validated on both clinical [247] and non-clinical [255] populations, is a 49-item, trans-diagnostic PRO measure that employs a response scale ranging from 0 (not characteristic of me/does not apply to me) to 8 (extremely characteristic of me/applies to me very much) to assess nine ID-related symptom domains, originally proposed by Brown and Barlow [256], including (1) Neurotic Temperament (5 items), (2) Positive Temperament (5 items), (3) Depression (5 items), (4) Autonomic Arousal (5 items), (5) Somatic Anxiety (5 items), (6) Intrusive Cognition (6 items), (7) Social Concerns (5 items), (8) Traumatic Re-experiencing (5 items), and (9) Avoidance (8 items). Although validated clinical severity thresholds for the MEDI total subscale scores are still lacking, the authors of this measure have suggested that average subscale scores >4 (or <4 for positive temperament), >6 (or <2 for positive temperament), and 7–8 (or 0–1 for positive temperament) may reflect moderate, severe, and extreme severities, respectively [257].
Inventory of depression and anxiety symptoms – second version (IDAS-II)
The IDAS-II is a valid and reliable 99-item, trans-diagnostic PRO measure that uses a response scale ranging from 1 (not at all) to 5 (extremely) to assess 19 current (past 2 weeks) ID-related symptom domains including general depression (20 items), dysphoria (10 items), lassitude (6 items), insomnia (6 items), suicidality (6 items), appetite loss (3 items), appetite gain (3 items), well-being (8 items), ill temper (5 items), mania (5 items), euphoria (5 items), panic (8 items), social anxiety (6 items), claustrophobia (5 items), traumatic intrusions (4 items), traumatic avoidance (4 items), checking (3 items), ordering (5 items), and cleaning (7 items) [249, 250]. Notably, in contrast to the other domains, the general depression domain is a composite of all 10 items from the dysphoria domain, as well as 2 items each from the suicidality, lassitude, insomnia, appetite loss and well-being domains. Recently, severity (mild, moderate, severe) thresholds have been introduced for 12 of the subscales including general depression, dysphoria, lassitude, insomnia, suicidality, appetite loss, appetite gain, well-being, ill-temper, panic, social anxiety, and traumatic intrusions [258].
Intolerance of uncertainty scale – Short form (IUS-12)
The IUS-12 is a valid and reliable 12-item, transdiagnostic PRO measure that assesses the degree to which an individual considers the possibility of a negative event occurring unacceptable, irrespective of its probability of occurrence [251, 259]. Whereas the original IUS-27 was GAD-specific, the IUS-12 has been distilled in order to measure the core intolerance of uncertainty construct [260]. The IUS-12 uses a response scale from 1 (“not at all characteristic of me”) to 5 (“entirely characteristic of me”) with total scores that can range from 12 to 60 [251]. Although some researchers have claimed that the IUS-12 is a unidimensional construct and recommend using only the IUS-12 total score [261,262,263], there has been considerable support for a two-factor IUS-12 structure: (1) a 7-item prospective IU scale related to action/approach-oriented strategies in order to increase certainty (e.g. seeking more information), and (2) a 5-item inhibitory IU associated with inaction/avoidance-oriented thoughts and behaviours (e.g. delayed decision-making) [251, 259, 264,265,266,267,268,269,270,271]. Intolerance of uncertainty (IU) is a common trait shared across the ID spectrum [271,272,273,274].
Repetitive thinking questionnaire (RTQ-10)
The RTQ-10 is a 10-item, trans-diagnostic PRO measure [253] distilled from three disorder-specific scales: 1) the MDD-associated Ruminative Responses Scale (RRS [275]), 2) the GAD-associated Penn State Worry Questionnaire (PSWQ [276]), and 3) the SOC-associated Post-Event Processing Questionnaire-Revised (PEPQ-R [277]). Participants are asked to rate the truthfulness of each statement with respect to their experience when they are “distressed or upset.” All items are rated along a 5-point scale: not at all true = 1, somewhat true = 3, or very true = 5 allowing total scores that can range from 10 (low levels of latent repetitive negative thinking) to 50 (extremely high levels of repetitive negative thinking). Repetitive negative thinking (i.e. rumination and worry) is a characteristic feature of IDs [278,279,280].
Heart rate variability (HRV)
Heart rate variability (HRV) is the phenomenon of cyclical beat-to-beat changes in the interbeat interval (i.e. RR interval), the dynamics of which can give insight into cardiac autonomic function [281]. ANS function is purportedly modulated by the core-ICNs and one of the most robust ANS disturbances found in IDs is cardiac dysautonomia in the form of reduced HRV [52, 96, 103, 280, 282,283,284,285,286,287,288,289,290,291,292,293]. This has significant clinical implications considering that cardiovascular disease is the leading cause of mortality in people with mental illness [52, 294, 295]. HRV is typically measured via the standard time-domain (i.e. standard deviation of normal-to-normal intervals, SDNN (ms), root mean square of successive differences between normal-to-normal intervals, RMSSD (ms)) and frequency-domain (i.e. LF-HRV = 0.04–0.15 Hz and HF-HRV = 0.15–0.4 Hz absolute power (ms2)) indices, however, non-linear analyses (e.g. Poincaré plot) are fast emerging as a way to characterize the complex, non-linear dynamics of cardiac-ANS interactions [296]. The raw ECG signals will be extracted in EEGLAB and saved as EDF (.edf) files. Using MATLAB 2021a and modifications to the open-source code for HRVTool version 1.07 (https://github.com/MarcusVollmer/HRV) [297], ECG time-series will be band-pass IIR filtered (10–35 Hz, 4th order Butterworth) to remove signal drift and line noise, undergo automated annotation of R-peaks, and be interpolated using the shape-preserving piecewise cubic Hermite interpolating polynomial (Pchip) method to correct for artefacts (e.g. ectopic or missing beats). Pchip interpolation was chosen because appears to perform best across the spectrum of HRV metrics as it preserves the linear trend as well as the non-linear contributions in the R-R timeseries [298]. Using the HRVTool graphical user interface (GUI), Poincaré plots will also be examined to look for evidence of regularities (e.g. ‘comet’ shape) with those showing marked irregularities suggestive of cardiac arrhythmias (e.g. ‘fan’ shape) excluded from further analyses. Next, the first 10 s of ECG traces will be truncated to allow for signal stabilization followed by manual inspection/correction of the succeeding 5 min (i.e. 10–310 s) to ensure proper annotation of R-peaks generated from normal sinus rhythm with traces requiring >5% R-R interval interpolation excluded from further analyses. Finally, using the GUI’s R-R tachogram (time-series and spectrum), consecutive 60-s epochs (i.e. 1–5 min) from spontaneous breathing ECGs will be examined with those suggesting mean respiration rates outside of 0.15–0.4 Hz (i.e. 9–24 breaths per min) excluded from further analyses. Indices for both spontaneous and paced breathing conditions will be reported.
Sample size
This is the first study examining the effects of sLORETA ISF-NFB in an ID population. Due to its novelty, there was no existing information around standard deviations for the measurements of interest. Therefore, no formal sample size or power calculations were made. Our group has previously carried out an sLORETA ISF-NFB trial in obese females [159]; however, this is a different population than the one in our trial and therefore not considered comparable for this study. Importantly, due to the pilot nature of this trial, only potential efficacy (or lack thereof) can be established via statistical analyses.
Statistical considerations
Objective 1
Between-group (sham vs. ISF1 and ISF2) comparisons post-6 sessions will be performed for the PROs and neurophysiological measures. For this analysis, we will use LORETA-Key software and a Bayesian model with random effects to allow for baseline differences and non-specific temporal effects between participants. A sensitivity analysis will be carried out to compare this approach with an ANCOVA that includes a linear baseline adjustment.
Objective 2
Regression methods will be used to explore the relationship between changes in the primary PRO subscales (i.e. HADS-A, HADS-D) and targeted ROI activity and connectivity for all participants post 6 sessions.
Objective 3
Within-group (ISF1 and ISF2) comparisons between post-6 sessions and post-12 sessions will be performed for all outcome measures. The same model will be applied as in Objective 1 where random effects will now allow for post-6 session differences between participants.
Objective 4
Within-group (ISF1 and ISF2) comparisons between post-12 sessions and 1-month follow-up will be performed for all outcome measures. This will use the same model as Objective 1 where random effects will now allow for post 12-session differences between participants.
Objective 5
Regression analysis from Objective 2 will be extended to post 12-session and 1-month follow-up for the ISF1 and ISF2 groups.
Analyses were performed using LORETA-KEY software, R, JAGS [299] and Stan [300] with analysis-specific details described below. For all endpoints, responses were modelled assuming a normal distribution (e.g. with group-specific means and variances or via regression). When normality assumptions were not met, appropriate transformations were performed. Additionally, potential endpoint covariates were examined (e.g. age); however, none exhibited correlations that were strong enough to warrant inclusion for denoising purposes in the models. For the Bayesian analysis, JAGS or Stan was linked to R using the rjags or rstanarm library with estimates based on 3 chains of 25,000 iterations with a burn-in/warm-up = 10,000 iterations. Vague priors were used throughout.
Bayes’ theorem postulates that the probability of an event A given event B is proportional to the probability of event B given event A multiplied by the prior probability of event A:
Bayesian statistical analysis is based on the concept of prior knowledge/beliefs regarding random variables P(A) that are combined with a model relating data to those variables P(B|A) to generate a posterior probability distribution reflecting updated knowledge/beliefs about the variables given the collected data P(A|B) [301].
The describe_posterior() function in the BayestestR package [302] was used to generate posterior summary statistics including the distribution mean (M), 95% credible interval (i.e. highest density interval, HDI), probability of direction (pd), and the percentage of the full posterior within the region-of-practical-equivalence (% in ROPE).
-
The HDI is the range of parameter values with a higher probability density than values outside the HDI [303]. As such, a 95% HDI can be interpreted as a 95% probability that the true (unknown) estimate lies within the interval, given the observed data and priors [304]. In other words, it is an index of the top 95% most credible parameter values.
-
The pd is an index of the existence of an effect and is represented by the certainty (50-100%) in the direction, positive or negative [302, 305]. Put simply, it is the percentage of the posterior on the same side as the posterior’s measure of central tendency (e.g. the mean). For pd interpretation, the following reference values have been suggested:
-
≤95% = uncertain
-
>95% = possibly existing
-
>97% = likely existing
-
>99% = probably existing
-
>99.9% = certainly existing
-
-
The percentage in ROPE indexes the magnitude of an effect where the ROPE is the range of effect size values considered to be practically equivalent to the null [302, 305]. There is no uniquely correct ROPE; however, by convention, the ROPE range is often set at half the size of Cohen’s definition of small effect size (i.e. 0.2 [306];) resulting in ROPE values of ±0.1 and ±0.05 for standardized mean differences (e.g. Cohen’s d = group 1 mean – group 2 mean/pooled standard deviation) and standardized regression coefficients (i.e. sβ = coefficient from a regression on standardized variables), respectively [303]. As an aside, Cohen’s d values can be interpreted as follows: d < ±0.10 = negligible, ±0.10 < d < ±0.20 = very small, ±0.20 < d < ±0.50 = small, ±0.50 < d < ±0.80 = medium, and d > ±0.80 = large [306] whereas sβs can be interpreted as follows: sβ < ±0.20 = weak association, ±0.20 < sβ < ±0.50 = moderate association, sβ > ±0.50 = strong association [307]. For percentage in ROPE interpretation, the following reference values have been suggested:
-
>99% = negligible
-
>97.5% = probably negligible
-
≤97.5 and ≥2.5% = undecided
-
<2.5% = probably significant
-
<1% = significant
-
For all outputs, checks for the validity of assumptions regarding the residuals (i.e. normal distribution, constant and equal variances), chain convergence (e.g. trace plots), and posterior predictive model fit (e.g. Bayesian p-value) will be performed. Of note, a Bayesian p-value can be defined as “the probability, given the data, that a future observation is more extreme (as measured by some test variable) than the data” with values near 0.5 indicative of good model fit [308].
Interim analyses
Not applicable: no interim analyses will be performed, and no stopping guidelines will be established.
Methods in analysis to handle protocol non-adherence and any statistical methods to handle missing data
We will utilize complete-case analysis for all endpoints and objectives. For post 6-session and/or post 12-session/follow-up data to be included in the analysis, participants must attend a minimum of 5 out of 6 ISF-NFB sessions in each respective 6-session block. Further, we will report the number and percentages of withdrawal in each of the groups. Based on our lab group’s prior feasibility study using ISF-NFB in an obese female population [159], discontinuation/loss-to-follow-up following randomization is expected to be 10-15%.
Adverse event reporting and harms
We will systematically monitor adverse effects from the therapy for the duration of the trial using the Discontinuation-Emergent Signs and Symptoms checklist (DESS [309]) created, verbatim, in Qualtrics and completed by participants on an iPad during EEG set-ups in the interventional and post-interventional phases. Initially developed for drug trials [309], the DESS is a structured 43-item self-report that utilizes the following scale: 1=new symptom, 2=old symptom but worse, 3=old symptom but improved, 4=old symptom but unchanged, 5=symptom not present. The DESS has been used for the assessment of treatment-related side-effects in ID populations [310, 311] and, recently, has been employed to monitor adverse-effects specifically associated with NFB therapy [312]. Participants may be withdrawn from the trial by the investigators, even without their request, in the event of serious adverse effects. As detailed in the PIS, a brief (4-item) interview used during our group’s prior sLORETA ISF-NFB feasibility trial revealed that, although unusual or vivid dreams were experienced by some participants, there were no serious adverse effects [160].
Data management and processing
Participant paper files, including case-report-forms (CRFs) and MINI assessments, are to be kept in numerical order and stored in a locked room accessible only to the researchers. PROs will be electronically stored in Qualtrics with a back-up copy automatically generated and sent to the lead researcher’s trial email address. All data collected will be entered into Microsoft Excel (version 2112) and double-checked for accuracy by the data analyst at the time of entry. Participant data will be maintained for a period of not less than 10 years after the completion of the study.
Confidentiality
All information generated in this study will be considered highly confidential and is not to be shared with any persons not directly concerned with the study. For de-identification purposes, participants will be assigned unique study numbers upon enrolment. All electronic records will be identified solely using assigned study numbers and stored locally in a password-protected database. All paper records will be stored on-site in a locked office accessible only to the researchers directly involved in the trial. Furthermore, paper documents that contain personal identifiers (i.e. informed consent forms), will be stored separately from de-identified paper records (i.e. CRFs and MINIs).
Access to data
The final trial dataset will be password protected and housed locally at the research lab. Other team members will be provided access to this dataset by TMP upon request. To ensure confidentiality, data dispersed to project team members will be blinded of any identifying participant information.
Plans for collection, laboratory evaluation and storage of biological specimens for genetic or molecular analysis in this trial/future use
Not applicable: no specimens collected
Plans to give access to the full protocol, participant level-data and statistical code
The full protocol will be submitted for publication to a peer-reviewed, open-source journal prior to analyses commencement. No more than 2 years following the final data collection, we will deliver the completed, de-identified dataset and statistical code to the appropriate data archive for sharing purposes in line with the scientific imperatives of increased transparency, reproducibility, and interpretation of trials.
Oversight and monitoring
Composition of the coordinating Centre and trial steering committee
Not applicable: no coordinating centre or trial steering committee for this trial
Composition of the data monitoring committee, its role and reporting structure
Due to the relatively short duration of recruitment, non-invasive make-up of the procedures/interventions, and non-serious nature of adverse effects reported in our prior feasibility trial, no formal data monitoring committee will be established.
Frequency and plans for auditing trial conduct
Not applicable: no auditing of trial conduct will be performed.
Plans for communicating important protocol amendments to relevant parties (e.g. trial participants, ethical committees)
Substantive protocol amendments which may impact on the conduct of the study including changes to the study objectives, design, population, sample sizes, or procedures will be agreed upon by the research team, updated in the trial registry, submitted to the ethics committee for approval, and updated on our online trial advertisements and web pages.
Dissemination policy
Every effort will be made to minimize the interval between the completion of data collection and release of study results. We estimate this process to take 12 months. Irrespective of magnitude or direction of effect, results from the study will be written up and submitted to international peer-reviewed scientific journals, presented at scientific conferences, and may form part of grant applications. In addition, once compiled, all participants will be provided with a digital copy of the results.
Discussion
Approximately one in five New Zealanders is dealing with a mental illness at any given time with the majority of the population expected to experience psychopathology at some point in their lifetime [7]. Alarmingly, New Zealand’s suicide numbers are increasing with the 2017–2018 rate the highest it’s been in 20 years [7] contributing to a staggering reduction in life expectancy for mental illness sufferers of up to 25 years [7]. A recent government inquiry by the New Zealand government has shed light on the shortcomings of current treatment and called for wider implementation of non-pharmaceutical approaches in treatment of mental health problems [7]. Similarly, scientists in other parts of the world are calling for research into “novel interventions that may be based on altering plasticity or returning circuitry rather than neurotransmitter pharmacology” [313].
The implementation of safe, non-invasive neuromodulation techniques that have the potential to impact neuroplasticity within and between large-scale ICNs may offer new treatment opportunities for individuals who either do not want, respond to, or tolerate standard interventions. Additionally, these techniques may serve as adjuncts to traditional treatments, potentially enhancing their efficacy. To date, ours is the only research group studying the effects of sLORETA ISF-NFB in clinical populations. We believe targeting core ICNs via this novel therapy offers a promising new avenue in the treatment of IDs and other psychopathologies.
Trial status
Recruitment began on 15 February 2020 but was prematurely halted due to COVID-19 lockdown measures here in New Zealand. Recruitment efforts resumed on 15 June 2020, however, due to budgetary and time restrictions imposed by the lockdown, we amended our protocol. Specifically, our recruitment goal for clinical participants was changed from 80 (40 males and 40 females) to 60 females. Data collection is on track to be completed by the end of 2021.
Abbreviations
- aINS:
-
Anterior insula
- ANZCTR:
-
Australia New Zealand clinical trials registry
- CEN:
-
Central executive network
- d :
-
Cohen’s d
- dACC:
-
dorsal Anterior Cingulate Cortex
- DESS:
-
Discontinuation-emergent signs and symptoms checklist
- dlPFC:
-
Dorsolateral prefrontal cortex
- DMN:
-
Default mode network
- DSM-5:
-
Diagnostic and statistical manual of mental disorders – 5th Edition
- ECG:
-
Electrocardiography
- EEG:
-
Electroencephalography
- EEG-NFB:
-
Electroencephalographic neurofeedback
- eISFs:
-
Electrophysiological infraslow fluctuations
- eLORETA:
-
Exact low-resolution electromagnetic tomography
- EMG:
-
Electromyography
- EOG:
-
Electrooculography
- GAD:
-
Generalized anxiety disorder
- HADS:
-
Hospital Anxiety and Depression Scale
- HRV:
-
Heart rate variability
- ICN:
-
Intrinsic connectivity network
- ID:
-
Internalizing disorder
- IDAS-II:
-
Inventory of Depression and Anxiety Symptoms – Second Version
- ISAD:
-
Infraslow closed-loop brain training for Anxiety and Depression
- ISF-NFB:
-
Infraslow fluctuation neurofeedback
- ISF1:
-
1-region Infraslow neurofeedback
- ISF2:
-
2-region Infraslow neurofeedback
- IUS-12:
-
Intolerance of Uncertainty Scale – 12-item version
- MDD:
-
Major depressive disorder
- MEDI:
-
Multidimensional Emotional Disorder Inventory
- MINI:
-
Mini International Neuropsychiatric Interview
- MNI:
-
Montreal Neurological Institute
- mPFC:
-
Medial prefrontal cortex
- PCC:
-
Posterior cingulate cortex
- pd:
-
Probability of direction
- PROs:
-
Patient-reported outcomes
- ROPE:
-
Region of practical equivalence
- RTQ-10:
-
Repetitive Thinking Questionnaire – 10-item trait version
- sLORETA:
-
Standardized low-resolution electromagnetic tomography
- SN:
-
Salience network
- SOC:
-
Social anxiety disorder
References
Kessler RC, Aguilar-Gaxiola S, Alonso J, Chatterji S, Lee S, Ormel J, et al. The global burden of mental disorders: an update from the WHO World Mental Health (WMH) surveys. Epidemiologia e psichiatria sociale. 2009;18(1):23–33.
Pfeifer JH, Allen NB. Puberty initiates cascading relationships between neurodevelopmental, social, and internalizing processes across adolescence. Biol Psychiatry. 2020;89(2):99–108.
Haidt J, Allen N. Scrutinizing the effects of digital technology on mental health: Nature Publishing Group; 2020.
Keyes KM, Dahsan G, Patrick MOM, Hamilton A, Schulenberg J. Recent increases in depressive symptoms among US adolescents: trends from 1991 to 2018. Soc Psychiatry Psychiatr Epidemiol. 2019;54(8):987–96.
Duffy ME, Twenge JM, Joiner TE. Trends in mood and anxiety symptoms and suicide-related outcomes among U.S. undergraduates, 2007–2018: evidence from two National Surveys. J Adolesc Health. 2019;65(5):590–8.
Twenge JM, Cooper AB, Joiner TE, Duffy ME, Binau SG. Age, period, and cohort trends in mood disorder indicators and suicide-related outcomes in a nationally representative dataset, 2005–2017. J Abnorm Psychol. 2019;128(3):185.
Kris N. ‘Once in a generation’: the crucial passages from the mental health inquiry; 2018.
Oakley-Browne M, Wells JE, Scott KM, New Zealand. Ministry of H. Te Rau hinengaro the New Zealand mental health survey. Wellington: Ministry of Health; 2006.
Demyttenaere K, Bruffaerts R, Posada-Villa J, Gasquet I, Kovess V, Lepine JP, et al. Prevalence, severity, and unmet need for treatment of mental disorders in the World Health Organization world mental health surveys. JAMA. 2004;291(21):2581–90.
Kessler RC, Angermeyer M, Anthony JC, De Graaf R, Demyttenaere K, Gasquet I, et al. Lifetime prevalence and age-of-onset distributions of mental disorders in the World Health Organization's world mental health survey initiative. World Psych. 2007;6(3):168.
Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62(6):593.
Buchan H, Sunderland M, Carragher N, Batterham P, Slade T. Investigating age-related differences in responses to screening items for internalising disorders in three national surveys. J Affect Disord. 2014;152-154:229–36.
Kotov R, Krueger RF, Watson D, Achenbach TM, Althoff RR, Bagby RM, et al. The hierarchical taxonomy of psychopathology (HiTOP): a dimensional alternative to traditional Nosologies. J Abnorm Psychol. 2017;126(4):454–77.
Krueger RF, Eaton NR. Transdiagnostic factors of mental disorders. World Psych. 2015;14(1):27–9.
Carragher N, Krueger R, Eaton N, Slade T. Disorders without borders: current and future directions in the meta-structure of mental disorders. Soc Psychiatry Psychiatr Epidemiol. 2015;50(3):339–50.
Rhee SH, Lahey BB, Waldman ID. Comorbidity among dimensions of childhood psychopathology: converging evidence from behavior genetics; 2015. p. 26–31.
de Graaf R, ten Have M, Tuithof M, van Dorsselaer S. First-incidence of DSM-IV mood, anxiety and substance use disorders and its determinants: results from the Netherlands mental health survey and incidence Study-2. J Affect Disord. 2013;149(1):100–7.
McDowell RD, Ryan A, Bunting BP, O'Neill SM, Alonso J, Bruffaerts R, et al. Mood and anxiety disorders across the adult lifespan: a European perspective. Pychol Med. 2014;44(4):707–22.
Kessler RC, Sampson NA, Berglund P, Gruber MJ, Al-Hamzawi A, Andrade L, et al. Anxious and non-anxious major depressive disorder in the World Health Organization world mental health surveys. Epidemiol Psych Sci. 2015;24(3):210.
Altemus M, Sarvaiya N, Neill Epperson C. Sex differences in anxiety and depression clinical perspectives. Front Neuroendocrinol. 2014;35(3):320–30.
Asher M, Asnaani A, Aderka IM. Gender differences in social anxiety disorder: a review. Clin Psychol Rev. 2017;56:1–12.
Bandelow B, Michaelis S. Epidemiology of anxiety disorders in the 21st century. Dialogues Clin Neurosci. 2015;17(3):327.
Hammen C. Risk factors for depression: an autobiographical review. Annu Rev Clin Psychol. 2018;14(1):1–28.
Hoertel N, McMahon K, Olfson M, Wall MM, Rodríguez-Fernández JM, Lemogne C, et al. A dimensional liability model of age differences in mental disorder prevalence: evidence from a national sample. J Psychiatr Res. 2015;64:107–13.
de Graaf R, ten Have M, van Gool C, van Dorsselaer S. Prevalence of mental disorders and trends from 1996 to 2009. Results from the Netherlands mental health survey and incidence Study-2. Soc Psychiatry Psychiatr Epidemiol. 2012;47(2):203–13.
Blanco C, Vesga-López O, Stewart JW, Liu S-M, Grant BF, Hasin DS. Epidemiology of major depression with atypical features: results from the National Epidemiologic Survey on alcohol and related conditions (NESARC). J Clin Psych. 2012;73(2):224.
Ohayon MM, Schatzberg AF. Social phobia and depression: prevalence and comorbidity. J Psychosom Res. 2010;68(3):235–43.
Stein DJ, Lim CCW, Roest AM, de Jonge P, Aguilar-Gaxiola S, Al-Hamzawi A, et al. The cross-national epidemiology of social anxiety disorder: data from the world mental health survey initiative. BMC Med. 2017;15(1):143.
Kessler RC, Bromet EJ. The epidemiology of depression across cultures. Annu Rev Public Health. 2013;34(1):119–38.
Kim Y-K, Yoon H-K. Common and distinct brain networks underlying panic and social anxiety disorders. Prog Neuro-Psychopharmacol Biol Psychiatry. 2018;80(Pt B):115–22.
Menon V. Large-scale brain networks and psychopathology: a unifying triple network model. Trends Cogn Sci. 2011;15(10):483–506.
Xu J, Van Dam NT, Feng C, Luo Y, Ai H, Gu R, et al. Anxious brain networks: a coordinate-based activation likelihood estimation meta-analysis of resting-state functional connectivity studies in anxiety. Neurosci Biobehav Rev. 2019;96:21–30.
Hamilton JP, Farmer M, Fogelman P, Gotlib IH. Depressive rumination, the default-mode network, and the dark matter of clinical neuroscience. Biol Psychiatry. 2015;78(4):224–230.
Buckner RL, Andrews-Hanna JR, Schacter DL. The Brain's default network. Ann N Y Acad Sci. 2008;1124(1):1–38.
Raichle M. The Brain's default mode network. Annu Rev Neurosci. 2015;38:433.
Uddin LQ, Supekar KS, Ryali S, Menon V. Dynamic reconfiguration of structural and functional connectivity across Core neurocognitive brain networks with development. J Neurosci. 2011;31(50):18578.
Sridharan D, Levitin DJ, Menon V. Critical role for the right fronto-insular cortex in switching between central-executive and default-mode networks. P Natl Acad Sci USA. 2008;105(34):12569–74.
Elton A, Gao W. Divergent task-dependent functional connectivity of executive control and salience networks. Cortex. 2014;51(1):56–66.
Uddin LQ. Salience processing and insular cortical function and dysfunction. Nat Rev Neurosci. 2014;16:55.
Liang X, Zou Q, He Y, Yang Y. Topologically reorganized connectivity architecture of default-mode, executive-control, and salience networks across working memory task loads. Cereb Cortex. 2016;26(4):1501–11.
Dosenbach NUF, Fair DA, Cohen AL, Schlaggar BL, Petersen SE. A dual-networks architecture of top-down control; 2008. p. 99–105.
Dai L, Zhou H, Xu X, Zuo Z. Brain structural and functional changes in patients with major depressive disorder: a literature review. PeerJ. 2019;7:e8170.
Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, et al. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci. 2007;27(9):2349–56.
Menon V. Salience network; 2015. p. 597–611.
Ben Simon E, Rossi A, Harvey AG, Walker MP. Overanxious and underslept. Nature human. Behaviour. 2020;4:100–110
Cersosimo MG, Benarroch EE. Chapter 5 - central control of autonomic function and involvement in neurodegenerative disorders. In: Buijs RM, Swaab DF, editors. Handbook of clinical neurology. 117: Elsevier; 2013. p. 45–57.
Critchley HD, Nagai Y, Gray MA, Mathias CJ. Dissecting axes of autonomic control in humans: insights from neuroimaging. Auton Neurosci-Basic. 2011;161(1-2):34–42.
Palma EJ-A, Benarroch EE. Neural control of the heart: recent concepts and clinical correlations. Neurology. 2014;83(3):261–71.
Beissner F, Meissner K, Bar KJ, Napadow V. The autonomic brain: an activation likelihood estimation meta-analysis for central processing of autonomic function. J Neurosci. 2013;33(25):10503.
Sie J-H, Chen Y-H, Shiau Y-H, Chu W-C. Gender- and age-specific differences in resting-state functional connectivity of the central autonomic network in adulthood. Front Hum Neurosci. 2019;13:369.
Beauchaine TP. Respiratory sinus arrhythmia: a Transdiagnostic biomarker of emotion Dysregulation and psychopathology. Curr Opin Psychol. 2015;3:43.
Alvares GA, Quintana DS, Hickie IB, Guastella AJ. Autonomic nervous system dysfunction in psychiatric disorders and the impact of psychotropic medications: a systematic review and meta-analysis. J Psych Neurosci. 2016;41(2):89–104.
Niv S. Clinical efficacy and potential mechanisms of neurofeedback. Personal Individ Differ. 2013;54(6):676.
Menon B. Towards a new model of understanding - the triple network, psychopathology and the structure of the mind. Med Hypotheses. 2019;133:109385.
Menon V. Brain networks and cognitive impairment in psychiatric disorders. World Psych. 2020;19(3):309–10.
Janiri D, Moser DA, Doucet GE, Luber MJ, Rasgon A, Lee WH, et al. Shared neural phenotypes for mood and anxiety disorders: a meta-analysis of 226 task-related functional imaging studies. JAMA Psych. 2020;77(2):172–9.
Sha Z, Wager TD, Mechelli A, He Y. Common dysfunction of large-scale neurocognitive networks across psychiatric disorders. Biol Psychiatry. 2019;85(5):379–88.
Rayner G, Jackson G, Wilson S. Cognition-related brain networks underpin the symptoms of unipolar depression: evidence from a systematic review. Neurosci Biobehav Rev. 2016;61:53–65.
Yang X, Liu J, Meng Y, Xia M, Cui Z, Wu X, et al. Network analysis reveals disrupted functional brain circuitry in drug-naive social anxiety disorder. Neuroimage. 2019;190:213–23.
Perez TM, Glue P, Adhia DB, Dillingham P, Zeng J, Navid MS, et al. Transdiagnostic, brain-Centred approaches to psychopathology: a study in Internalzing disorders. Dunedin: University of Otago; 2022.
Aoki Y, Ishii R, Pascual-Marqui RD, Canuet L, Ikeda S, Hata M, et al. Detection of EEG-resting state independent networks by eLORETA-ICA method. Front Hum Neurosci. 2015;9:31.
Pascual-Marqui RD. Standardized low resolution brain electromagnetic tomography (SLORETA): technical details. Methods Find Exp Clin Pharmacol. 2002;24 Suppl D:5–12.
Vanhatalo S, Voipio J, Kaila K. Full-band EEG (FbEEG): a new standard for clinical electroencephalography. Clin EEG Neurosci. 2005;36(4):311–7.
Aladjalova N, Kol'tsova A. Very slow rhythmic variations in the potential of the nuclei of the hypothalamus and the thalamus. Bull Exp Biol Med. 1958;46(4):1153–7.
Aladjalova NA. Infra-slow rhythmic oscillations of the steady potential of the cerebral cortex. Nature. 1957;179(4567):957–9.
Aladjalova NA. Slow electrical processes in the brain. Amsterdam: Elsevier Pub. co.; 1964.
Vanhatalo S, Palva JM, Holmes MD, Miller JW, Voipio J, Kaila K. Infraslow oscillations modulate excitability and interictal epileptic activity in the human cortex during sleep. P Natl Acad Sci USA. 2004;101(14):5053–7.
He BJ, Raichle ME. The fMRI signal, slow cortical potential and consciousness; 2009. p. 302–9.
Niedermeyer E, Schomer DL, Lopes da Silva FH. Niedermeyer's electroencephalography : basic principles, clinical applications, and related fields. 6th ed. Philadelphia: Wolters Kluwer/Lippincott Williams & Wilkins Health; 2011.
Smith M. Infra-slow fluctuation training; on the down-low in neuromodulation; 2013.
He BJ, Snyder AZ, Zempel JM, Smyth MD, Raichle ME. Electrophysiological correlates of the brain's intrinsic large-scale functional architecture. P Natl Acad Sci USA. 2008;105(41):16039.
Khader P, Schicke T, Röder B, Rösler F. On the relationship between slow cortical potentials and BOLD signal changes in humans. Int J Psychophysiol. 2008;67(3):252–61.
Pan WJ, Thompson GJ, Magnuson ME, Jaeger D, Keilholz S. Infraslow LFP correlates to resting-state fMRI BOLD signals. Neuroimage. 2013;74:288–97.
Leistner HS, Sander HT, Wuebbeler HG, Link HA, Elster HC, Curio HG, et al. Magnetoencephalography discriminates modality-specific infraslow signals less than 0.1 Hz. NeuroReport. 2010;21(3):196–200.
Krishnan G, González O, Bazhenov M. Origin of slow spontaneous resting-state neuronal fluctuations in brain networks. P Natl Acad Sci USA. 2018;115(26):6858.
Watson BO. Cognitive and physiologic impacts of the Infraslow oscillation. Front Syst Neurosci. 2018;12:44.
Timofeev I, Grenier F, Bazhenov M, Sejnowski T, Steriade M. Origin of slow cortical oscillations in Deafferented cortical slabs. Cereb Cortex. 2000;10(12):1185.
Lőrincz ML, Geall F, Bao Y, Crunelli V, Hughes SW. ATP-dependent infra-slow (<0.1 Hz) oscillations in thalamic networks (infra-slow oscillations). PLoS One. 2009;4(2):e4447.
Hughes SW, Lőrincz ML, Parri HR, Crunelli V. Infraslow (< 0.1 Hz) oscillations in thalamic relay nuclei: basic mechanisms and significance to health and disease states. Prog Brain Res. 2011;193:145–62.
van Putten MJAM, Tjepkema-Cloostermans MC, Hofmeijer J. Infraslow EEG activity modulates cortical excitability in postanoxic encephalopathy. J Neurophysiol. 2015;113(9):3256–67.
Filippov IV, Williams WC, Frolov VA. Very slow potential oscillations in locus coeruleus and dorsal raphe nucleus under different illumination in freely moving rats. Neurosci Lett. 2004;363(1):89–93.
Dash MB, Ajayi S, Folsom L, Gold PE, Korol DL. Spontaneous Infraslow fluctuations modulate hippocampal EPSP-PS coupling. eNeuro. 2018;5(1):ENEURO.0403-17.2017.
Abbas A, Belloy M, Kashyap A, Billings J, Nezafati M, Schumacher EH, et al. Quasi-periodic patterns contribute to functional connectivity in the brain. Neuroimage. 2019;191:193–204.
Grooms JK, Thompson GJ, Pan WJ, Billings J, Schumacher EH, Epstein CM, et al. Infraslow electroencephalographic and dynamic resting state network activity. Brain Connect. 2017;7(5):265–80.
Buzsáki G, Draguhn A. Neuronal oscillations in cortical networks. Science (New York, NY). 2004;304(5679):1926–9.
Palva JM, Palva S. Roles of multiscale brain activity fluctuations in shaping the variability and dynamics of psychophysical performance. Prog Brain Res. 2011;193:335–50.
Palva JM, Palva S. Infra-slow fluctuations in electrophysiological recordings, blood-oxygenation-level-dependent signals, and psychophysical time series. Neuroimage. 2012;62(4):2201–11.
Engel Andreas K, Gerloff C, Hilgetag Claus C, Nolte G. Intrinsic coupling modes: multiscale interactions in ongoing brain activity. Neuron. 2013;80(4):867–86.
Weaver KE, Wander JD, Ko AL, Casimo K, Grabowski TJ, Ojemann JG, et al. Directional patterns of cross frequency phase and amplitude coupling within the resting state mimic patterns of fMRI functional connectivity. Neuroimage. 2016;128:238–51.
Florin E, Baillet S. The brain's resting-state activity is shaped by synchronized cross-frequency coupling of neural oscillations. Neuroimage. 2015;111:26–35.
Wang L, Saalmann Yuri B, Pinsk Mark A, Arcaro Michael J, Kastner S. Electrophysiological low-frequency coherence and cross-frequency coupling contribute to BOLD connectivity. Neuron. 2012;76(5):1010–20.
Perlman K, Benrimoh D, Israel S, Rollins C, Brown E, Tunteng J-F, et al. A systematic meta-review of predictors of antidepressant treatment outcome in major depressive disorder. J Affect Disord. 2019;243:503–15.
Fornaro M, Anastasia A, Novello S, Fusco A, Pariano R, De Berardis D, et al. The emergence of loss of efficacy during antidepressant drug treatment for major depressive disorder: an integrative review of evidence, mechanisms, and clinical implications. Pharmacol Res. 2019;139:494–502.
Iosifescu D. Electroencephalography-derived biomarkers of antidepressant response. Harvard Rev Psych. 2011;19(3):144–54.
Furukawa TA, Kato T, Shinagawa Y, Miki K, Fujita H, Tsujino N, et al. Prediction of remission in pharmacotherapy of untreated major depression: development and validation of multivariable prediction models. Psychol Med. 2019;49(14):2405–13.
Choi KW, Jeon HJ. Heart rate variability for the prediction of treatment response in major depressive disorder. Front Psych. 2020;11:607.
Nicholson AA, Ros T, Jetly R, Lanius RA. Regulating posttraumatic stress disorder symptoms with neurofeedback: regaining control of the mind. J Milit Vet Fam Health. 2020;6(S1):3–15.
Seibell PJ, Hollander E. Management of obsessive-compulsive disorder. F1000 Prime Rep. 2014;6:68.
Haller SPW, Cohen Kadosh K, Scerif G, Lau JYF. Social anxiety disorder in adolescence: how developmental cognitive neuroscience findings may shape understanding and interventions for psychopathology. Dev Cognit Neurosci. 2015;13(C):11–20.
Möller H-J, Bandelow B, Volz H-P, Barnikol UB, Seifritz E, Kasper S. The relevance of ‘mixed anxiety and depression’ as a diagnostic category in clinical practice. Eur Arch Psychiatry Clin Neurosci. 2016;266(8):725–36.
Andrade LH, Alonso J, Mneimneh Z, Wells JE, Al-Hamzawi A, Borges G, et al. Barriers to mental health treatment: results from the WHO world mental health surveys. Psychol Med. 2014;44(6):1303–17.
Schoenberg P, David A. Biofeedback for psychiatric disorders: a systematic review. Appl Psychophysiol Biofeedback. 2014;39(2):109–35.
Pinter A, Szatmari S Jr, Horvath T, Penzlin AI, Barlinn K, Siepmann M, et al. Cardiac dysautonomia in depression - heart rate variability biofeedback as a potential add-on therapy. Neuropsychiatr Dis Treat. 2019;15:1287–310.
Gøtzsche PC. Why I think antidepressants cause more harm than good. Lancet Psychiatry. 2014;1(2):104.
Andersohn F, Schade R, Suissa S, Garbe E. Long-term use of antidepressants for depressive disorders and the risk of diabetes mellitus. Am J Psychiatry. 2009;166(5):591–8.
Cannon R, Congedo M, Lubar J, Hutchens T. Differentiating a network of executive attention: Loreta Neurofeedback in anterior cingulate and dorsolateral prefrontal cortices. Int J Neurosci. 2009;119(3):404–41.
Rance M, Walsh C, Sukhodolsky DG, Pittman B, Qiu M, Kichuk SA, et al. Time course of clinical change following neurofeedback. Neuroimage. 2018;181:807–13.
Schabus M, Griessenberger H, Gnjezda MT, Heib DPJ, Wislowska M, Hoedlmoser K. Better than sham? A double-blind placebo-controlled neurofeedback study in primary insomnia. Brain. 2017;140(4):1041–52.
Thibault RT, Raz A. Neurofeedback: the power of psychosocial therapeutics. Lancet Psychiatry. 2016;3(11):e18.
Thibault RT, Lifshitz M, Raz A. The climate of neurofeedback: scientific rigour and the perils of ideology. Brain. 2018;141(2):e11-e.
Thibault RT, Raz A. When can neurofeedback join the clinical armamentarium? Lancet Psychiatry. 2016;3(6):497–8.
Thibault RT, Lifshitz M, Birbaumer N, Raz A. Neurofeedback, self-regulation, and brain imaging: clinical science and fad in the service of mental disorders. Psychother Psychosom. 2015;84(4):193–207.
Thibault RT, Lifshitz M, Raz A. The self-regulating brain and neurofeedback: experimental science and clinical promise. Cortex. 2016;74:247–61.
Thibault RT, Lifshitz M, Raz A. Neurofeedback or neuroplacebo? Brain. 2017;140(4):862–4.
Thibault RT, Raz A. The psychology of Neurofeedback: clinical intervention even if applied placebo. Am Psychol. 2017;72(7):679–88.
Schönenberg M, Wiedemann E, Schneidt A, Scheeff J, Logemann A, Keune PM, et al. Neurofeedback, sham neurofeedback, and cognitive-behavioural group therapy in adults with attention-deficit hyperactivity disorder: a triple-blind, randomised, controlled trial. Lancet Psychiatry. 2017;4(9):673–84.
Schönenberg M, Wiedemann E, Schneidt A, Scheeff J, Logemann A, Keune PM, et al. In: Schönenberg M, editor. Confusion regarding operant conditioning of the EEG - Authors' reply; 2017. p. 897–8.
Arnold LE, Arns M, Barterian J, Bergman R, Black S, Conners CK, et al. Double-blind placebo-controlled randomized clinical trial of Neurofeedback for attention-deficit/hyperactivity disorder with 13 month follow-up. J Am Acad Child Adolesc Psychiatry. 2021;60(7):841–55.
Ghaziri J, Thibault RT. 19 - Neurofeedback: an inside perspective. In: Raz A, Thibault RT, editors. Casting light on the dark side of brain imaging: Academic; 2019. p. 113–6.
Kerson C. A proposed multisite double-blind randomized clinical trial of Neurofeedback for ADHD: need, rationale, and strategy. J Atten Disord. 2013;17(5):420–36.
Arns M, Heinrich H, Strehl U. Evaluation of neurofeedback in ADHD: the long and winding road. Biol Psychol. 2014;95:108–15.
Witte M, Kober SE, Wood G. Noisy but not placebo: defining metrics for effects of neurofeedback. Brain. 2018;141(5):e40-e.
Zuberer A, Brandeis D, Drechsler R. Are treatment effects of neurofeedback training in children with ADHD related to the successful regulation of brain activity? A review on the learning of regulation of brain activity and a contribution to the discussion on specificity. Front Hum Neurosci. 2015;9:135.
Szewczyk RŁ, Ratomska M, Jaśkiewicz M. The neglected problem of the Neurofeedback learning (in)ability. Biomedical engineering and neuroscience. Cham: Springer International Publishing; 2018.
Sherlin LH, Arns M, Lubar J, Heinrich H, Kerson C, Strehl U, et al. Neurofeedback and basic learning theory: implications for research and practice. J Neurother. 2011;15(4):292–304.
Holtmann M, Sonuga-Barke E, Cortese S, Brandeis D. Neurofeedback for ADHD: a review of current evidence. Child Adolesc Psychiatr Clin N Am. 2014;23(4):789–806.
Trullinger M, Novian A, Russell-Chapin L, Pradhan D. Perspectives on type III statistical errors: exaggerating the effects of placebo in Neurofeedback. NeuroRegulation. 2019;6(1):38.
Pigott HE, Cannon R, Trullinger M. The fallacy of sham-controlled Neurofeedback trials: a reply to Thibault and colleagues (2018). J Atten Disord. 2021;25(3):448–57.
Weber LA, Ethofer T, Ehlis A-C. Predictors of neurofeedback training outcome: a systematic review. NeuroImage: Clin. 2020;27:102301.
Vaitl D, Birbaumer N, Gruzelier J, Jamieson GA, Kotchoubey B, Kubler A, et al. Psychobiology of altered states of consciousness. Psychol Bull. 2005;131(1):98–127.
Imperatori C, Valenti EM, Della Marca G, Amoroso N, Massullo C, Carbone GA, et al. Coping food craving with neurofeedback. Evaluation of the usefulness of alpha/theta training in a non-clinical sample. Int J Psychophysiol. 2017;112:89–97.
Kluetsch RC, Ros T, Theberge J, Frewen PA, Calhoun VD, Schmahl C, et al. Plastic modulation of PTSD resting-state networks and subjective wellbeing by EEG neurofeedback. Acta Psychiatr Scand. 2014;130(2):123–36.
Lackner N, Unterrainer HF, Skliris D, Shaheen S, Dunitz-Scheer M, Wood G, et al. EEG neurofeedback effects in the treatment of adolescent anorexia nervosa. Eat Disord. 2016;24(4):354–74.
Raymond J, Varney C, Parkinson LA, Gruzelier JH. The effects of alpha/theta neurofeedback on personality and mood. Cogn Brain Res. 2005;23(2-3):287–92.
Trudeau DL. EEG biofeedback for addictive disorders - the state of the art in 2004. J Adult Dev. 2005;12(2-3):139–46.
Cheon EJ, Choi JH, Lee GW, Koo BH, Seo WS, Kim HG, et al. P.2.F.009 - Neurofeedback treatment on depressive symptoms and functional recovery and brain-derived neurotrophic factor in treatment-resistant major depression. Eur Neuropsychopharmacol. 2017;27:S851.
Liu H. Neurofeedback training intervention for persons with major depression disorder: reducing depressive symptoms. NeuroQuantology. 2017;15(3):1091.
Wang S-Y, Lin IM, Fan S-Y, Tsai Y-C, Yen C-F, Yeh Y-C, et al. The effects of alpha asymmetry and high-beta down-training neurofeedback for patients with the major depressive disorder and anxiety symptoms. J Affect Disord. 2019;257:287–96.
Chiba T, Kanazawa T, Koizumi A, Ide K, Taschereau-Dumouchel V, Boku S, et al. Current status of Neurofeedback for post-traumatic stress disorder: a systematic review and the possibility of decoded Neurofeedback. Front Hum Neurosci. 2019;13:233.
Gapen M, van der Kolk BA, Hamlin E, Hirshberg L, Suvak M, Spinazzola J. A pilot study of Neurofeedback for chronic PTSD. Appl Psychophysiol Biofeedback. 2016;41(3):251–61.
Chrapusta A, Pąchalska M, Wilk-Frańczuk M, Starczyńska M, Kropotov JD. Evaluation of the effectiveness of neurofeedback in the reduction of posttraumatic stress disorder (PTSD) in a patient following high-voltage electric shock with the use of ERPs. Ann Agric Environ Med. 2015;22(3):556–63.
Van Der Kolk BA, Hodgdon H, Gapen M, Musicaro R, Suvak MK, Hamlin E, et al. A randomized controlled study of neurofeedback for chronic PTSD. PLoS One. 2016;11(12):e0166752.
Wang SY, Lin IM, Peper E, Chen YT, Tang TC, Yeh YC, et al. The efficacy of neurofeedback among patients with major depressive disorder: preliminary study. NeuroRegulation. 2016;3(3):127–34.
Cheon E-J, Koo B-H, Choi J-H. The efficacy of Neurofeedback in patients with major depressive disorder: an open labeled prospective study. Appl Psychophysiol Biofeedback. 2016;41(1):103–10.
Bell AN, Moss D, Kallmeyer RJ. Healing the neurophysiological roots of trauma: a controlled study examining loreta z-score neurofeedback and HRV biofeedback for chronic PTSD. NeuroRegulation. 2019;6(2):54–70.
Askovic M, Watters AJ, Aroche J, Harris AW. Neurofeedback as an adjunct therapy for treatment of chronic posttraumatic stress disorder related to refugee trauma and torture experiences: two case studies. Australasian Psych. 2017;25(4):358–63.
Askovic M, Watters AJ, Coello M, Aroche J, Harris AWF, Kropotov J. Evaluation of Neurofeedback for posttraumatic stress disorder related to refugee experiences using self-report and cognitive ERP measures. Clin EEG Neurosci. 2020;51(2):79–86.
Cheon E-J, Koo B-H, Seo W-S, Lee J-Y, Choi J-H, Song S-H. Effects of Neurofeedback on adult patients with psychiatric disorders in a naturalistic setting. Appl Psychophysiol Biofeedback. 2015;40(1):17–24.
Zhang P, Cheng L. A randomized controlled trial of a Neurofeedback-based training for improvement in social phobia disorder. NeuroQuantology. 2017;15(4):133–38.
Lu Y, Wang C, Su L, Ma Z, Li S, Fan Y. Effects of Neurofeedback on panic disorder patients’ anxiety. NeuroQuantology. 2017;15(3):172–78.
Ros T, Frewen P, Théberge J, Michela A, Kluetsch R, Mueller A, et al. Neurofeedback tunes scale-free dynamics in spontaneous brain activity. Cereb Cortex. 2017;27(10):4911–22.
Orndorff-Plunkett F, Singh F, Aragón O, Pineda J. Assessing the effectiveness of Neurofeedback training in the context of clinical and social neuroscience. Brain Sci. 2017;7(8):95.
Reiter BK, Andersen BS, Carlsson BJ. Neurofeedback treatment and posttraumatic stress disorder: effectiveness of Neurofeedback on posttraumatic stress disorder and the optimal choice of protocol. J Nerv Ment Dis. 2016;204(2):69–77.
Panisch LS, Hai AH. The effectiveness of using Neurofeedback in the treatment of post-traumatic stress disorder: a systematic review. Trauma Violence Abuse. 2018;21(3):541–50.
Hou Y, Zhang S, Li N, Huang Z, Wang L, Wang Y. Neurofeedback training improves anxiety trait and depressive symptom in GAD. Brain Behavior. 2021;11(3):e02024.
Othmer S, Othmer S. Post traumatic stress disorder-the Neurofeedback remedy. Biofeedback. 2009;37(1):24–31.
Grin-Yatsenko VA, Othmer S, Ponomarev VA, Evdokimov SA, Konoplev YY, Kropotov JD. Infra-low frequency neurofeedback in depression: three case studies. NeuroRegulation. 2018;5(1):30–42.
Villanueva M, Benson A, LaDou T. Clinical practice and observations of infralow neurofeedback as an adjunctive treatment within Camp Pendleton’s deployment health center. NCCOSC Confer. 2011:27–37
Leong SL, Vanneste S, Lim J, Smith M, Manning P, De Ridder D. A randomised, double-blind, placebo-controlled parallel trial of closed-loop infraslow brain training in food addiction. Sci Rep. 2018;8(1):11659
Leong SL, Vanneste S, Lim J, Smith M, Manning P, De Ridder D, et al. Effects and side effects of Infraslow network Neurofeedback (ISF-NF): a randomised double blind placebo controlled trial. Int J Psychophysiol. 2018;131:S151.
Goldstein-Piekarski AN, Williams LM, Humphreys K. A trans-diagnostic review of anxiety disorder comorbidity and the impact of multiple exclusion criteria on studying clinical outcomes in anxiety disorders. Transl Psychiatry. 2016;6(6):e847.
Ehring T, Watkins E. Repetitive negative thinking as a Transdiagnostic process. Int J Cogn Ther. 2008;1:192–205.
Epkins C, Heckler D. Integrating etiological models of social anxiety and depression in youth: evidence for a cumulative interpersonal risk model. Clin Child Fam Psychol Rev. 2011;14(4):329–76.
Cummings CM, Caporino NE, Kendall PC. Comorbidity of anxiety and depression in children and adolescents: 20 years after. Psychol Bull. 2014;140(3):816–45.
Lahey BB, Krueger RF, Rathouz PJ, Waldman ID, Zald DH. A hierarchical causal taxonomy of psychopathology across the life span. Psychol Bull. 2017;143(2):142–86.
Zald DH, Lahey BB. Implications of the hierarchical structure of psychopathology for psychiatric neuroimaging. Biol Psych Cognit Neurosci Neuroimaging. 2017;2(4):310–7.
Dickinson D. “If the shoe fits …”: the hierarchical structure of psychopathology and psychiatric neuroimaging. Biol Psych Cogn Neurosci Neuroimaging. 2017;2(4):303–4.
Bui E, Fava M. From depression to anxiety, and back. Acta Psychiatr Scand. 2017;136(4):341–2.
Wenzel A, Jager-Hyman S. Chapter 9 - social anxiety disorder and its relation to clinical syndromes in adulthood. In: Hofmann SG, DiBartolo PM, editors. Social anxiety. 3rd ed. San Diego: Academic; 2014. p. 227–51.
Scholten WD, Batelaan NM, Penninx BWJH, Balkom AJLM, Smit JH, Schoevers RA, et al. Diagnostic instability of recurrence and the impact on recurrence rates in depressive and anxiety disorders. J Affect Disord. 2016;195:185–90.
McTeague LM, Goodkind MS, Etkin A. Transdiagnostic impairment of cognitive control in mental illness. J Psychiatr Res. 2016;83:37–46.
Begemann MJ, Florisse EJ, van Lutterveld R, Kooyman M, Sommer IE. Efficacy of EEG neurofeedback in psychiatry: a comprehensive overview and meta-analysis. Transl Brain Rhythmicity. 2016;1(1):19–29.
Arns M, Batail JM, Bioulac S, Congedo M, Daudet C, Drapier D, et al. Neurofeedback: one of today's techniques in psychiatry? L'Encéphale. 2017;43(2):135–45.
Omejc N, Rojc B, Battaglini PP, Marusic U. Review of the therapeutic neurofeedback method using electroencephalography: EEG Neurofeedback. Bosnian J Basic Med Sci. 2019;19(3):213.
Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, et al. The Mini-international neuropsychiatric interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psych. 1998;59(Suppl 20):22.
Lecrubier Y, Sheehan DV, Weiller E, Amorim P, Bonora I, Harnett Sheehan K, et al. The MINI international neuropsychiatric interview (MINI). A short diagnostic structured interview: reliability and validity according to the CIDI. Eur Psych. 1997;12(5):224–31.
Sheehan DV, Lecrubier Y, Harnett Sheehan K, Janavs J, Weiller E, Keskiner A, et al. The validity of the MINI international neuropsychiatric interview (MINI) according to the SCID-P and its reliability. Eur Psych. 1997;12(5):232–41.
Posner K, Brown GK, Stanley B, Brent DA, Yershova KV, Oquendo MA, et al. The Columbia-suicide severity rating scale: initial validity and internal consistency findings from three multisite studies with adolescents and adults. Am J Psychiatr. 2011;168(12):1266–77.
Barnett AG, van der Pols JC, Dobson AJ. Regression to the mean: what it is and how to deal with it. Int J Epidemiol. 2004;34(1):215–20.
Shrout PE, Stadler G, Lane SP, McClure MJ, Jackson GL, Clavél FD, et al. Initial elevation bias in subjective reports. Proc Natl Acad Sci. 2018;115(1):E15.
Jobert M, Wilson FJ, Ruigt GSF, Brunovsky M, Prichep LS, Drinkenburg WHIM. Guidelines for the recording and evaluation of Pharmaco-EEG data in man: the international Pharmaco-EEG society (IPEG). Neuropsychobiology. 2012;66(4):201–20.
Heathers J. Everything hertz: methodological issues in short-term frequency-domain HRV. Front Physiol. 2014;5:177.
Laborde S, Mosley E, Thayer JF. Heart rate variability and cardiac vagal tone in psychophysiological research - recommendations for experiment planning, data analysis, and data reporting. Front Psychol. 2017;8:213.
Shannon BJ, Dosenbach RA, Su Y, Vlassenko AG, Larson-Prior LJ, Nolan TS, et al. Morning-evening variation in human brain metabolism and memory circuits. J Neurophysiol. 2013;109(5):1444–56.
van Eekelen APJ, Houtveen JH, Kerkhof GA. Circadian variation in cardiac autonomic activity: reactivity measurements to different types of stressors. Chronobiol Int. 2004;21(1):107–29.
Lohmann G, Margulies DS, Horstmann A, Pleger B, Lepsien J, Goldhahn D, et al. Eigenvector centrality mapping for analyzing connectivity patterns in fMRI data of the human brain (eigenvector centrality maps). PLoS One. 2010;5(4):e10232.
Rossi P, Andriesse GI, Oey PL, Wieneke GH, Roelofs JMM, Akkermans LMA. Stomach distension increases efferent muscle sympathetic nerve activity and blood pressure in healthy humans. J Neurol Sci. 1998;161(2):148–55.
Young HA, Cousins A, Johnston S, Fletcher JM, Benton D. Autonomic adaptations mediate the effect of hydration on brain functioning and mood: evidence from two randomized controlled trials. Sci Rep. 2019;9(1):16412.
Fagius J, Karhuvaara S. Sympathetic activity and blood pressure increases with bladder distension in humans. Hypertension. 1989;14(5):511–7.
Zimmermann-Viehoff F, Thayer J, Koenig J, Herrmann C, Weber CS, Deter H-C. Short-term effects of espresso coffee on heart rate variability and blood pressure in habitual and non-habitual coffee consumers–a randomized crossover study. Nutr Neurosci. 2016;19(4):169–75.
Sondermeijer HP, van Marle AGJ, Kamen P, Krum H. Acute effects of caffeine on heart rate variability. Am J Cardiol. 2002;90(8):906–7.
Liao W, Fan Y-S, Yang S, Li J, Duan X, Cui Q, et al. Preservation effect: cigarette smoking acts on the dynamic of influences among unifying neuropsychiatric triple networks in schizophrenia. Schizophr Bull. 2019;45(6):1242.
Sjoberg N, Saint DA. A single 4 mg dose of nicotine decreases heart rate variability in healthy nonsmokers: implications for smoking cessation programs. Nicotine Tob Res. 2011;13(5):369–72.
Quintana DS, McGregor IS, Guastella AJ, Malhi GS, Kemp AH. A meta-analysis on the impact of alcohol dependence on Short-term resting-state heart rate variability: implications for cardiovascular risk. Alcohol Clin Exp Res. 2013;37(S1):E23–E9.
Quintana DS, Guastella AJ, McGregor IS, Hickie IB, Kemp AH. Moderate alcohol intake is related to increased heart rate variability in young adults: implications for health and well-being. Psychophysiology. 2013;50(12):1202–8.
Qualtrics. December, 2020 ed2005-2020.
Lane SJ, Heddle NM, Arnold E, Walker I. A review of randomized controlled trials comparing the effectiveness of hand held computers with paper methods for data collection. BMC Med Inform Decis Making. 2006;6(1):23.
Dale O, Hagen KB. Despite technical problems personal digital assistants outperform pen and paper when collecting patient diary data. J Clin Epidemiol. 2007;60(1):8–17.
Litchfield J, Freeman J, Schou H, Elsley M, Fuller R, Chubb B. Is the future for clinical trials internet-based? A cluster randomized clinical trial. Clin Trials. 2005;2(1):72–9.
Corsi-Cabrera M, Galindo-Vilchis L, Del-Río-Portilla Y, Arce C, Ramos-Loyo J. Within-subject reliability and inter-session stability of EEG power and coherent activity in women evaluated monthly over nine months. Clin Neurophysiol. 2007;118(1):9–21.
van Diessen E, Numan T, van Dellen E, van Der Kooi AW, Boersma M, Hofman D, et al. Opportunities and methodological challenges in EEG and MEG resting state functional brain network research. Clin Neurophysiol. 2015;126(8):1468–81.
Ross S, Grant A, Counsell C, Gillespie W, Russell I, Prescott R. Barriers to participation in randomised controlled trials: a systematic review. J Clin Epidemiol. 1999;52(12):1143–56.
Dallal GE. [updated 23 Dec, 2020. Available from: (http://www.randomization.com).
Scharnowski F, Veit R, Zopf R, Studer P, Bock S, Diedrichsen J, et al. Manipulating motor performance and memory through real-time fMRI neurofeedback. Biol Psychol. 2015;108:85–97.
Sepulveda P, Sitaram R, Rana M, Montalba C, Tejos C, Ruiz S. How feedback, motor imagery, and reward influence brain self-regulation using real-time fMRI. Hum Brain Mapp. 2016;37(9):3153–71.
Strehl U. What learning theories can teach us in designing neurofeedback treatments. Front Hum Neurosci. 2014;8:894.
Kober S, Witte M, Ninaus M, Neuper C, Wood G. Learning to modulate one's own brain activity: the effect of spontaneous mental strategies. Front Hum Neurosci. 2013;7:695.
Micoulaud-Franchi JA, McGonigal A, Lopez R, Daudet C, Kotwas I, Bartolomei F. Electroencephalographic neurofeedback: level of evidence in mental and brain disorders and suggestions for good clinical practice. Neurophysiologie Clinique/Clin Neurophysiol. 2015;45(6):423–33.
Gruzelier JH. EEG-neurofeedback for optimising performance. III: a review of methodological and theoretical considerations. Neurosci Biobehav Rev. 2014;44:159.
Sulzer J, Haller S, Scharnowski F, Weiskopf N, Birbaumer N, Blefari ML, et al. Real-time fMRI neurofeedback: Progress and challenges. Neuroimage. 2013;76(1):386–99.
Vanneste S, Joos K, Ost J, De Ridder D. Influencing connectivity and cross-frequency coupling by real-time source localized neurofeedback of the posterior cingulate cortex reduces tinnitus related distress. Neurobiol Stress. 2018;8:211–24.
Audacity. 2.3.2 ed1999-2020.
Sorger B, Scharnowski F, Linden DEJ, Hampson M, Young KD. Control freaks: towards optimal selection of control conditions for fMRI neurofeedback studies. Neuroimage. 2019;186:256–65.
Cannon R. LORETA Neurofeedback: odd reports, observations, and findings associated with spatial specific Neurofeedback training. J Neurother. 2012;16:164–7.
Hoffmann TC, Glasziou PP, Boutron I, Milne R, Perera R, Moher D, et al. Better reporting of interventions: template for intervention description and replication (TIDieR) checklist and guide. BMJ: Brit Med J. 2014;348:g1687.
Health USDo, Human Services FDACfDE, Research, Health USDo, Human Services FDACfBE, Research, et al. Guidance for industry: patient-reported outcome measures: use in medical product development to support labeling claims: draft guidance. Health Qual Life Outcomes. 2006;4(1):79.
Doward LC, Gnanasakthy A, Baker MG. Patient reported outcomes: looking beyond the label claim. Health Qual Life Outcomes. 2010;8:89.
Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67(6):361–70.
Bjelland I, Dahl AA, Haug TT, Neckelmann D. The validity of the hospital anxiety and depression scale: an updated literature review. J Psychosom Res. 2002;52(2):69–77.
Breeman S, Cotton S, Fielding S, Jones GT. Normative data for the hospital anxiety and depression scale. Qual Life Res. 2015;24(2):391–8.
Snaith RP. The hospital anxiety and depression scale. Health Qual Life Outcomes. 2003;1:29.
Herrmann C. International experiences with the hospital anxiety and depression scale-a review of validation data and clinical results. J Psychosom Res. 1997;42(1):17–41.
Hinz A, Brähler E. Normative values for the hospital anxiety and depression scale (HADS) in the general German population. J Psychosom Res. 2011;71(2):74–8.
Singer S, Kuhnt S, Götze H, Hauss J, Hinz A, Liebmann A, et al. Hospital anxiety and depression scale cutoff scores for cancer patients in acute care. Br J Cancer. 2009;100(6):908–12.
Cosco TD, Doyle F, Ward M, McGee H. Latent structure of the hospital anxiety and depression scale: a 10-year systematic review. J Psychosom Res. 2012;72(3):180–4.
Turk DC, Dworkin RH, Trudeau JJ, Benson C, Biondi DM, Katz NP, et al. Validation of the hospital anxiety and depression scale in patients with acute low Back pain. J Pain. 2015;16(10):1012–21.
Wynne S, Patel S, Barker RE, Jones SE, Walsh JA, Kon SS, et al. The hospital anxiety and depression scale (HADS) in bronchiectasis: response to pulmonary rehabilitation (PR) and minimum clinically important difference (MCID). Eur Respir J. 2019;54(suppl 63):PA3407.
Lemay KR, Tulloch HE, Pipe AL, Reed JL. Establishing the minimal clinically important difference for the hospital anxiety and depression scale in patients with cardiovascular disease. J Cardiopulmon Rehabil Prev. 2019;39(6):E6–E11.
Puhan MA, Frey M, Büchi S, Schünemann HJ. The minimal important difference of the hospital anxiety and depression scale in patients with chronic obstructive pulmonary disease. Health Qual Life Outcomes. 2008;6:46.
Delorme A, Makeig S. EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J Neurosci Methods. 2004;134(1):9–21.
Lopez-Calderon J, Luck SJ. ERPLAB: an open-source toolbox for the analysis of event-related potentials. Front Hum Neurosci. 2014;8:213.
Bigdely-Shamlo N, Mullen T, Kothe C, Su K-M, Robbins KA. The PREP pipeline: standardized preprocessing for large-scale EEG analysis. Front Neuroinform. 2015;9:16.
Navid MS, Niazi IK, Lelic D, Drewes AM, Haavik H. The effects of Filter’s class, cutoff frequencies, and independent component analysis on the amplitude of somatosensory evoked potentials recorded from healthy volunteers. Sensors. 2019;19(11):2610.
Navid MS, Niazi IK, Lelic D, Nedergaard RB, Holt K, Amjad I, et al. Investigating the effects of chiropractic spinal manipulation on EEG in stroke patients. Brain Sci. 2020;10(5):253.
Onton JA, Makeig S. High-frequency broadband modulation of electroencephalographic spectra. Front Hum Neurosci. 2009;3:61.
Palmer J, Kreutz-Delgado K, Makeig S. AMICA: an adaptive mixture of independent component analyzers with shared components; 2011.
Delorme A, Palmer J, Onton J, Oostenveld R, Makeig S. Independent EEG sources are dipolar. PLoS One. 2012;7(2):e30135.
Pion-Tonachini L, Kreutz-Delgado K, Makeig S. ICLabel: an automated electroencephalographic independent component classifier, dataset, and website. Neuroimage. 2019;198:181–97.
Jung TP, Makeig S, Humphries C, Lee TW, Mckeown MJ, Iragui V, et al. Removing electroencephalographic artifacts by blind source separation. Psychophysiology. 2000;37(2):163–78.
Chaumon M, Bishop DVM, Busch NA. A practical guide to the selection of independent components of the electroencephalogram for artifact correction. J Neurosci Methods. 2015;250:47–63.
de Goede AA, van Putten M. Infraslow activity as a potential modulator of corticomotor excitability. J Neurophysiol. 2019;122(1):325–35.
Pascual-Marqui RD. Discrete, 3D distributed, linear imaging methods of electric neuronal activity. Part 1: exact, zero error localization. arXiv preprint arXiv:07103341. 2007.
Pascual-Marqui RD, Lehmann D, Koukkou M, Kochi K, Anderer P, Saletu B, et al. Assessing interactions in the brain with exact low-resolution electromagnetic tomography. Philos Trans R Soc A Math Phys Eng Sci. 1952;2011(369):3768–84.
Krönke K-M, Wolff M, Shi Y, Kräplin A, Smolka MN, Bühringer G, et al. Functional connectivity in a triple-network saliency model is associated with real-life self-control. Neuropsychologia. 2020;149:107667.
Team RC. R: a language and environment for statistical computing. Vienna, Austria; 2020.
Pascual-Marqui RD, Biscay RJ, Bosch-Bayard J, Lehmann D, Kochi K, Kinoshita T, et al. Assessing direct paths of intracortical causal information flow of oscillatory activity with the isolated effective coherence (iCoh). Front Hum Neurosci. 2014;8:448.
Rosellini AJ, Brown TA. The multidimensional emotional disorder inventory (MEDI): assessing Transdiagnostic dimensions to validate a profile approach to emotional disorder classification. Psychol Assess. 2019;31(1):59–72.
Rosellini AJ, Boettcher H, Brown TA, Barlow DH. A Transdiagnostic temperament-phenotype profile approach to emotional disorder classification: an update. J Exper Psychopathol. 2015;a2(1):110.
Watson D, O’hara MW, Naragon-Gainey K, Koffel E, Chmielewski M, Kotov R, et al. Development and validation of new anxiety and bipolar symptom scales for an expanded version of the IDAS (the IDAS-II). Assessment. 2012;19(4):399–420.
Watson D, O'Hara MW. Understanding the emotional DisordersA symptom-level approach based on the IDAS-II: a symptom-level approach based on the IDAS-II: Oxford University Press; 2017. 2017-06
Carleton RN, Norton MAPJ, Asmundson GJG. Fearing the unknown: a short version of the intolerance of uncertainty scale. J Anxiety Disord. 2007;21(1):105–17.
McEvoy PM, Mahoney AEJ, Moulds ML. Are worry, rumination, and post-event processing one and the same? J Anxiety Disord. 2010;24(5):509–19.
McEvoy PM, Thibodeau MA, Asmundson GJG. Trait repetitive negative thinking: a brief Transdiagnostic assessment. Journal of experimental. Psychopathology. 2014;5(3):jep.037813.
McEvoy PM, Hyett MP, Ehring T, Johnson SL, Samtani S, Anderson R, et al. Transdiagnostic assessment of repetitive negative thinking and responses to positive affect: structure and predictive utility for depression, anxiety, and mania symptoms. J Affect Disord. 2018;232:375–84.
Osma J, Martínez-Loredo V, Quilez-Orden A, Peris-Baquero Ó, Suso-Ribera C. Validity evidence of the multidimensional emotional disorders inventory among non-clinical Spanish University students. Int J Environ Res Public Health. 2021;18(16):8251.
Brown TA, Barlow DH. A proposal for a dimensional classification system based on the shared features of the DSM–IV anxiety and mood disorders: implications for assessment and treatment. Psychol Assess. 2009;21(3):256–71.
Boettcher H, Correa J, Cassiello-Robbins C, Ametaj A, Rosellini AJ, Brown TA, et al. Dimensional assessment of emotional disorder outcomes in Transdiagnostic treatment: a clinical case study. Cogn Behav Pract. 2020;27(4):2020:442–53.
Stasik-O’brien SM, Brock RL, Chmielewski M, Naragon-Gainey K, Koffel E, McDade-Montez E, et al. Clinical utility of the inventory of depression and anxiety symptoms (IDAS). Assessment. 2019;26(5):944–60.
McEvoy PM, Mahoney AEJ. Achieving certainty about the structure of intolerance of uncertainty in a treatment-seeking sample with anxiety and depression.(report). J Anxiety Disord. 2011;25(1):112.
Carleton RN, Mulvogue MK, Thibodeau MA, McCabe RE, Antony MM, Asmundson GJG. Increasingly certain about uncertainty: intolerance of uncertainty across anxiety and depression. J Anxiety Disord. 2012;26(3):468–79.
Hale W, Richmond M, Bennett J, Berzins T, Fields A, Weber D, et al. Resolving uncertainty about the intolerance of uncertainty Scale-12: application of modern psychometric strategies. J Pers Assess. 2016;98(2):200–8.
Lauriola M, Mosca O, Carleton RN. Hierarchical factor structure of the intolerance of uncertainty scale short form (IUS-12) in the Italian version. TPM: Test Psychometr Methodol Appl Psychol. 2016;23(3):377–94.
Shihata S, McEvoy PM, Mullan BA. A Bifactor model of intolerance of uncertainty in undergraduate and clinical samples: do we need to reconsider the two-factor model? Psychol Assess. 2018;30(7):893–903.
Birrell J, Meares K, Wilkinson A, Freeston M. Toward a definition of intolerance of uncertainty: a review of factor analytical studies of the intolerance of uncertainty scale. Clin Psychol Rev. 2011;31(7):1198–208.
Hong RY, Lee SSM. Further clarifying prospective and inhibitory intolerance of uncertainty: factorial and construct validity of test scores from the intolerance of uncertainty scale. Psychol Assess. 2015;27(2):605–20.
Fetzner M, Horswill S, Boelen P, Carleton R. Intolerance of uncertainty and PTSD symptoms: exploring the construct relationship in a community sample with a heterogeneous trauma history. Cogn Ther Res. 2013;37(4):725–34.
Helsen K, Van Den Bussche E, Vlaeyen JWS, Goubert L. Confirmatory factor analysis of the Dutch intolerance of uncertainty scale: comparison of the full and short version. J Behav Ther Exp Psychiatry. 2013;44(1):21–9.
McEvoy PM, Mahoney AEJ. To be sure, to be sure: intolerance of uncertainty mediates symptoms of various anxiety disorders and depression. Behav Ther. 2012;43(3):533–45.
Khawaja NG, Yu LNH. A comparison of the 27-item and 12-item intolerance of uncertainty scales. Clin Psychol. 2010;14(3):97–106.
Jacoby RJ, Fabricant LE, Leonard RC, Riemann BC, Abramowitz JS. Just to be certain: confirming the factor structure of the intolerance of uncertainty scale in patients with obsessive-compulsive disorder. J Anxiety Disord. 2013;27(5):535–42.
Boelen PA, Lenferink LIM. Latent class analysis of indicators of intolerance of uncertainty. Scand J Psychol. 2018;59(3):243–51.
Oglesby M, Allan N, Short N, Raines A, Schmidt N. Factor mixture modeling of intolerance of uncertainty. Psychol Assess. 2017;29(4):435–45.
Correa KA, Liu H, Shankman SA. The role of intolerance of uncertainty in current and remitted internalizing and externalizing psychopathology. J Anxiety Disord. 2019;62:68–76.
Shihata S, McEvoy PM, Mullan BA, Carleton RN. Intolerance of uncertainty in emotional disorders: what uncertainties remain? J Anxiety Disord. 2016;41:115–24.
Nolen-Hoeksema S, Morrow J. A prospective study of depression and posttraumatic stress symptoms after a natural disaster: the 1989 Loma Prieta earthquake. J Pers Soc Psychol. 1991;61(1):115–21.
Meyer TJ, Miller ML, Metzger RL, Borkovec TD. Development and validation of the penn state worry questionnaire. Behav Res Ther. 1990;28(6):487–95.
McEvoy PM, Kingsep P. The post-event processing questionnaire in a clinical sample with social phobia. Behav Res Ther. 2006;44(11):1689–97.
Spinhoven P, van Hemert AM, Penninx BW. Repetitive negative thinking as a predictor of depression and anxiety: a longitudinal cohort study. J Affect Disord. 2018;241:216–25.
Kaplan DM, Palitsky R, Carey AL, Crane TE, Havens CM, Medrano MR, et al. Maladaptive repetitive thought as a transdiagnostic phenomenon and treatment target: an integrative review. J Clin Psychol. 2018;74(7):1126–36.
Bailey T, Shahabi L, Tarvainen M, Shapiro D, Ottaviani C. Moderating effects of the valence of social interaction on the dysfunctional consequences of perseverative cognition: an ecological study in major depression and social anxiety disorder. Anxiety Stress Coping. 2019;32(2):179–95.
Hayano J. Introduction to heart rate variability. In: Iwase S, Hayano J, Orimo S, editors. Clinical assessment of the autonomic nervous system. Tokyo: Springer Japan; 2017. p. 109–27.
Ottaviani C, Thayer JF, Verkuil B, Lonigro A, Medea B, Couyoumdjian A, et al. Physiological concomitants of perseverative cognition: a systematic review and meta-analysis. Psychol Bull. 2016;142(3):231–59.
Iseger TA, van Bueren NER, Kenemans JL, Gevirtz R, Arns M. A frontal-vagal network theory for major depressive disorder: implications for optimizing neuromodulation techniques. Brain Stimul. 2020;13(1):1–9.
Koenig J, Kemp AH, Beauchaine TP, Thayer JF, Kaess M. Depression and resting state heart rate variability in children and adolescents—a systematic review and meta-analysis. Clin Psychol Rev. 2016;46:136–50.
Chen X, Yang R, Kuang D, Zhang L, Lv R, Huang X, et al. Heart rate variability in patients with major depression disorder during a clinical autonomic test. Psychiatry Res. 2017;256:207–11.
Chalmers JA, Heathers JAJ, Abbott MJ, Kemp AH, Quintana DS. Worry is associated with robust reductions in heart rate variability: a transdiagnostic study of anxiety psychopathology. BMC Psychol. 2016;4(1):32.
Ottaviani C. Brain-heart interaction in perseverative cognition. Psychophysiology. 2018;55(7):n/a-n/a.
Mulcahy JS, Larsson DEO, Garfinkel SN, Critchley HD. Heart rate variability as a biomarker in health and affective disorders: a perspective on neuroimaging studies. Neuroimage. 2019;202:116072.
Shinba T. Major depressive disorder and generalized anxiety disorder show different autonomic dysregulations revealed by heart-rate variability analysis in first-onset drug-naïve patients without comorbidity. Psychiatry Clin Neurosci. 2017;71(2):135–45.
Campbell AA, Wisco BE, Silvia PJ, Gay NG. Resting respiratory sinus arrhythmia and posttraumatic stress disorder: a meta-analysis. Biol Psychol. 2019;144(125-135):125–35.
Alvares GA, Quintana DS, Kemp AH, Van Zwieten A, Balleine BW, Hickie IB, et al. Reduced heart rate variability in social anxiety disorder: associations with gender and symptom severity. PLoS One. 2013;8(7):e70468.
Lesnewich LM, Conway FN, Buckman JF, Brush CJ, Ehmann PJ, Eddie D, et al. Associations of depression severity with heart rate and heart rate variability in young adults across normative and clinical populations. Int J Psychophysiol. 2019;142:57–65.
Huang M, Shah A, Su S, Goldberg J, Lampert RJ, Levantsevych OM, et al. Association of Depressive Symptoms and Heart Rate Variability in Vietnam war–era twins: a longitudinal twin difference study. JAMA Psychiatry. 2018;75(7):705–12.
Miller BJ, Paschall CB, Svendsen DP. Mortality and medical comorbidity among patients with serious mental illness. Psych Serv (Washington, DC). 2006;57(10):1482.
Hert M, Correll CU, Bobes J, Cetkovich-Bakmas M, Cohen D, Asai I, et al. Physical illness in patients with severe mental disorders. I. Prevalence, impact of medications and disparities in health care. World Psych. 2011;10(1):52–77.
Henriques T, Ribeiro M, Teixeira A, Castro L, Antunes L, Costa-Santos C. Nonlinear methods Most applied to heart-rate time series: a review. Entropy. 2020;22(3):309.
Vollmer M. HRVTool–an open-source Matlab toolbox for analyzing heart rate Variability2019; 2019. IEEE
Benchekroun M, Chevallier B, Zalc V, Istrate D, Lenne D, Vera N. Analysis of the impact of inter-beat-interval interpolation on real-time HRV feature estimation for e-health applications. Toulouse: Colloque JETSAN 2021; 20-21 May; 2021.
Plummer M. JAGS: a program for analysis of Bayesian graphical models using Gibbs sampling. Vienna: Proceedings of the 3rd international workshop on distributed statistical computing; 2003.
Stan Development T. Stan modeling language users guide and reference manual, v. 2.22; 2021. p. 1.
Westbury CF. Bayes' rule for clinicians: an introduction. Front Psychol. 2010;1:192.
Makowski D, Ben-Shachar MS, Lüdecke D. bayestestR: describing effects and their uncertainty, existence and significance within the Bayesian framework. J Open Source Softw. 2019;4(40):1541.
Kruschke JK. Rejecting or accepting parameter values in Bayesian estimation. Adv Methods Pract Psychol Sci. 2018;1(2):270–80.
Hespanhol L, Vallio CS, Costa LM, Saragiotto BT. Understanding and interpreting confidence and credible intervals around effect estimates. Braz J Phys Ther. 2019;23(4):290–301.
Makowski D, Ben-Shachar MS, Chen SHA, Lüdecke D. Indices of effect existence and significance in the Bayesian framework. Front Psychol. 2019;10:2767.
Cohen J. Statistical power analysis for the behavioral sciences. In: Conner BE, editor. The box in the barn. Columbus: highlights for …; 1988: Erlbaum; 1988.
Acock AC. A gentle introduction to Stata. 5th ed. College Station: A Stata Press Publication, StataCorp LP; 2016.
Gelman A. Comment: fuzzy and Bayesian p-values and u-values. Stat Sci. 2005;20(4):380–1.
Rosenbaum JF, Fava M, Hoog SL, Ascroft RC, Krebs WB. Selective serotonin reuptake inhibitor discontinuation syndrome: a randomized clinical trial. Biol Psychiatry. 1998;44(2):77–87.
Montgomery SA, Fava M, Padmanabhan SK, Guico-Pabia CJ, Tourian KA. Discontinuation symptoms and taper/poststudy-emergent adverse events with desvenlafaxine treatment for major depressive disorder. Int Clin Psychopharmacol. 2009;24(6):296–305.
Baldwin DS, Montgomery SA, Nil R, Lader M. Discontinuation symptoms in depression and anxiety disorders. Int J Neuropsychopharmacol. 2007;10(1):73–84.
Rogel A, Guez J, Getter N, Keha E, Cohen T, Amor T, et al. Transient adverse side effects during Neurofeedback training: a randomized, sham-controlled, double blind study. Appl Psychophysiol Biofeedback. 2015;40(3):209–18.
Insel TR, Wang PS. Rethinking mental illness. JAMA. 2010;303(19):1970–1.
Acknowledgements
We are grateful to all the trial participants for their willingness and commitment to participate in this trial.
Public and patient involvement
The Ngāi Tahu Research Consultation Committee was consulted during the protocol development stage.
Protocol amendments
Notably, recruitment began in February 2020 but was prematurely halted in March 2020 due to COVID-19 lockdown measures here in New Zealand. Recruitment efforts resumed in June 2020, however, due to time and staffing restrictions resulting from the lockdown, we amended our original protocol. Specifically, our trial recruitment goal was changed from 40 males and 40 females to 60 females. Additionally, rather than multiple PROs (i.e. HADS, MEDI, IDAS-II), the HADS was selected as the primary PRO endpoint because it has an established minimal clinically important difference (MCID) value. Finally, electrodermal activity (EDA) assessment was removed due to equipment malfunction during testing. All amendments occurred prior to the start of data analyses.
Funding
This clinical trial is part of a doctoral thesis supported by the Department of Surgical Sciences, University of Otago, Dunedin, New Zealand. This sponsor has no role in the study design, collection, management, analysis, interpretation of data, or dissemination of results.
Author information
Authors and Affiliations
Contributions
TMP conceived of the study and drafted the protocol. All authors contributed to the refinement of the trial protocol and provided feedback on the protocol manuscript. The authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Ethical approval (19/CEN/179) has been obtained from the Health & Disability Ethics Committee (HDEC), New Zealand (see Additional file 3). Written, informed consent to participate will be obtained from all participants.
Consent for publication
Examples of the participant information and informed consent forms can be provided upon request.
Competing interests
MS is the owner of Neurofeedback Therapy Services of New York which provides ISF-NFB therapy for patients and training for clinicians. He created the ISF-NFB software programs for this trial, provided guidance to the principal investigator in their utilization, and participated in editing of the final version of the manuscript. MS did not participate in the trial conceptualisation, design, implementation, or analyses. All other authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1.
MCC voxelsR1.
Additional file 2.
PCC voxelsR1.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Perez, T.M., Glue, P., Adhia, D.B. et al. Infraslow closed-loop brain training for anxiety and depression (ISAD): a protocol for a randomized, double-blind, sham-controlled pilot trial in adult females with internalizing disorders. Trials 23, 949 (2022). https://doi.org/10.1186/s13063-022-06863-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13063-022-06863-z