Abstract
Background
Polycystic ovary syndrome (PCOS) is a complex endocrine syndrome with poorly understood mechanisms. To provide patients with PCOS with individualized therapy, it is critical to precisely diagnose the phenotypes of the disease. However, the criteria for diagnosing the different phenotypes are mostly based on symptoms, physical examination and laboratory results. This study aims to compare the accuracy and efficacy of diagnosing PCOS by integrating metabolomic markers with common clinical characteristics.
Methods
This is a prospective, multicenter, analyst-blinded, randomized controlled trial. Participants will be grouped into (1) people without PCOS (healthy control group), (2) patients diagnosed with PCOS based on clinical indices (experimental group 1), and (3) patients diagnosed with PCOS based on metabolomic indices (experimental group 2). A total of 276 participants, including 60 healthy people and 216 patients with PCOS, will be recruited. The 216 patients with PCOS will be randomly assigned to the two experimental groups in a 1:1 ratio, and each group will receive a different 6-month treatment. The primary outcome for the experimental groups will be the effect of PCOS treatment.
Discussion
The results of this trial should help to determine whether using metabolomic indices is more accurate and effective than using clinical characteristics in diagnosing the phenotypes of PCOS. The results could provide a solid foundation for the accurate diagnosis of different PCOS subgroups and for future research on individualized PCOS therapy.
Trial registration
Chinese Clinical Trial Registry, ID: ChiCTR-INR-1800016346. Registered 26 May 2018.
Similar content being viewed by others
Background
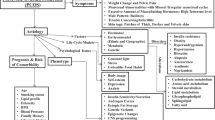
Polycystic ovary syndrome (PCOS) is a complex endocrine syndrome [1, 2] that exhibits chronic ovulatory disorders, hyperandrogenism, insulin resistance, and metabolic disorders [3]. PCOS has a prevalence of 5%–10% globally [4, 5], and it is one of the most common endocrine and metabolic disorders in Chinese women. PCOS is strongly associated with insulin resistance and dyslipidemia, which affect patients with obesity, type 2 diabetes, and metabolic syndrome (MetS) [6,7,8,9,10]. Evidence also suggests that patients with PCOS may have a higher prevalence of asthma [11], nonalcoholic fatty liver disease [12], and mental disorders [13] such as depression and anxiety [14]. However, the underlying mechanisms of PCOS are still poorly understood, making clinical diagnosis and treatment very difficult.
To determine whether a patient has PCOS, careful assessment of hyperandrogenism, hyperandrogenemia, ovulatory function, and ovarian morphology is required. The recent diagnostic criteria for PCOS are mostly based on a combination of other criteria/statements that are still disputed, and because of the different clinical manifestations and complex pathogenesis of the disease, the criteria are mainly aimed at ruling out other diseases. The accuracy of the diagnosis and the evaluation methods for assessing the individual criteria is even more critical.
PCOS has diverse phenotypes [15,16,17] and thus requires individualized treatment. The European Society of Endocrinology [2] proposed four phenotypes, including a type without hyperandrogenemia. When diagnosing the disease, it was suggested that abnormalities in steroidogenic enzymes, prolactin excess, and thyroid problems need to be ruled out. In China, the classification of subtypes and treatment of PCOS are mainly based on clinical indices, such as sex hormones (e.g., follicle-stimulating hormone (FSH), luteinizing hormone (LH), LH/FSH ratio, free testosterone, dehydroepiandrosterone (DHEAS), androstenedione, sex-hormone-binding globulin), serum lipids (e.g., total cholesterol, triglycerides, low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), apolipoprotein (Apo) A, Apo B), and the results of oral glucose tolerance tests and immunoreactive trypsinogen tests [1]. This heterogeneity in diagnostic criteria could significantly lead to misdiagnoses. Therefore, it is urgent to explore a new detection method to standardize the classification of phenotypes and establish an efficient diagnostic and treatment strategy for PCOS. The development and application of new technologies, such as genomics, proteomics, and metabolomics, provide a new direction for diagnosing PCOS phenotypes. However, in genomics, although the findings of genome-wide association studies have implications, the direct pathogenic gene for PCOS has not been found yet. In proteomics, some scholars have identified potential biomarkers for PCOS phenotyping, but their clinical application is limited because proteins, or amino acids, are highly susceptible to interference, which can bias the results. Lastly, due to the high sensitivity and specificity of metabolomic methods, it can be assumed that some metabolic markers can be used to distinguish whether patients with PCOS have high androgen levels.
In the attempt to classify PCOS phenotypes, recent studies have adopted various measurements such as nuclear magnetic resonance [18], liquid chromatography/mass spectrometry [19], and others [20, 21]. In our previous study [3], using ultra-high-performance liquid chromatography/quadrupole time-of-flight mass spectrometry, we found two subgroups of patients with PCOS: one with hormone metabolism disorders and the other with lipid metabolism disorders.
This trial aims to compare the accuracy and efficacy between using metabolomic indices and using clinical characteristics in diagnosing PCOS phenotypes. Our findings could provide a basis for further research on individualized PCOS therapy.
Methods
Study design
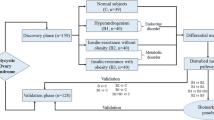
This is a prospective, multicenter, analyst-blinded, randomized controlled trial. Subjects will be grouped into one healthy control group and two parallel experiment arms, as follows: (1) people without PCOS (healthy control group), (2) patients diagnosed with PCOS based on clinical indices (experimental group 1), and (3) patients diagnosed with PCOS based on metabolomic indices (experimental group 2). A total of 276 participants, including 60 people without PCOS and 216 patients with PCOS, will be recruited. The 216 patients with PCOS will be randomly assigned to the two experimental (diagnosis) groups in a 1:1 ratio. After group assignment, medical detection results, relative information, and biological samples will be collected from all three groups. Additionally, the blood samples from all participants will be tested using metabolomic methods for further study, while the analysis results will be totally blinded to all participants and their research physicians. Based on our previous study [3], we will categorize patients with PCOS into two subgroups: patients with hormone metabolism disorders (subgroup 1) and patients with lipid metabolism disorders (subgroup 2).
The two diagnosis groups will be automatically divided into the two different subgroups according to patient characteristics. The main difference is that in group 1, diagnosis will be based on clinical indices [1], such as clinical signs and symptoms and ultrasonography findings, while in group 2, diagnosis will be based on metabolomic indicators. All patients will receive treatment according to their subtype group, and the treatment will consist of Diane-35 or metformin. The intervention period for all experimental groups will be 6 months, and the results will be evaluated after the completion of three constant treatment sessions (3 months).
Outcomes will be assessed, and the data will be analyzed by someone blinded to the assignment of participants. The study design is shown in Fig. 1, and the timeline is presented in Figs. 2 and 3.
The schedule of forms and procedures, according to the Protocol Items.
Physical examination includes: ultrasonography of bilateral ovarian anad clinical biochemical indicators. Clinical biochemical indicators routine urine tests, routine blood tests, liver function, pregnancy tests, LH/FSH ratio, sex hormone related indicators blood lipid related indicators and endocrine related indicators. There are twenty-five clinical biochemical indexes including eight sex hormone-related indexes: FSH, LH, PRL, E2, P, Ts, AMH, AND vitD; nine blood lipid indicators: TC, TG, HDL, LDL, ApoA1, Apo B, Lp(a), FFA, hsCRP; seven endocrine-related indicators: GLU (0h, 30min, 120min), INS (0h, 30min, 120min), BUA. The main elevation index was LH/FSh ratio. Note: ultrasonography examination is scheduled for the 14th day of menstruation, if the patient with rare ovulation has follicular diameter > 10mn or luteal appearance, should be reviewed in the subsequent menstrual cycle. Unmarried women without sexual life suggested abdominal B ultra. All blood samples will be collected on an empty stomach within 72 hours from the first day of menstruation
The main treatment center will be the Department of Endocrinology and Metabolism of the Affiliated Wuxi People’s Hospital of Nanjing Medical University, which will enroll 60 participants for the healthy control group and 108 participants who, in the experimental group (108/216), will be randomly assigned between the two experimental groups. Two other treatments centers—Wuxi Maternal and Child Health Centers Clinical Hospital and Jiangyin People’s Hospital—will enroll the other 108 participants, who will also be randomly assigned between the two experimental groups.
Participants and recruitment
People without PCOS will be enrolled in the healthy control group only if they do not have any other disorders that may influence sex-related hormones and metabolic conditions. For the experimental groups, PCOS will be diagnosed according to the 2018 Chinese Endocrine Society clinical practice consensus on PCOS [1], which is based on the widely accepted criteria for PCOS published by Rotterdam [22, 23]. Patients who are of reproductive age and can follow the procedure, including strict contraception for 6 months, will be informed of this trial. If a potential participant expresses interest, a face-to-face interview regarding the entire trial process will be conducted in a reception room in the three hospitals. Patients who meet the inclusion criteria will be enrolled if they provide written informed consent. Potential participants will be recruited in two ways: (1) the experimental group participants will be recruited by approaching patients with PCOS who are admitted to an outpatient department or inpatient ward of each center; the official microblog and WeChat platforms of each center will also be used; and (2) the healthy control group participants will be directly recruited by approaching volunteers in the health examination center of Wuxi People’s Hospital.
Inclusion criteria
Participants who meet all of the following criteria will be enrolled in the experimental groups:
- 1.
Diagnosed with PCOS according to the 2018 Chinese Endocrine Society clinical practice consensus, i.e., presence of at least two of the following three conditions, excluding other etiologies: (a) clinical or biochemical hyperandrogenism, (b) oligo-anovulation (menstrual cycle length of > 35 days and having eight or fewer menstrual cycles per year), and (c) a polycystic ovarian morphology characterized by at least 25 small follicles (2–9 mm) in the entire ovary and/or an increase in ovarian volume of ≥ 10 mL
- 2.
A reproductive woman aged 18–45 years
- 3.
Able and willing to comply with the interventions and follow-up evaluations
Exclusion criteria
The exclusion criteria are as follows:
- 1.
Currently receiving treatment in another experimental study or having just finished a trial in the past 30 days
- 2.
Treated with Diane-35, metformin, or other forms of estrogen, progesterone, and lipid-regulating and hypoglycemic drugs (e.g., glucocorticoids, spironolactone, antibiotics, bacterial regulators, anti-inflammatory drugs) within the past 12 weeks
- 3.
An allergy or intolerance to Diane-35, metformin, or any of its components
- 4.
Presence of congenital adrenal cortical hyperplasia, hypercorticism, androgen-secreting tumors, Cushing’s syndrome, thyroid dysfunction, gonadotropin deficiency, hyperprolactinemia, premature ovarian insufficiency, functional hypothalamic amenorrhea, or diabetes
- 5.
A medical history of malignant tumors, particularly a history of surgery, radiation, or chemotherapy for a gynecological malignant tumor
- 6.
Presence of liver dysfunction (alanine aminotransferase and aspartate transaminase levels > 1.5-times the normal values) or chronic liver disease
- 7.
Presence of hematopenia or thrombotic disease
- 8.
Is pregnant or expects to be pregnant during the study
- 9.
Has an unstable medical, physical, or mental status
- 10.
Has a medical history of symptomatic ventricular arrhythmias with torsion ventricular tachycardia (torsade de points)
- 11.
Unable to complete the procedures of the study
- 12.
Any other situation that would interfere with the study evaluation, procedures, or completion
Subject dropout
Subject dropout occurs when:
- 1.
The subject quits the study
- 2.
Safety issues arise (e.g., adverse events (AEs), failure of contraceptive measures, accidents)
- 3.
The subject is lost to follow-up
- 4.
Researchers remove the subject from the trial due to poor compliance, severe liver dysfunction, complications, or serious AEs
Suspension of the trial
The trial may be suspended if the following events occur:
- 1.
A significant safety problem is found, such as serious impairment of the patients’ liver function or the sudden onset of other life-threatening illnesses. In this case, the trial will be suspended, and the participants will have to undergo medical treatment until recovery
- 2.
The diagnostic and therapeutic effects are poor, such as no improvement in menstrual/metabolic disorders (to be evaluated at the third month of therapy). In this case, the researchers will evaluate the physical condition of the participants and decide whether to suspend the trial if necessary
- 3.
A major mistake (e.g., patients taking the wrong doses, patients taking or using medicine without following the physician’s advice) is made that will affect the results of the trial
- 4.
There is a major problem in funding or management, such as withdrawal of funding, which can cause the trial to be discontinued early
Randomization
The Department of Good Clinical Practice of Wuxi People’s Hospital will lead the randomization process by using a random number generator in the Statistical Package for Social Sciences (SPSS) version 21.0 (SPSS Inc., Chicago, IL, USA).
Random sequences will be placed in opaque envelopes, numbered in order, and then sent to a clinical researcher and physicians.
The envelopes will be opened sequentially to decide upon the allocation of participants. In this trial, the physicians and trial participants will be blinded to the group assignment.
Blinding
In the experimental groups, participants will not be informed of the group assignment, the type of diagnosis, or the treatment that they will receive. Data managers and the statistician will also be blinded. Only the trial administrator (a steering committee of the Department of Good Clinical Practice, Wuxi People’s Hospital), who will be responsible for assigning the participants into different subgroups and monitoring the entire trial process, will be not blinded. Moreover, the clinical research physicians will be blinded to the assignment of group 1, whose treatment will be decided based on their clinical practice, while they will have access to the diagnostic and treatment allocation data for group 2. The clinical physicians will be responsible for learning how to use the blinding method to communicate with the participants to ensure diagnosis and treatment blinding. The blinding procedure will be conducted until the data are locked and the trial is completed. Unblinding will only be permitted in case of a medical emergency or when a participant quits the trial. All cases of unblinding will be documented.
Interventions
Healthy control group
As mentioned above, participants will only be recruited in the healthy control group if they do not have PCOS, metabolic conditions, or any other disorders that may influence sex-related hormones and they are not using any drugs that could also influence sex hormones. A medical evaluation will be conducted to confirm eligibility, after which the trial team will collect information regarding the patient’s medical history, conduct a physical examination, and collect blood samples from eligible participants for further investigation.
Clinical index group
For the clinical index group, blood samples and medical information of all eligible participants will be collected. Based on several guidelines and clinical experience, subgroup 1 (participants with hormone metabolism disorders) will receive orally administered Diane-35 once a day for 21 days, after which the treatment will be paused for 7 days. Subgroup 2 (participants with lipid metabolism disorders) will receive orally administered metformin (2000–2500 mg) once a day based on their clinical condition. The entire treatment period will be at least 6 months. During the trial, blood samples will be collected three times (week 0, week 15 ± 1, and week 26 ± 1) for metabolomic evaluation to assess changes. However, the detection information will be blinded to all participants and clinical research physicians to avoid influencing the results. All clinical research physicians will be required to have majored in endocrinology and metabolism or gynecological endocrinology for at least 10 years. They must also be employed as an attending physician for > 5 years. Finally, they must receive professional training on clinical trials and pass a test to ensure consistent performance in the study methods.
Metabolomic index group
Most of the interventions for the metabolomic index group will be similar to those for the clinical index group. The main difference is that the division of the subgroups will be based on metabolomic indices. The physicians in this group will be informed of the allocation of participants to the subgroups and will provide the corresponding treatment scheme to all participants. The physicians will not be allowed to change the scheme during the trial and will remain blinded to the metabolomic results to minimize the possibility of any influence on treatment decisions.
Concurrent treatment of patients
All other treatments for PCOS will be banned during the trial, including oral contraceptives, broad-spectrum lipid drugs, and any other drugs that might influence the results. However, participants may receive any treatment not related to PCOS and will be instructed to use condoms during sexual intercourse. Any change in concurrent treatment will be recorded at every visit.
Outcome measurements
The healthy control group will remain enrolled in the trial until all their samples are collected. For the experimental groups, the primary outcome will be the changes in their relative PCOS condition evaluated using clinical indices. The main clinical index that will be evaluated is the LH/FSH ratio.
Secondary outcomes will be assessed using other indices, signs, and symptoms, as described in the 2018 Chinese Endocrine Society clinical practice consensus on PCOS [1] such as sex hormone-related indices (FSH, LH, prolactin, estradiol, progesterone, testosterone, anti-Müllerian hormone (AMH), and vitamin D), blood lipid-related indicators (total cholesterol, triglycerides, HDL, LDL, Apo A1, Apo B, lipoprotein(a), free fatty acids, and high-sensitivity C-reactive protein), and endocrine-related indicators (glucose and insulin (at 0, 30, and 120 min) and blood uric acid). To help evaluate the outcomes, three metabolic markers will also be used, namely, palmitoyl sphingomyelin, cyclic guanosine monophosphate, and DHEAS. Ultrasound results will also be evaluated, and the number and diameter of follicles in both ovaries will be measured.
Safety outcomes will include any severe impairment in liver function or inability of the patient to tolerate symptoms (e.g., nausea and vomiting caused by metformin) related to the therapeutic drugs. Such outcomes will be monitored after each treatment session (1 month) using the hepatic function test. An exploratory outcome will be complete recovery from PCOS after treatment within 26 weeks.
Safety evaluation
AEs will be evaluated at every visit and will include any unexpected or unfavorable response that occurs during or after treatment. Although these events may not have a causal relationship with the variables in this study, the investigators will need to ensure that all AEs are reported and recorded in the subject’s medical records. In this trial, AEs are defined as disorders that (1) hinder one’s ability to work or are life-threatening (especially liver dysfunction) or (2) lead to hospitalization or prolong department or hospital stay. Remedial treatment should be given immediately to resolve any observed AE, and all AEs will be reported to the responsible units, ethical committees, and trial administrator to decide whether the participant ought to remain in or drop out of the trial. Regardless of the decision, all participants with an AE will be followed up until the event has been resolved or the condition has become chronic or stable.
The study should not cause any additional harm to the participants. In case of serious unexpected harm to a participant related to the study, we will shoulder the relevant expenses (including the diagnosis and treatment) and follow up the condition of the participant until recovery.
Follow-up
To evaluate the accuracy and efficacy of diagnosis, safety, and the superior effects of individual interventions, a follow-up via telephone (or via face-to-face interviews, emails, text messages, or WeChat) will be conducted 6 months after the trial. During the follow-up, no participants will undergo special therapy except for routine cervical care. At weeks 30, 34, 38, and 41, the outcome assessor will call the participants to investigate their PCOS condition, asking mainly about menstrual cycle length and frequency, the appearance of hyperandrogenism (e.g., weight loss, acne, excessive facial and body hair), and pregnancy. Participants are welcome to inform the assessors of their clinical symptoms and AEs via face-to-face meetings, emails, telephone calls, text messages, or WeChat at relevant time points.
Blinding and credibility tests
Metabolomic evaluation with blinding will be completed at weeks 0, 15, and 26. The implementation of the blinding strategy will be crucial for the trial. The credibility rating for both the diagnostic methods and different types of treatment will be estimated using a credibility test at weeks 15, 26, and 41 during the follow-up period.
Data collection and monitoring
Data on basic patient characteristics will be collected and recorded by screeners when participants are recruited. Clinical signs and symptoms, physical examination findings, metabolomic evaluation results, short- and long-term outcomes, assessment of diagnosis accuracy and treatment efficiency, and details of the AEs will be recorded by trial assessors and clinical researchers in case report forms (CRFs).
Completed CRFs will be checked and reviewed by a five-person steering committee, which is composed of two supervisors of the major trial center, a data statistician, and two data administrators. The committees are completely independent from the research team and will be constantly blinded to the group allocation.
All data entry and management procedures will be conducted in an OpenClinica System (version 3.12) database. The committee members will need to be qualified in data analysis and will have been trained uniformly.
To ensure the accuracy of data, two data administrators will independently perform data entry and validation. Any issues with the information in the CRFs will be reported by the data administrators to the steering committee. Any revisions will be modified by the administrators according to feedback from the committee. Once the accuracy of data is confirmed, the electronic database will be locked while real-time tracking and monitoring will remain open.
The steering committee and the Department of Good Clinical Practice in Wuxi People’s Hospital will monitor the entire trial (e.g., checking the progress of recruitment, participant data including CRFs, the protocols for researchers, informed consent forms, and any other study-specific files) and audit the trial conduct at least once a month.
All information will be made available to the investigators whose proposed use of the data has been approved by the relevant committee for up to 15 years following publication. The findings will be presented at conferences and published in peer-reviewed journals. A summary of the findings will be provided to the participants upon request via telephone.
Sample collection and handling
The actual dates and times of sample collection will be recorded in the laboratory requisition form. Instructions for the collection, handling, and storage of samples are found in the laboratory manual that will be provided.
The standard procedure for plasma separation will be performed as follows:
- 1)
Fresh anticoagulated peripheral blood will be collected in the EDTA anticoagulant tube
- 2)
The fresh peripheral blood will be centrifuged at 1700 G at 4 °C for 10 min, and the upper plasma will be carefully collected to avoid touching the lower layers of red blood cells and white blood cells
- 3)
The collected plasma will be centrifuged at 2000 G at 4 °C for 10 min, and the supernatant will be decanted
- 4)
The plasma will be divided and placed in separate containers and frozen at − 80 °C in a refrigerator for later use
Precautions for plasma separation
-
1)
The plasma separation process shall be performed on ice to maintain the integrity of the blood cells
-
2)
The entire separation process will be completed within 4 h
-
3)
The blood collection and plasma separation process will be handled carefully to avoid the risk of hemolysis
After the study, all samples will be safely destroyed.
Statistical analyses
The dataset will include a safety set, a full analysis set (FAS), and a per-protocol set. The safety set is designed for participants who will be randomly assigned to receive at least one session of treatment. The FAS will include all medical data related to the trial and will indicate the intervention conducted; however, participants who miss the primary outcome evaluation will be excluded. In the per-protocol set, participants will be included if they receive at least three constant sessions of treatment.
In this trial, the FAS will be used for the basic analysis. For missing data, we will employ the imputation adjustment approach, and the last observation analysis will be selectively used to handle the missing data.
A sensitivity analysis will then be used to compare the results of the per-protocol analysis and the different intervention analyses to evaluate the impact of the missing data on the trial results.
Descriptive statistics about quantitative indicators, such as means, standard deviations, medians, minimums, and maximums, will be presented. Variance analysis will be used to compare the quantitative indices among the different groups (healthy control group and experimental groups 1 and 2), and the Student-Newman-Keuls test will be used to analyze the two subgroups.
Classification indicators will be described by the number and percentage of each category. The chi-squared test or exact probability method will be used to compare the qualitative indices among the three groups, and the chi-squared segmentation method will be used to compare the two pairwise subgroups.
SPSS version 21.0 will be used to analyze all trial data. All statistical tests will be double-sided, and statistical significance will be considered when the p value is ≤ 0.05.
Sample size calculation
Based on the literature and findings from a small pilot study that used metabolomic techniques, the sensitivity and specificity required to classify the control and PCOS groups will be 100% and 86%, respectively. The corresponding values between the control group and subgroup 1 will be 96.7% and 100%, respectively, while those between the control group and subgroup 2 will be 100% and 86.2%, respectively. The sensitivity and specificity for dividing PCOS into two different subtypes will be 90.9% and 87.5%, respectively. To meet the lower statistical limit, each subgroup will include at least 30 participants.
According to a presumptive maximum dropout tolerance of 20%, with a significance level of 0.05 and a power of 0.80, we calculate the required sample size for the experimental groups to be 216. In this trial, 60 people will be assigned to the healthy control group and 108 people to each experimental group across the three centers.
Quality control
During the trial, quality control will be carried out by the steering committee. All researchers are required to attend training on trial methods, techniques, and protocol regulations to maintain data consistency and validity. Any modifications or corrections required will be discussed, decided upon, and submitted to the steering and ethics committees. Patient details will be kept confidential throughout the trial.
Discussion
PCOS is a syndrome characterized by various signs and symptoms, such as androgen excess, ovarian dysfunction, and a polycystic ovarian morphology. Considering its high prevalence among reproductive women in China and given that its complications may lead to endometriosis, endometrial cancer, or some malignant diseases, PCOS might be one of the most common and severe endocrine and metabolic disorders.
The diagnosis of PCOS still depends on ruling out other diseases [24,25,26,27,28,29] including thyroid dysfunction, nonclassical congenital adrenal hyperplasia, and androgen-secreting tumors. PCOS has several recognized phenotypes that present with distinct characteristics and require individualized treatment [1]. However, with the lack of classification criteria, it is very difficult for physicians to make a precise diagnosis and develop a suitable treatment strategy.
Of note, researchers have attempted to determine whether there are biomarkers that could be used as predictive markers for the different PCOS phenotypes. Zhang et al. [30] found that the phenotype with hyperandrogenism is associated with a higher score in the homeostatic model assessment of insulin resistance (HOMA-IR) than the other phenotypes (without androgen excess). Further detection showed that serum irisin levels are associated with hyperandrogenism but not with oligo-anovulation or PCOS morphology. Additionally, Minooee et al. [31] pointed out that the testosterone-to-androstenedione ratio is associated with insulin resistance (sensitivity 0.83, specificity 0.42) and MetS (sensitivity 0.85, specificity 0.70) among patients with PCOS, indicating the strong link between insulin resistance and hyperandrogenism. This finding may assist clinicians and researchers in classifying the specific phenotypes of PCOS. However, Bozic-Antic et al. [32] designed a cross-sectional clinical study of 365 women with PCOS and 125 healthy controls from a Caucasian population. In their study, patients with PCOS were divided into groups based on four phenotypes, according to the criteria of the European Society of Human Reproduction and Embryology (ESHRE)/American Society for Reproductive Medicine (ASRM) [33]. Their findings suggested that all phenotypes are associated with the same prevalence of MetS, but phenotype B was associated with a different cardiovascular risk. This difference might partly be related to epigenetic and environmental influences. Moreover, the free androgen index [34], serum AMH level [35,36,37,38], PPARGC1A (a leukocyte methylation promoter) [39], and some other indices were also found to be potential biomarkers for predicting PCOS and classifying its phenotypes. Although there have been many efforts to address the predicament surrounding the diagnosis of PCOS, the precise classification criteria have yet to be developed. However, there has been a turning point in the detection methodology with the advent of metabolomics.
This trial is designed to explore a more specific and sensitive method to determine the subtypes of PCOS. Based on the preliminary study, our team found that clinical indicators such as the LH/FSH ratio and Apo A1, AMH, and insulin levels had a good correlation with metabolomic results. This finding indicates the potential of developing a diagnostic and therapeutic strategy for PCOS based on an integrated biomarker system.
To further investigate the findings of this trial, we will conduct a prospective cohort study, expanding the sample size of patients with PCOS and analyzing the detection frequencies. This study is a multicenter, randomized, open-label, blinded clinical study aiming to standardize the diagnostic classification and treatment of PCOS. The sensitivity and specificity of integrating metabolomic indices in the diagnostic criteria will be compared with diagnosing the disease with clinical indices alone. Our findings may help improve the empirical classification, find predictive biomarkers, and assist in establishing an integrated biomarker system for developing precise diagnostic and treatment strategies for different PCOS subgroups in the future.
Study limitations
The main difficulty inherent to this trial is making a consistent diagnosis in experimental group 1 because the diagnosis will be made by several different physicians. To address this problem as much as possible, our group will organize a diagnosis team consisting of three professional physicians who have majored in endocrinology and metabolism or gynecological endocrinology for at least 10 years and achieved the position of assistant director . If the clinical research physicians cannot diagnose the participant’s subtype, the case will be submitted to the team for a final diagnosis. Another methodological difficulty is ensuring that the treatment complies with the scheme. In this trial, we will ensure that medication reminders are sent and participants who miss treatment for seven consecutive days will be excluded from the trial.
Trial status
This trial will use protocol version 2.0. Recruitment was initiated in February 2019 and completed in August 2019. The trial is currently in the screening phase, and some participants are undergoing observation. Ethical approval was granted in 2018, and the study is expected to be completed at the end of December 2019.
Availability of data and materials
The datasets (including the metadata and protocol) analyzed in this study will be available from the corresponding author upon reasonable request and through a public clinical trial management platform (http://www.medresman.org). Sponsor members of the research team, namely Qing He and Lan Xu, and core members, namely Cheng-Ming Ni and Xue-Mei Fan, are eligible to write papers relevant to this trial. We do not intend to use any professional writers.
Abbreviations
- AE:
-
Adverse event
- AMH:
-
Anti-Müllerian hormone
- CRF:
-
Case report form
- DHEAS:
-
Dehydroepiandrosterone
- FAS:
-
Full analysis set
- FSH:
-
Follicle-stimulating hormone
- LH:
-
Luteinizing hormone
- MetS:
-
Metabolic syndrome
- PCOS:
-
Polycystic ovary syndrome
- SPSS:
-
Statistical Package for Social Sciences
References
Chinese Medical Doctor Association of Endocrine and Metabolic Diseases. Consensus of Chinese endocrinologists for the diagnosis and treatment of polycystic ovary syndrome. Chin J Endocrine Metabolism. 2018;34(1):1–7.
Conway G, Dewailly D, Diamanti-Kandarakis E, et al. The polycystic ovary syndrome: a position statement from the European Society of Endocrinology. Eur J Endocrinol. 2014;171(4):P1–29.
Fan X, Jiang J, Huang Z, et al. UPLC/QTOFMS based plasma metabolomics and clinical characteristics of polycystic ovarian syndrome. Mol Med Rep. 2019;19(1):280–92.
Marcondes RR, Maliqueo M, Fornes R, et al. Exercise differentially affects metabolic functions and white adipose tissue in female letrozole- and dihydrotestosterone-induced mouse models of polycystic ovary syndrome. Mol Cell Endocrinol. 2017;448:66–76.
Zhai KL, Zhuo G, Chi HB, Lan Z. Comparisons of prevalence and clinical and environmental characteristics between Tibetan and Han women with polycystic ovarian syndrome in Tibetan Plateau. Zhonghua Yi Xue Za Zhi. 2017;97(37):2928–31.
Lazaridou S, Dinas K, Tziomalos K. Prevalence, pathogenesis and management of prediabetes and type 2 diabetes mellitus in patients with polycystic ovary syndrome. Hormones (Athens). 2017;16(4):373–80.
Boyle JA, Cunningham J, Norman RJ, et al. Polycystic ovary syndrome and metabolic syndrome in Indigenous Australian women. Intern Med J. 2015;45(12):1247–54.
Hillman JK, Johnson LN, Limaye M, et al. Black women with polycystic ovary syndrome (PCOS) have increased risk for metabolic syndrome and cardiovascular disease compared with white women with PCOS. Fertil Steril. 2014;101(2):530–5.
Ranasinha S, Joham AE, Norman RJ, et al. The association between Polycystic Ovary Syndrome (PCOS) and metabolic syndrome: a statistical modelling approach. Clin Endocrinol. 2015;83(6):879–87.
Lim SS, Norman RJ, Davies MJ, Moran LJ. The effect of obesity on polycystic ovary syndrome: a systematic review and meta-analysis. Obes Rev. 2013;14(2):95–109.
Htet TD, Teede HJ, de Courten B, et al. Asthma in reproductive-aged women with polycystic ovary syndrome and association with obesity. Eur Respir J. 2017;49(5). https://doi.org/10.1183/13993003.01334-2016.
Kumarendran B, O’Reilly MW, Manolopoulos KN, et al. Polycystic ovary syndrome, androgen excess, and the risk of nonalcoholic fatty liver disease in women: a longitudinal study based on a United Kingdom primary care database. PLoS Med. 2018;15(3):e1002542.
Damone AL, Joham AE, Loxton D, et al. Depression, anxiety and perceived stress in women with and without PCOS: a community-based study. Psychol Med. 2019;49(9):1510–1520. PMID: 30131078. https://doi.org/10.1017/S0033291718002076.
Kogure GS, Ribeiro VB, Lopes IP, et al. Body image and its relationships with sexual functioning, anxiety, and depression in women with polycystic ovary syndrome. J Affect Disord. 2019;253:385–93.
Lizneva D, Suturina L, Walker W, et al. Criteria, prevalence, and phenotypes of polycystic ovary syndrome. Fertil Steril. 2016;106(1):6–15.
Huang CC, Tien YJ, Chen MJ, et al. Symptom patterns and phenotypic subgrouping of women with polycystic ovary syndrome: association between endocrine characteristics and metabolic aberrations. Hum Reprod. 2015;30(4):937–46.
Torchen LC, Legro RS, Dunaif A. Distinctive reproductive phenotypes in peripubertal girls at risk for polycystic ovary syndrome. J Clin Endocrinol Metab. 2019;104(8):3355–61.
Castiglione Morelli MA, Iuliano A, Schettini SCA, et al. NMR metabolic profiling of follicular fluid for investigating the different causes of female infertility: a pilot study. Metabolomics. 2019;15(2):19.
Li J, Xie LM, Song JL, et al. Alterations of sphingolipid metabolism in different types of polycystic ovary syndrome. Sci Rep. 2019;9(1):3204.
Gent R, du Toit T, Bloem LM, Swart AC. The 11beta-hydroxysteroid dehydrogenase isoforms: pivotal catalytic activities yield potent C11-oxy C19 steroids with 11betaHSD2 favouring 11-ketotestosterone, 11-ketoandrostenedione and 11-ketoprogesterone biosynthesis. J Steroid Biochem Mol Biol. 2019;189:116–26.
Zhang B, Shen S, Gu T, et al. Increased circulating conjugated primary bile acids are associated with hyperandrogenism in women with polycystic ovary syndrome. J Steroid Biochem Mol Biol. 2019;189:171–5.
Rotterdam, Eshre Asrm-Sponsored Pcos Consensus Workshop Group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril. 2004;81(1):19–25.
Rotterdam, Eshre Asrm-Sponsored Pcos Consensus Workshop Group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS). Hum Reprod. 2004;19(1):41–7.
Kyritsi EM, Dimitriadis GK, Kyrou I, et al. PCOS remains a diagnosis of exclusion: a concise review of key endocrinopathies to exclude. Clin Endocrinol. 2017;86(1):1–6.
Azziz R, Carmina E, Chen Z, et al. Polycystic ovary syndrome. Nat Rev Dis Primers. 2016;2:16057.
Azziz R, Carmina E, Dewailly D, Task Force on the Phenotype of the Polycystic Ovary Syndrome of The Androgen and PCOS Society, et al. The Androgen Excess and PCOS Society criteria for the polycystic ovary syndrome: the complete task force report. Fertil Steril. 2009;91(2):456–88.
Bozdag G, Mumusoglu S, Zengin D, et al. The prevalence and phenotypic features of polycystic ovary syndrome: a systematic review and meta-analysis. Hum Reprod. 2016;31(12):2841–55.
Lie Fong S, Laven SJSE, Duhamel A, Dewailly D. Polycystic ovarian morphology and the diagnosis of polycystic ovary syndrome: redefining threshold levels for follicle count and serum anti-Mullerian hormone using cluster analysis. Hum Reprod. 2017;32(8):1723–31.
Stankiewicz M, Norman R. Diagnosis and management of polycystic ovary syndrome: a practical guide. Drugs. 2006;66(7):903–12.
Zhang L, Fang X, Li L, et al. The association between circulating irisin levels and different phenotypes of polycystic ovary syndrome. J Endocrinol Investig. 2018;41(12):1401–7.
Minooee S, Ramezani Tehrani F, Tohidi M, Azizi F. Role of androgen ratios in the prediction of the metabolic phenotype in polycystic ovary syndrome. Int J Gynaecol Obstet. 2017;137(2):110–5.
Bozic-Antic I, Ilic D, Bjekic-Macut J, et al. Lipid accumulation product as a marker of cardiometabolic susceptibility in women with different phenotypes of polycystic ovary syndrome. Eur J Endocrinol. 2016;175(6):551–60.
Fauser BC, Tarlatzis BC, Rebar RW, et al. Consensus on women’s health aspects of polycystic ovary syndrome (PCOS): the Amsterdam ESHRE/ASRM-Sponsored 3rd PCOS Consensus Workshop Group. Fertil Steril. 2012;97(1):28–38.e25.
Ozga K, Krzyczkowska-Sendrakowska M, Hubalewska-Dydejczyk A, et al. The value of Free Androgen Index depends on the phenotype of polycystic ovary syndrome, one centre experience. Endokrynol Pol. 2019;70(4):330–5.
Romualdi D, Di Florio C, Tagliaferri V, et al. The role of anti-Mullerian hormone in the characterization of the different polycystic ovary syndrome phenotypes. Reprod Sci. 2016;23(5):655–61.
Song DK, Oh JY, Lee H, Sung YA. Differentiation between polycystic ovary syndrome and polycystic ovarian morphology by means of an anti-Mullerian hormone cutoff value. Korean J Intern Med. 2017;32(4):690–8.
Tal R, Seifer DB, Khanimov M, et al. Characterization of women with elevated anti-Mullerian hormone levels (AMH): correlation of AMH with polycystic ovarian syndrome phenotypes and assisted reproductive technology outcomes. Am J Obstet Gynecol. 2014;211(1):59.e51–8.
Gupta M, Yadav R, Mahey R, et al. Correlation of body mass index (BMI), anti-Mullerian hormone (AMH), and insulin resistance among different polycystic ovary syndrome (PCOS) phenotypes—a cross-sectional study. Gynecol Endocrinol.2019;35(11):970–973. PMID: 31081410. https://doi.org/10.1080/09513590.2019.1613640.
Zhao H, Zhao Y, Ren Y, et al. Epigenetic regulation of an adverse metabolic phenotype in polycystic ovary syndrome: the impact of the leukocyte methylation of PPARGC1A promoter. Fertil Steril. 2017;107(2):467–474.e465.
Acknowledgements
We thank Lu Yu and Chao Liu of the Affiliated Wuxi People’s Hospital of Nanjing Medical University for their helpful advice.
Funding
This study is supported by the Science and Technology Development Fund of Wuxi (Z201807). The funders had no role in the design or conduct of the trial.
Author information
Authors and Affiliations
Contributions
C-MN and W-LH participated in designing the study protocol and drafting the manuscript. YJ and JX participated in designing the assessment of outcomes. Y-LZ participated in assessing treatment efficacy. X-PZ participated in recruiting and screening patients in the outpatient department. Y-YL was responsible for generating and distributing the random numbers. RD was responsible for monitoring the data during the trial. X-MF, G-AL, and YW were responsible for metabolomic evaluation and analysis. LX and QH participated in designing the trial and in the critical revision of the manuscript. They are the sponsors of the trial and have ultimate authority over the trial. All authors have read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Central ethical approval was confirmed from the ethics committee of the Affiliated Wuxi People’s Hospital of Nanjing Medical University (approval no. 2017-IIT-08-01). The participants provided written informed consent before participating in the trial.
Consent for publication
Not applicable
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Ni, CM., Huang, WL., Jiang, YM. et al. Improving the accuracy and efficacy of diagnosing polycystic ovary syndrome by integrating metabolomics with clinical characteristics: study protocol for a randomized controlled trial. Trials 21, 169 (2020). https://doi.org/10.1186/s13063-020-4060-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13063-020-4060-6