Abstract
Background
Approximately 1 million individuals experience a mild traumatic brain injury (TBI) and cost the United States nearly $17 billion each year. Many trauma survivors with mild TBI have debilitating and long-term physical, emotional, and cognitive impairments that are unrecognized at trauma centers. Early intervention studies are needed to address these impairments, especially cognitive deficits in executive functioning. Goal management training (GMT) is a structured cognitive rehabilitation program that has been found to improve executive functioning in patients with moderate to severe TBI. The current study adapted the GMT program for telephone delivery in order to improve the accessibility of rehabilitation services in a patient population with multiple barriers to care and significant yet unrecognized cognitive impairment. The primary objective of this study is to examine the efficacy of telephone-based GMT for improving executive functioning, functional status, and psychological health in trauma survivors with mild TBI.
Methods/design
This study is a three-group randomized controlled trial being conducted at a Level I trauma center. Ninety trauma survivors with mild TBI and cognitive deficits in executive functioning will be randomized to receive telephone-based GMT, telephone-based education, or usual care. GMT and education programs will be delivered by a physical therapist. The first in-person session is 1 h and the remaining six telephone sessions are 30 min. A battery of well-established cognitive tests will be conducted and validated questionnaires will be collected that measure executive functioning, functional status, and depressive and posttraumatic stress disorder symptoms at 6 weeks, 4 months, and 7 months following hospital discharge.
Discussion
This study supports a telephone-delivery approach to rehabilitation services in order to broaden the availability of evidence-based cognitive strategies.
Trial registration
This trial was registered with Clinicaltrials.gov on 10 October 2012, registration number: NCT01714531.
Similar content being viewed by others
Background
Approximately 2.5 million individuals are hospitalized each year due to traumatic injuries [1, 2], with over half experiencing a brain injury. Patients with moderate to severe traumatic brain injury (TBI) have evident debilitating cognitive and functional impairments. However, mild TBI is a silent epidemic that can result in long-term or permanent impairment and disability that is under-managed at trauma centers [3, 4]. The Centers for Disease Control (CDC) estimates that more than 1 million individuals experience a mild TBI and cost the United States (U.S.) nearly $17 billion each year [4].
There are considerable symptoms as a consequence of mild TBI including poor concentration, lethargy, confusion, disorientation, and irritability [5]. Physical, emotional, and cognitive deficits as a result of these symptoms can become chronic and disabling leading to vocational and social disabilities [5]. Physical symptoms such as impaired gait, persistent headaches, fatigue, and dizziness may continue for several months up to many years, delaying one’s ability to return to work [6, 7]. Depressive and posttraumatic stress disorder (PTSD) symptoms are extremely common in individuals with cognitive impairment [8, 9], with mild TBI being the triggering event for an episode of depression in some individuals [10]. Thirty to forty percent of trauma survivors with mild TBI have depressive symptoms and 20 % to 30 % have PTSD within the first year of recovery. Long-term cognitive consequences of mild TBI include deficits in attention, memory, and most importantly, executive functioning [11, 12, 7].
Executive functions are those involved in complex cognitions such as planning, initiating activities, and monitoring and inhibiting, which enable individuals to engage in purposeful, goal-directed behaviors (for example, balancing a checkbook and understanding social cues) [13, 14]. Deficits in executive functioning are the most disabling of all cognitive impairments and affect a person’s ability to manage effectively in one’s personal and professional life. Current literature demonstrates that deficits in executive functioning contribute to reduced quality of life, difficulty in returning to work, and persistent psychological distress in patients following head injury [15, 16]. Deficits in executive functioning may also contribute to the development and maintenance of depression and PTSD [17], with studies suggesting that cognitive impairment and psychological distress share neuroanatomic and pathophysiologic correlates [17, 18].
Current literature supports the effectiveness of cognitive rehabilitation for improving cognitive, functional, and psychological health in patients with identified brain injury [19, 20]. Cognitive rehabilitation retrains previously learned skills, increases awareness and acceptance of cognitive impairments, and teaches self-confidence and self-efficacy for coping with emotional distress. Data show that cognitive interventions are effective in a variety of settings (for example, inpatient, outpatient, and home) and when delivered by various professionals in different disciplines [21, 20]. Cognitive rehabilitation has not been traditionally offered or studied in patients with mild TBI. This population of trauma survivors has limited access to care due to underdiagnosis, as well as financial constraints and mobility issues that typically render clinic-based rehabilitation impractical.
Goal management training (GMT) is a structured form of cognitive rehabilitation that has been found to improve executive functioning in patients with moderate to severe brain injury and older adults with cognitive impairment [22, 23]. GMT uses metacognitive strategies to improve patients’ ability to organize and achieve goals in ‘real-life’ situations. GMT participants are taught to be reflective (that is, to ‘stop and think’) prior to making decisions and executing specific tasks, and to achieve success by dividing tasks into manageable units, so as to increase the likelihood that these tasks are completed.
The purpose of this study is to examine the efficacy of a telephone-based GMT program for improving executive functioning, functional status, and depressive and PTSD symptoms in trauma survivors with mild TBI. The GMT program will be compared to a telephone-based education program and usual care at 4 months (treatment completion) and 7 months following hospital discharge. Emerging research suggests that telephone rehabilitation may be a feasible and effective alternative (with much broader applicability) to clinic-based interventions [24–27]. Researchers have also suggested that rehabilitation conducted in a patient’s well-known and natural environment may facilitate and enhance the transfer of skills to the everyday living setting [28].
Methods/design
Study design
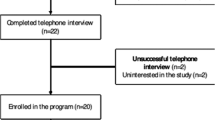
This study is a three-group randomized controlled trial conducted at a Level I trauma center. Figure 1 depicts the overall study design with assessments at 6 weeks (baseline) and 4 and 7 months after hospital discharge (see ClinicalTrials.gov and NCT01714531 for more information). The investigators, participating surgeons, research personnel conducting the assessments, and patients will be blinded to group assignment. Potential subjects will be informed that they will be randomly assigned to one of two different educational treatments or usual care. Participants will be asked not to discuss study procedures with their treating surgeon, medical staff, and research personnel.
Study population
Ninety English-speaking adults with mild TBI and cognitive deficits in executive functioning who are admitted to a Level I trauma center will be recruited for this study. Mild TBI will be determined through a medical chart review and patient interview questions using American Congress of Rehabilitation Medicine guidelines [29]. The guidelines include at least one of the following: (1) any period of loss of consciousness; (2) any loss of memory for events immediately before or after the accident; (3) any alteration in mental state at the time of the accident; and (4) focal neurological deficit(s) that may or may not be transient; but where the severity of the injury does not exceed the following: (1) posttraumatic amnesia not greater than 24 h; (2) after 30 min, an initial Glasgow Coma Scale of 13 to 15; and (3) loss of consciousness of approximately 30 min or less. Eligible participants with mild TBI will also be screened for presence of cognitive deficits in executive functioning. Deficits are defined for this study as one standard deviation (SD) below the norm referenced mean on any one of the following neuropsychological tests: Delis-Kaplan Executive Function System (D-KEFS) Tower Test, Trail Making Test B (Trails B), and FAS test [13, 30].
Exclusion criteria
Patients will be excluded from the study if they meet any of the following criteria: (1) documented evidence of moderate to severe TBI; (2) current alcohol or substance dependence within the last 6 months; (3) preexisting cognitive impairment as determined by a score greater than 3.3 on the short form of the Informant Questionnaire of Cognitive Decline in the Elderly [12, 31]; (4) neurological history other than TBI (for example, premorbid epilepsy, multiple sclerosis, Alzheimer’s disease); (5) history of schizophrenia, psychotic disorder, or suicidal intent; and (6) inability to provide a telephone number and a stable address.
Procedures
Written informed consent will be obtained from all study participants prior to study enrollment. Participants will be screened for mild TBI, preexisting cognitive impairment, alcohol and substance dependence, and cognitive deficits in executive functioning. Those that pass the screening phase will be asked to complete a baseline assessment (6 weeks after hospital discharge) and follow-up assessments at 4 and 7 months following hospitalization. Table 1 summarizes the data collection procedures across the baseline and follow-up time points. Assessments will consist of cognitive tests and questionnaires that measure cognitive functioning, functional status, and depressive and PTSD symptoms. Patients will also be asked to answer demographic and health questions at the baseline assessment. Clinical characteristics will be extracted from the medical record. All data will be entered into the Research Electronic Data Capture system (REDCap, a secure, web-based application designed exclusively to support data capture) [32].
Randomization
Participants will be randomized to one of the three groups (telephone-based GMT, telephone-based education, usual care) in a 1:1:1 ratio. Block size will be determined randomly with the patient as the unit of randomization. A randomization list will be computer generated and administered thought the REDCap system. Randomization will occur immediately after the baseline assessment at 6 weeks. Surgeons and research personnel conducting the assessments will be unaware of group assignment.
Interventions
Telephone-based GMT
The telephone-based GMT program will include seven sessions delivered by a physical therapist (Table 2). GMT was originally conceptualized by Robertson [33] and derived from Duncan’s [34] theory of goal neglect. Levine and colleagues expanded on GMT and tested a standardized protocol and treatment manual in patients with moderate to severe brain injury and in older adults with cognitive impairment [23, 35, 36, 21]. The GMT intervention targets cognitive deficits in executive functioning that impact a person’s ability to carry out daily tasks. This current study has adapted the GMT intervention to include mindfulness techniques [37] and to be delivered over the telephone, in collaboration with Dr. Brian Levine. The first session is 1 h and conducted in-person to provide participants with a session-by-session treatment manual. The remaining six sessions are 30 min and are conducted once a week over the phone. Sessions focus on increasing awareness of one’s thoughts and experiences and increasing self-efficacy. Participants learn how to use mindful attention and goal setting to recognize and stop ‘absentmindedness’ and ‘automatic pilot’ in order to reduce daily errors and ‘slips’ (Fig. 2). Each session builds upon the content of the previous session. Weekly homework is personally tailored based on patient goals.
Telephone-based education
The telephone-based education group will receive an educational program that is matched to the GMT intervention in terms of session length and contact with the study therapist. The education program includes seven sessions delivered by a physical therapist (Table 3). Material was developed by Levine et al. [23] based on material commonly employed in rehabilitation centers and has been successfully used in several studies as a comparison to the GMT intervention [35, 38]. Sessions address education on brain function and cognitive principles of memory, attention, language, perception, and motor skills. Education on stress reduction, sleep hygiene, energy management, exercise, communication, and nutrition are also provided. The first session is conducted in-person to provide participants with a session-by-session treatment manual. The remaining six sessions are conducted once a week over the phone.
Usual care
Participants in the usual care group will receive usual care as determined by the treating surgeon. Usual care may include referral to a physical therapist, occupational therapist, psychiatrist, and/or psychologist. At the end of the intervention phase, participants will be asked whether they had, on their own initiative, followed a course to improve their cognitive functioning.
Quality assurance
One study physical therapist will complete training in both the GMT and education programs. Formal training will occur with the principal investigator (PI) of the study (KRA) and an experienced neuropsychologist (JCJ). Written and skills competency tests will be completed at the end of training. After passing both tests (scores >85), the GMT and education treatments will be implemented with study staff and a pretest of both programs will occur with one patient. All sessions during the pretest will be audiotaped and reviewed to evaluate adherence to the treatment protocol and structured manual.
Our treatment integrity protocol includes detailed session-by-session treatment manuals for the telephone-based groups and ongoing supervision to ensure accurate and consistent treatment delivery (provided via weekly clinical team meetings). The study physical therapist’s adherence to procedures will be assessed by audio recording all sessions and randomly selecting sessions for the investigators to review using standardized fidelity checklists. The study physical therapist will also complete a checklist of all the components delivered during each session and make note of any protocol deviations. If the integrity of the treatments is compromised, the study therapist will be retrained and 100 % of audiotapes will be reviewed until problems are addressed.
Primary outcome measures
Executive functioning
Executive functioning will be measured using a battery of widely used and previously validated cognitive tests and patient-reported questionnaires (Table 4).
The D-KEFS Tower Test assesses the ability to plan and strategize efficiently and requires participants to move discs across three pegs until a tower is built using the fewest number of moves possible [30]. D-KEFS Tower Test is timed, but participants are unaware of specific time constraints. If the tower is not built within the allotted time, participants receive a score of 0. Completed D-KEFS Tower Test scores are adjusted for age and converted into a scaled score that ranges from 1 to 19, with higher scores reflecting better performance. The D-FEKS Tower Test has demonstrated moderate correlations with self-reported executive functioning and has been found to be sensitive and specific for brain lesion diagnosis [30].
Trails B is a time-based test that measures set shifting and cognitive flexibility [30]. Participants are asked to draw a line between a series of alternating numbers and letters according to a specified sequence. Trails B has acceptable test-retest reliability [39] and good convergent and predictive validity with significant associations with self-reported executive functioning and functional status in patients with TBI and older adults [40, 41]. The FAS assesses verbal (letter) fluency and is a valid and sensitivity measure of frontal lobe function [13]. Participants are given 1 min to generate as many words as they can for each of the letters F, A, and S. The FAS exhibits moderate correlation with measures of executive functioning after TBI and good sensitivity and specificity for patients with dementia [42, 43]. Trails B and FAS scores are adjusted for age, education, and gender and converted to T-scores, with a norm referenced mean of 50.
The Sustained Attention to Response Test (SART) is a go/no-go computer test that identifies failures of sustained attention [44]. Participants are instructed to respond to randomly presented single numbers (one through nine) every 1.15 s, except for a single no-go number (for example ‘three’). The number of errors (commission and omission) and reaction time are recorded and used as scores for the SART. The SART has good sensitivity at discriminating attention error rates of TBI patients [45, 46] and good convergent validity through associations with self- and informant-reported measures of everyday attention failure and lapses [46, 47].
The Hotel Task is a measure of planning and organizational ability and involves the participant modeling a real-life multitasking situation as a hotel manager [48]. The participant is asked to try and complete five different tasks: compiling bills; sorting a charity collection; looking up telephone numbers; sorting conference labels; proofreading the hotel leaflet. In order to complete all five tasks, the participants must distribute their time equally across the total 15-min allotment (that is, 3 min per task). Scoring of the Hotel Task is the total deviation from optimal time allocation. The Hotel Task is a sensitive measure for detecting frontal dysfunction in various conditions [48, 49].
Patient-reported executive functioning will be measured using the Dysexecutive Questionnaire (DEX) [50] and the Cognitive Failures Questionnaire (CFQ) [51]. The DEX is a 20-item questionnaire that assesses behavioral changes in executive functioning related to the areas of inhibition, memory, intention, and affect. Items on the DEX are scored using a 5-point Likert scale ranging from 0 (never) to 4 (very often) with total scores ranging from 0 to 80. The DEX has demonstrated good internal consistency and moderate correlations with other measures of patient-reported executive functioning in adults with dementia [52]. The CFQ is a 25-item questionnaire that assesses daily mental errors associated with distractibility, blunders, names, and memory [51]. Items on the CFQ are scored using a 5-point Likert scale ranging from 0 (never) to 4 (very often) with total scores ranging from 0 to 100. CFQ scores greater than 38 have been reported to indicate persistent cognitive difficulties [53]. The CFQ has been shown to have excellent psychometric properties and moderate to high correlations with cognitive tests and questionnaires in patients following head injury [54].
Secondary outcome measures
Functional status
The Functional Activities Questionnaire (FAQ) [55] and Quality of Life after Brain Injury Overall Scale (QOLIBRI-OS) [56] will be used to assess functional status. The FAQ is a 10-item questionnaire measuring a person’s ability to perform daily tasks such as writing checks, shopping, preparing meals, and others [13, 55]. Items on the FAQ are scored using a 4-point Likert scale ranging from 0 (normal) to 3 (dependent). The FAQ has excellent inter-rater reliability and is highly correlated with other instrumental activities of daily living (IADL) measures such as the Lawton and Brody’s IADL [55]. The QOLIBRI-OS is a brief 6-item measure that assesses overall satisfaction with physical condition, cognition, emotions, function, personal/social life, and current situation/future prospects in people with TBI [56]. Items on the QOLIBRI-OS are scored using a 5-point Likert ranging from 1 (not at all) to 5 (very). Scores are summed and converted to a percentage where 0 % represents the lowest and 100 % the highest possible health-related quality of life. The QOLIBRI-OS is a unidimensional scale that demonstrates good reliability and correlates highly with the full 37-item QOLIBRI scale and other measures of health-related function [56].
Psychological health
The 9-item Patient Health Questionnaire (PHQ-9) will assess depressive symptoms with items scored using a 4-point Likert scale from 0 (not at all) to 3 (nearly every day) [57]. Total scores on the PHQ-9 can range from 0 to 27. Scores of 10 or greater are commonly used cutoff points for clinically significant depressive symptoms [58]. In a psychometric study of the PHQ-9 in persons with TBI, the instrument demonstrated acceptable test-retest reliability and is a sensitive and specific measure when compared to a diagnosis of major depression [59]. The PTSD Checklist-Civilian Version (PCL-C) is a 17-item questionnaire that will be used to measure PTSD symptoms [60]. Patients rate questions about how much they are bothered by particular symptoms during the past month using a 5-point Likert scale from 1 (not at all) to 5 (extremely). The PCL-C has demonstrated acceptable test-retest reliability and internal consistency values, and good convergent validity with moderate to high correlations with other PTSD instruments and measures of anxiety and depression in patients with traumatic injury [61]. Studies have also found that trauma survivors with PCL-C scores equal to or greater than 45 have a 75 % probability of developing symptoms consistent with a diagnosis of PTSD [62, 63].
Sample size
We estimated power based on a target of 90 participants (30 per group) with complete follow-up data on 72 (85 %) by the 7-month follow-up. Power was estimated by generating simulated data, and then using simulated data to try and estimate the original model parameters. Simulated datasets were generated from available pilot data. Control subjects were resampled from control individuals in the pilot data, and treatment subjects were also resampled from control individuals, but with the target effect size added to the sampled values. Power was estimated by fitting Bayesian models to each of the simulated datasets for each response variable and recording the proportion of calculated 95 % credible intervals for effect sizes that excluded zero. There will be sufficient power to detect the following effect sizes: 2.0 points on the D-KEFS Tower test, 10.0 points on the Trails B and FAS tests, 4.0 and 6.0 points on the DEX and CFQ instruments, respectively, and minimum detectable differences of 23 % for depressive symptoms and 19 % for PTSD symptoms.
Data analysis
All data will be explored numerically and graphically for normality and appropriateness of parametric statistical testing. Analyses will be conducted using models with either original data or suitably transformed data (for example, log-linear transformation) or nonparametric analyses if necessary. Baseline variables will be summarized using appropriate descriptive statistics and compared across groups. The characteristics of the patients who are lost to follow-up will be compared to those who complete the follow-up assessments. For each outcome, we will perform longitudinal mixed-effects regression analyses, with a random intercept for patient to account for the correlation among observations from the same patient. We will examine possible nonlinear effects of the treatment over time. A random slope over time may be included to allow a separate slope to be estimated for each patient. We will fit the model with an independent conditional covariance structure and an autoregressive structure and choose the best data-supported model based on the deviance information criteria or a related criterion. The primary analysis will be intent-to-treat; missing observations due to dropout and other reasons not related to the treatments will be handled with multiple imputation methodology [64]. Statistical significance will be P <0.05. All analyses and reporting will be consistent with Consolidated Standards of Reporting Trials (CONSORT) guidelines. The data analysis plan will be fully specified and approved prior to completion of data collection.
Ethics
Ethical approval has been received from Vanderbilt Institutional Review Board (IRB# 111484) at the participating center and prospectively registered at www.ClinicalTrials.gov (NCT01714531).
Discussion
The proposed study will focus on a patient population that has significant yet clinically unrecognized and unmanaged cognitive impairment in the vital domain of executive functioning. Assessment and treatment of cognitive impairment in trauma survivors at Level I trauma centers is currently limited to patients with moderate to severe TBI. We propose to identify patients with mild TBI and clinically significant impairment in executive functioning and implement a targeted evidence-based cognitive rehabilitation program. Since previous investigations have suggested that deficits in executive functioning may contribute to the development and maintenance of depression and PTSD [17, 18], our intervention also has the potential to ameliorate depressive and PTSD symptoms during the first year of recovery following major trauma. Innovative rehabilitation interventions such as our GMT program have the potential to address poor return to work rates and profound functional and psychological disability noted in trauma survivors with mild TBI.
This study will have a direct impact on traditional rehabilitation practice. Our interventional approach broadens the availability of evidence-based cognitive strategies by expanding implementation from traditional providers, such as occupational therapists, speech-language pathologists, and neuropsychologists, to physical therapists. Compelling data are needed to support the expanding role of the physical therapist in integrating cognitive and functional strategies into patient management. This is especially important since trauma survivors are commonly referred to physical therapists during the early recovery period to address physical impairments and disability. Physical therapists are in a unique position to assess and manage both the physical and cognitive consequences of injury.
Our cognitive rehabilitation intervention will also serve to accelerate a telephone-delivery approach to rehabilitation services. Teletherapy has been used effectively in adults with chronic medical conditions and depression [65–68]. In patients with brain injury, Salazar and colleagues [69] found no significant differences in outcomes between in-hospital and telephone-based cognitive rehabilitation in military personnel with moderate to severe closed head injury. Additional research is needed to overcome common perceptions that visual contact is necessary for effective treatment. Telephone-based rehabilitation appears to be a promising approach to service delivery in patients with cognitive deficits and multiple barriers to effective treatment (that is, insurance and transportation limitations, work instability, and lack of social support and community resources). The proposed study extends the telephone-delivery model in order to improve the accessibility of effective cognitive strategies for trauma survivors.
We anticipate several difficulties in implementing the study protocol. First, the cognitive tests are time intensive and require in-person visits, which may negatively affect patient enrollment and retention. Second, we anticipate patients having a lack of awareness regarding cognitive deficits. This diminished understanding of the need for cognitive rehabilitation may impact enrollment as well as engagement in the study programs. Third, we also anticipate that completing the in-person screening and baseline assessment during the first 6 weeks following hospital discharge may be difficult due to high levels of opioid use, moderate to severe pain levels, injury to the hand or arm, and financial and geographic constraints. However, we were interested in testing our interventional approach during the early postoperative period. The National Academy of Sciences Committee on Cognitive Rehabilitation Therapy for Traumatic Brain Injury recommends that further research is needed to test the efficacy of cognitive rehabilitation therapy in individuals with milder injuries and during the subacute phase [20].
A limitation of the design of this study includes the 7-month follow-up, which impacts the ability to assess sustainability of study results. However, the priority was having adequate statistical power to detect efficacy rather than longitudinal follow-up. Serial neuropsychological assessments can result in practice effects and this will be addressed methodologically using the Reliable Change Index [13]. A potential limitation of a longitudinal study in trauma survivors is that intervening events could affect outcomes. Therefore, an intervening events questionnaire will be used to track rehospitalization, additional surgery, complications, and new or continuing use of opioid or psychoactive medications. We will use these data to control for effects of intervening events on outcomes across groups. Finally, dose–response is an important issue for the proposed study. Secondary analyses to examine the number of sessions completed will begin to explore the dose–response relationship. A next step will be to conduct a multicenter trial to further validate the telephone-based GMT intervention and improve generalizability of findings.
This study will be the first to investigate systematically a physical therapist-delivered, telephone-based cognitive rehabilitation program in patients with mild head injuries. Innovative rehabilitation interventions and delivery methods are needed to improve outcomes in trauma survivors with significant yet unrecognized cognitive impairment. Early interventional studies are also needed to address the moderate to severe cognitive, physical, and emotional impairments associated with mild TBI, especially cognitive deficits in executive functioning. There are currently no standards of treatment and early assessment and management of mild TBI are critical for optimal recovery. Overall, this line of work has the potential to benefit a large population of trauma survivors by enhancing their ability to return to a productive life both inside and outside the home.
Trial status
Recruitment was completed in February 2015. This study is currently in the follow-up phase.
Abbreviations
- CDC:
-
Centers for Disease Control
- CFQ:
-
Cognitive Failures Questionnaire
- DEX:
-
Dysexecutive Questionnaire
- D-KEFS:
-
Delis-Kaplan Executive Function System
- FAQ:
-
Functional Activities Questionnaire
- GMT:
-
Goal Management Training
- IADL:
-
instrumental activities of daily living
- NIDDR:
-
National Institute on Disability and Rehabilitation Research
- PI:
-
principal investigator
- PCL-C:
-
PTSD Checklist-Civilian Version
- PHQ-9:
-
Patient Health Questionnaire
- PTSD:
-
posttraumatic stress disorder
- QOLIBRI-OS:
-
Quality of Life after Brain Injury Overall Scale
- SART:
-
Sustained Attention to Response Test
- SD:
-
standard deviation
- TBI:
-
traumatic brain injury
References
Segui-Gomez M, MacKenzie EJ. Measuring the public health impact of injuries. Epidemiol Rev. 2003;25:3–19.
Weir S, Salkever DS, Rivara FP, Jurkovich GJ, Nathens AB, Mackenzie EJ. One-year treatment costs of trauma care in the USA. Expert Rev Pharmacoecon Outcomes Res. 2010;10:187–97.
Powell JM, Ferraro JV, Dikmen SS, Temkin NR, Bell KR. Accuracy of mild traumatic brain injury diagnosis. Arch Phys Med Rehabil. 2008;89:1550–5.
National Center for Injury Prevention and Control. Report to Congress on mild traumatic brain injury in the United States: steps to prevent a serious public health problem. Atlanta: Centers for Disease Control and Prevention; 2003.
Bryant RA, Harvey AG. Postconcussive symptoms and posttraumatic stress disorder after mild traumatic brain injury. J Nerv Ment Dis. 1999;187:302–5.
Kraus J, Schaffer K, Ayers K, Stenehjem J, Shen H, Afifi AA. Physical complaints, medical service use, and social and employment changes following mild traumatic brain injury: a 6-month longitudinal study. J Head Trauma Rehabil. 2005;20:239–56.
Vanderploeg RD, Curtiss G, Belanger HG. Long-term neuropsychological outcomes following mild traumatic brain injury. J Int Neuropsychol Soc. 2005;11:228–36.
Carlson KF, Kehle SM, Meis LA, Greer N, Macdonald R, Rutks I, et al. Prevalence, assessment, and treatment of mild traumatic brain injury and posttraumatic stress disorder: a systematic review of the evidence. J Head Trauma Rehabil. 2011;26:103–15.
Vaishnavi S, Rao V, Fann JR. Neuropsychiatric problems after traumatic brain injury: unraveling the silent epidemic. Psychosomatics. 2009;50:198–205.
Busch CR, Alpern HP. Depression after mild traumatic brain injury: a review of current research. Neuropsychol Rev. 1998;8:95–108.
Jackson JC, Archer KR, Bauer R, Abraham CM, Song Y, Greevey R, et al. A prospective investigation of long-term cognitive impairment and psychological distress in moderately versus severely injured trauma intensive care unit survivors without intracranial hemorrhage. J Trauma. 2011;71:860–6.
Jackson JC, Obremskey W, Bauer R, Greevy R, Cotton BA, Anderson V, et al. Long-term cognitive, emotional, and functional outcomes in trauma intensive care unit survivors without intracranial hemorrhage. J Trauma. 2007;62:80–8.
Lezak MD. Neuropsychological assessment. 3rd ed. New York: Oxford University Press; 1995.
Royall DR, Lauterbach EC, Cummings JL, Reeve A, Rummans TA, Kaufer DI, et al. Executive control function: a review of its promise and challenges for clinical research. A report from the Committee on Research of the American Neuropsychiatric Association. J Neuropsychiatry Clin Neurosci. 2002;14:377–405.
Lannoo E, Colardyn F, Vandekerckhove T, De Deyne C, De Soete G, Jannes C. Subjective complaints versus neuropsychological test performance after moderate to severe head injury. Acta Neurochir (Wien). 1998;140:245–53.
Tate RL, Fenelon B, Manning ML, Hunter M. Patterns of neuropsychological impairment after severe blunt head injury. J Nerv Ment Dis. 1991;179:117–26.
Moore AD, Stambrook M. Cognitive moderators of outcome following traumatic brain injury: a conceptual model and implications for rehabilitation. Brain Inj. 1995;9:109–30.
Qureshi SU, Long ME, Bradshaw MR, Pyne JM, Magruder KM, Kimbrell T, et al. Does PTSD impair cognition beyond the effect of trauma? J Neuropsychiatry Clin Neurosci. 2011;23:16–28.
Cicerone KD, Langenbahn DM, Braden C, Malec JF, Kalmar K, Fraas M, et al. Evidence-based cognitive rehabilitation: updated review of the literature from 2003 through 2008. Arch Phys Med Rehabil. 2011;92:519–30.
Koehler R, Wilhelm EE, Shoulson I, Institute of Medicine (U.S.). Committee on Cognitive Rehabilitation Therapy for Traumatic Brain Injury. Cognitive rehabilitation therapy for traumatic brain injury: evaluating the evidence. Washington, DC: National Academies Press; 2011.
Tsaousides T, Gordon WA. Cognitive rehabilitation following traumatic brain injury: assessment to treatment. Mt Sinai J Med. 2009;76:173–81.
Levine B, Robertson IH, Clare L, Carter G, Hong J, Wilson BA, et al. Rehabilitation of executive functioning: an experimental-clinical validation of goal management training. J Int Neuropsychol Soc. 2000;6:299–312.
Levine B, Schweizer TA, O'Connor C, Turner G, Gillingham S, Stuss DT, et al. Rehabilitation of executive functioning in patients with frontal lobe brain damage with goal management training. Front Hum Neurosci. 2011;5:9.
Bombardier CH, Bell KR, Temkin NR, Fann JR, Hoffman J, Dikmen S. The efficacy of a scheduled telephone intervention for ameliorating depressive symptoms during the first year after traumatic brain injury. J Head Trauma Rehabil. 2009;24:230–8.
Bradbury CL, Christensen BK, Lau MA, Ruttan LA, Arundine AL, Green RE. The efficacy of cognitive behavior therapy in the treatment of emotional distress after acquired brain injury. Arch Phys Med Rehabil. 2008;89:S61–8.
Mozer E, Franklin B, Rose J. Psychotherapeutic intervention by telephone. Clin Interv Aging. 2008;3:391–6.
Perednia DA, Allen A. Telemedicine technology and clinical applications. JAMA. 1995;273:483–8.
Boman IL, Lindstedt M, Hemmingsson H, Bartfai A. Cognitive training in home environment. Brain Inj. 2004;18:985–95.
American Congress of Rehabilitation Medicine. Definition of mild traumatic brain injury. J Head Trauma Rehabil. 1993;8:86–7.
Delis DC, Kaplan E, Kramer JH. D-KEFS technical manual. San Antonio: Psych Corp; 2001.
Jorm AF. A short form of the Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE): development and cross-validation. Psychol Med. 1994;24:145–53.
Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)–a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–81.
Robertson LH. Goal management training: a clinical manual. Cambridge: PsyConsult; 1996.
Duncan J. Attention, intelligence, and the frontal lobes. In: Gazzaniga MS, editor. The cognitive neurosciences. Cambridge: MIT Press; 1995. p. 721–33.
Novakovic-Agopian T, Chen AJ, Rome S, Abrams G, Castelli H, Rossi A, et al. Rehabilitation of executive functioning with training in attention regulation applied to individually defined goals: a pilot study bridging theory, assessment, and treatment. J Head Trauma Rehabil. 2011;26:325–38.
van Hooren SA, Valentijn SA, Bosma H, Ponds RW, van Boxtel MP, Levine B, et al. Effect of a structured course involving goal management training in older adults: A randomised controlled trial. Patient Educ Couns. 2007;65:205–13.
McHugh L, Wood R. Stimulus over-selectivity in temporal brain injury: mindfulness as a potential intervention. Brain Inj. 2013;27:1595–9.
Chen AJ, Novakovic-Agopian T, Nycum TJ, Song S, Turner GR, Hills NK, et al. Training of goal-directed attention regulation enhances control over neural processing for individuals with brain injury. Brain. 2011;134:1541–54.
Homack S, Lee D, Riccio CA. Test review: Delis-Kaplan executive function system. J Clin Exp Neuropsychol. 2005;27:599–609.
Cahn-Weiner DA, Boyle PA, Malloy PF. Tests of executive function predict instrumental activities of daily living in community-dwelling older individuals. Appl Neuropsychol. 2002;9:187–91.
Mitchell M, Miller LS. Prediction of functional status in older adults: the ecological validity of four Delis-Kaplan Executive Function System tests. J Clin Exp Neuropsychol. 2008;30:683–90.
Monsch AU, Bondi MW, Butters N, Salmon DP, Katzman R, Thal LJ. Comparisons of verbal fluency tasks in the detection of dementia of the Alzheimer type. Arch Neurol. 1992;49:1253–8.
Ruff RM, Light RH, Parker SB, Levin HS. The psychological construct of word fluency. Brain Lang. 1997;57:394–405.
Robertson IH, Manly T, Andrade J, Baddeley BT, Yiend J. 'Oops!': performance correlates of everyday attentional failures in traumatic brain injured and normal subjects. Neuropsychologia. 1997;35:747–58.
Manly T, Owen AM, McAvinue L, Datta A, Lewis GH, Scott SK, et al. Enhancing the sensitivity of a sustained attention task to frontal damage: convergent clinical and functional imaging evidence. Neurocase. 2003;9:340–9.
Dockree PM, Kelly SM, Roche RA, Hogan MJ, Reilly RB, Robertson IH. Behavioral and physiological impairments of sustained attention after traumatic brain injury. Brain Res Cogn Brain Res. 2004;20:403–14.
Smilek D, Carriere JS, Cheyne JA. Failures of sustained attention in life, lab, and brain: ecological validity of the SART. Neuropsychologia. 2010;48:2564–70.
Manly T, Hawkins K, Evans J, Woldt K, Robertson IH. Rehabilitation of executive function: facilitation of effective goal management on complex tasks using periodic auditory alerts. Neuropsychologia. 2002;40:271–81.
Torralva T, Roca M, Gleichgerrcht E, Bekinschtein T, Manes F. A neuropsychological battery to detect specific executive and social cognitive impairments in early frontotemporal dementia. Brain. 2009;132:1299–309.
Galvin JE, Roe CM, Coats MA, Morris JC. Patient's rating of cognitive ability: using the AD8, a brief informant interview, as a self-rating tool to detect dementia. Arch Neurol. 2007;64:725–30.
Broadbent DE, Cooper PF, FitzGerald P, Parkes KR. The Cognitive Failures Questionnaire (CFQ) and its correlates. Br J Clin Psychol. 1982;21:1–16.
Bodenburg S, Dopslaff N. The Dysexecutive Questionnaire advanced: item and test score characteristics, 4-factor solution, and severity classification. J Nerv Ment Dis. 2008;196:75–8.
Theadom A, Mahon S, Barker-Collo S, McPherson K, Rush E, Vandal AC, et al. Enzogenol for cognitive functioning in traumatic brain injury: a pilot placebo-controlled RCT. Eur J Neurol. 2013;20:1135–44.
Chan RC, Shum D, Toulopoulou T, Chen EY. Assessment of executive functions: review of instruments and identification of critical issues. Arch Clin Neuropsychol. 2008;23:201–16.
Pfeffer RI, Kurosaki TT, Harrah Jr CH, Chance JM, Filos S. Measurement of functional activities in older adults in the community. J Gerontol. 1982;37:323–9.
von Steinbuechel N, Wilson L, Gibbons H, Muehlan H, Schmidt H, Schmidt S, et al. QOLIBRI Overall Scale: a brief index of health-related quality of life after traumatic brain injury. J Neurol Neurosur Psychiatry. 2012;83:1041–7.
Spitzer RL, Williams JB, Kroenke K, Linzer M, deGruy III FV, Hahn SR, et al. Utility of a new procedure for diagnosing mental disorders in primary care. The PRIME-MD 1000 study. JAMA. 1994;272:1749–56.
Manea L, Gilbody S, McMillan D. Optimal cut-off score for diagnosing depression with the Patient Health Questionnaire (PHQ-9): a meta-analysis. CMAJ. 2012;184:E191–6.
Fann JR, Bombardier CH, Dikmen S, Esselman P, Warms CA, Pelzer E, et al. Validity of the Patient Health Questionnaire-9 in assessing depression following traumatic brain injury. J Head Trauma Rehabil. 2005;20:501–11.
Weathers F, Litz B, Huska J. PTSD checklist-civilian version. Boston: National Center for PTSD. Behavioral Sciences Division; 1994.
Wilkins KC, Lang AJ, Norman SB. Synthesis of the psychometric properties of the PTSD checklist (PCL) military, civilian, and specific versions. Depress Anxiety. 2011;28:596–606.
Zatzick DF, Kang SM, Muller HG, Russo JE, Rivara FP, Katon W, et al. Predicting posttraumatic distress in hospitalized trauma survivors with acute injuries. Am J Psychiatry. 2002;159:941–6.
Zatzick DF, Rivara FP, Nathens AB, Jurkovich GJ, Wang J, Fan MY, et al. A nationwide US study of post-traumatic stress after hospitalization for physical injury. Psychol Med. 2007;37:1469–80.
Harrell FE. Regression modeling strategies: with applications to linear models, logistic regression, and survival analysis. New York: Springer Publishing Co.; 2001.
Simon GE, Ludman EJ, Tutty S, Operskalski B, Von Korff M. Telephone psychotherapy and telephone care management for primary care patients starting antidepressant treatment: a randomized controlled trial. JAMA. 2004;292:935–42.
Simon GE, VonKorff M, Rutter C, Wagner E. Randomised trial of monitoring, feedback, and management of care by telephone to improve treatment of depression in primary care. BMJ. 2000;320:550–4.
van Elderen-van KT, Maes S, van den Broek Y. Effects of a health education programme with telephone follow-up during cardiac rehabilitation. Br J Clin Psychol. 1994;33:367–78.
Weinberger M, Tierney WM, Cowper PA, Katz BP, Booher PA. Cost-effectiveness of increased telephone contact for patients with osteoarthritis. A randomized, controlled trial. Arthritis Rheum. 1993;36:243–6.
Salazar AM, Warden DL, Schwab K, Spector J, Braverman S, Walter J, et al. Cognitive rehabilitation for traumatic brain injury: A randomized trial. Defense and Veterans Head Injury Program (DVHIP) Study Group. JAMA. 2000;283:3075–81.
Acknowledgments
The authors would like to acknowledge Brian Levine, PhD and Charles Bombardier, PhD for their assistance with adapting GMT for telephone delivery and Rajesh Tummuru, MD, Christine Haug, Kenya Robinson, and Rosemary Sanders for procedural assistance and data management. This study has received funding from the National Institute on Disability and Rehabilitation Research (NIDDR) (H133G120052). The study sponsor is not involved in the concept, design, management, analysis, or dissemination procedures of this study.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
KRA is the principal investigator of the study, procured funding, and registered the trial. KRA, CMA, EWE, JCJ, ODG, and WTO were responsible for the concept/idea/research design. KRA, CMA, JCJ, and SWV adapted the treatment procedures for delivery over the telephone and are responsible for treatment integrity. KRA, CMA, and LRH developed the protocol and manual of operating procedures. KRA was responsible for the randomization scheme and data analysis plan. LRH and CMA are responsible for participant enrollment and data collection in collaboration with KRA, AEL, ODG, and WTO. AEL and RAC drafted the manuscript and KRA critically revised the manuscript for important intellectual content. All authors read and approved the manuscript.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Archer, K.R., Coronado, R.A., Haislip, L.R. et al. Telephone-based goal management training for adults with mild traumatic brain injury: study protocol for a randomized controlled trial. Trials 16, 244 (2015). https://doi.org/10.1186/s13063-015-0775-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13063-015-0775-1