Abstract
We report that engineered Cas9 variants with improved specificity—eCas9-1.1 and Cas9-HF1—are often poorly active in human cells, when complexed with single guide RNAs (sgRNAs) with a mismatch at the 5’ terminus, relative to target DNA sequences. Because the nucleotide at the 5’ end of sgRNAs, expressed under the control of the commonly-used U6 promoter, is fixed to a guanine, these attenuated Cas9 variants are not useful at many target sites. By using sgRNAs with matched 5’ nucleotides, produced by linking them to a self-cleaving ribozyme, the editing activity of Cas9 variants can be rescued without sacrificing high specificity.
Similar content being viewed by others
Background
Clustered, regularly interspaced, short palindromic repeats (CRISPR) – CRISPR-associated (Cas) RNA-guided endonucleases, derived from adaptive immune systems in bacteria and archaea, have been repurposed for targeted genome editing in various cells and organisms [1,2,3,4]. These nucleases cleave chromosomal DNA in a targeted manner, producing site-specific DNA double-strand breaks (DSBs), the repair of which via non-homologous end-joining (NHEJ) induces small insertions or deletions (indels) at target sites. Unfortunately, off-target DNA cleavage at sites that are highly homologous to on-target sites can lead to mutations at undesired genomic loci [5, 6] and to chromosomal rearrangements such as translocations and inversions [6,7,8]. Both S. pyogenes Cas9 [9, 10] and single guide RNAS (sgRNAs) [6, 11, 12] have been modified to minimize or eliminate these off-target effects. In particular, two groups have independently presented engineered Cas9 variants, termed enhanced Cas9-1.1 (eCas9-1.1) [9] and Cas9 high-fidelity variant 1 (Cas9-HF1) [10], with minimal or no detectable off-target effects in human cells. These high-specificity Cas9 variants contain alanine substitutions to weaken non-specific ionic interactions between the Cas9 protein and the non-target or target DNA strand.
Here, we show that these attenuated Cas9 variants are poorly active at sites with a mismatched 5’ nucleotide relative to their sgRNA sequences in human cells. By using sgRNAs with matched 5’ nucleotides relative to their target DNA sequences, generated by self-cleaving ribozyme fusion, the cleavage activity of the Cas9 variants was rescued in human cells without sacrificing their high specificities.
Results
We hypothesized that the attenuated Cas9 variants might be poorly active at sites with a mismatch at the 5’ terminus. Because the U6 promoter, which is commonly used to express sgRNAs in eukaryotic cells, requires a guanosine (G) nucleotide to initiate transcription, sgRNAs typically contain a G nucleotide at the 5’ terminus. Three out of four DNA target sites will contain a mismatch at this position and thus might be poorly edited in cells by attenuated Cas9 variants in complex with gX19 sgRNAs (Fig. 1a), where “g” or “G” is a mismatched or matched guanosine, respectively. Note that high-specificity Cas9 variants have been previously tested at target sites with a G nucleotide at the 5’ end of the target DNA strand using GX19 sgRNAs [9, 10].
High-specificity Cas9 variants with attenuated cleavage activity. a Schematics of Cas9-WT, engineered Cas9 variants, and sgRNA variants. Locations of the alanine substitutions introduced in Cas9-WT to create eCas9-1.1 or Cas9-HF1 are shown with blue or red asterisks, respectively. Red triangles indicate cleavage positions. GX19 sgRNA starts with a G matched to its protospacer (blue line). gX19 sgRNA has a mismatched G at its 5’-end. gX20 sgRNA includes an extra guanine at its 5’ terminus. The protospacer-adjacent motif (PAM) is shown by a red line labeled NGG. H, not G (A or C or T); D, not C (A or G or T). b, c Western blot (b) and indel frequencies at the EMX1 site (c) of HeLa cells co-transfected with plasmids encoding Cas9-WT or variants and EMX1-specific sgRNA. Cas9 variants were expressed by new plasmids used in this study or by old plasmids used in previous studies. Error bars, s.e.m of two or three biological replicates
Before testing this hypothesis, we compared expression levels of Cas9 variants in human cells and found that the two variants, especially Cas9-HF1, were poorly expressed in HeLa cells (Fig. 1b). We noted that our plasmid encoding wild-type Cas9 (termed Cas9-WT hereinafter) and the two plasmids encoding Cas9 variants [9, 10] contained different promoters, tags, and codon sequences [4, 6]. We performed site-directed mutagenesis in our Cas9-WT plasmid to obtain constructs encoding the two high-specificity Cas9 variants. Western blot analysis showed that all three proteins were highly expressed in HeLa cells (Fig. 1b). Consistent with this result, eCas9-1.1 and Cas9-HF1 expressed using the new constructs induced indels at the EMX1 site with efficiencies comparable to Cas9-WT (Fig. 1c). Based on these results, we used the newly cloned constructs to express eCas9-1.1 and Cas9-HF1 throughout this study.
Reduced editing activity of high-fidelity Cas9 variants at target sites with a mismatched 5’ nucleotide
To test whether the attenuated Cas9 variants are poorly active at sites with a mismatch at the 5’ terminus, we compared editing activities of eCas9-1.1 and Cas9-HF1 with those of Cas9-WT at 26 sites whose 5’ terminal nucleotides are not guanosine in HeLa cells using gX19 sgRNAs: the sites with a 5’ cytosine (C) were termed CX19 (seven sites); those with a 5’ thymine (T), TX19 (ten sites); and those with a 5’ adenosine (A), AX19 (nine sites) (Fig. 2a and b, Additional file 1: Table S1 and Table S2). As expected, Cas9-WT was not sensitive to the mismatch at the 5’ end, inducing indels at high frequencies (64 ± 5% at CX19 sites; 65 ± 5% at TX19 sites; 80 ± 2% at AX19 sites, on average). eCas9-1.1 showed much lower indel frequencies at CX19 sites (36 ± 10%) and TX19 sites (24 ± 10%), a 1.8-fold or 2.7-fold reduction in average indel frequencies at CX19 or TX19 sites, respectively. Cas9-HF1 was least active among the three Cas9 nucleases, with average indel frequencies of 9.0 ± 3% at CX19 sites and 20 ± 10% at TX19 sites, which corresponds to 7.1-fold and 3.2-fold reductions, respectively. At AX19 target sites, however, both eCas9-1.1 and Cas9-HF1 showed indel efficiencies (81 ± 3% and 79 ± 3%) comparable to that of Cas9-WT (80 ± 2%), suggesting that a G:T mismatch at the 5’ terminus may still form a wobble base pair. These results are in line with a previous report showing Cas9-HF1 activities with three and one gX19 sgRNAs at CX19 and AX19 sites, respectively [10].
Reduced editing activity of high-fidelity Cas9 variants at target sites with a non-G 5’ nucleotide. a Indel frequencies of Cas9-WT and Cas9 variants at 26 endogenous target sites with an HX19 sequence obtained using gX19 sgRNAs in HeLa cells (H: not G [C or T or A]). The sequences and indel frequencies (%) of the 26 target sites are described in Additional file 1: Table S1. * P < 0.05, ** P < 0.01, **** P < 0.0001. b Distributions of relative indel frequencies of eCas9-1.1 and Cas9-HF1 normalized to that of Cas9-WT. Means and medians of relative indel frequencies are represented in Additional file 1: Table S2. Box and whisker plots: center lines show the medians; crosses show the means. c Indel frequencies (measured by targeted deep sequencing) at six on-target sites with a 5’ C or 5’ T nucleotide obtained using gX19 and gX20 sgRNAs in HeLa cells. PAM sequences are shown in blue. Error bars, s.e.m. of three biological replicates
We chose six CX19 or TX19 target sites at which the two Cas9 variants were poorly active and tested gX20 sgRNAs with an extra guanosine at the 5’ terminus rather than gX19 sgRNAs (Fig. 2c). Note that gX20 sgRNAs, unlike gX19 sgRNAs, have matched nucleotides at the 5’ end. Use of gX20 sgRNAs enhanced the activity of Cas9 variants at AAVS1-01 and HBB-02 sites but reduced the activity at the other four sites, compared to gX19 sgRNAs. We also noted that Cas9-WT was more efficient with gX19 sgRNAs than with gX20 sgRNAs at all six sites. These results show that gX20 sgRNAs cannot rescue the genome editing activities of high-specificity Cas9 variants. These Cas9 variants in combination with gX19 and gX20 sgRNAs also showed lower indel frequencies than Cas9-WT in HEK293T, another human cell line (Additional file 1: Figure S1).
Rescue of high-specificity Cas9 variants using Hammerhead ribozyme-linked sgRNAs
To expand the utility of high-fidelity Cas9 variants, we produced sgRNAs with matched 5’ nucleotides by using a self-cleaving ribozyme. Thus, each sgRNA was fused to a Hammerhead (HH) ribozyme at its 5’-end [13], which generates mature 20-nucleotide (X20) sgRNAs after self-cleavage (Fig. 3a). HH ribozyme-fused sgRNAs with matched 5’ nucleotides (termed HH-X20) or the mismatched 5’ guanosine nucleotide (termed HH-gX19) were tested in combination with Cas9-WT and high-fidelity Cas9 variants in HeLa cells (Fig. 3b). Use of HH-X20 sgRNAs rescued the activity of the two Cas9 variants at all six target sites. Thus, indel frequencies obtained with eCas9-1.1 (64 ± 6%) and Cas9-HF1 (55 ± 7%) using HH-X20 sgRNAs were comparable to those obtained with Cas9-WT (69 ± 5% or 70 ± 3%) using HH-X20 sgRNAs or HH-gX19 sgRNAs, respectively (Fig. 3c and Additional file 1: Table S3a). The ratios of indel frequencies of Cas9 variants in complex with HH-X20 sgRNAs relative to that of Cas9-WT had medians of 0.9 for eCas9-1.1 and 0.8 for Cas9-HF1 (Additional file 1: Figure S2, Table S3b). The two Cas9 variants were poorly active when combined with HH-gX19 sgRNAs, demonstrating that the rescue of high-fidelity variants was due to matched nucleotides at the 5’ end rather than the ribozyme fusion itself. As expected, Cas9-WT was equally efficient with HH-X20, HH-X19, and gX19 sgRNAs (P = 0.36, HH-X20 vs HH-X19; P = 0.28, HH-X20 vs gX19; P = 0.31, HH-X19 vs gX19) (Fig. 3c and Additional file 1: Table S3a). Editing efficiencies of eCas9-1.1 and Cas9-HF1 were also increased with HH-X20 sgRNAs in HEK293T cells (Additional file 1: Figure S3 and Table S4).
Recovery of editing efficiency of high-fidelity Cas9 variants using HH ribozyme-linked sgRNAs. a A schematic of a self-processing ribozyme fused sgRNA. The pre-sgRNA contains a HH ribozyme at its 5’-end. The pre-sgRNA undergoes self-cleavage to release a mature sgRNA. The red arrow indicates the self-cleavage position. b HH ribozyme-fused sgRNAs with a matched 5’ nucleotide (HH-X20) or a mismatched guanosine (HH-gX19) were tested in combination with Cas9-WT and high-fidelity Cas9 variants at six target sites in HeLa cells. Indel frequencies were measured using targeted deep sequencing. The PAM is shown in blue. Error bars, s.e.m. of three biological replicates. Statistical significances were calculated by unpaired t-test. * P < 0.05, ** P < 0.01. c Mean indel frequencies ± s.e.m. at the six target sites in HeLa cells. Statistical significances were calculated by paired t-test. ** P < 0.01, *** P < 0.001
Specificities of Cas9-WT and high-fidelity Cas9 variants in combination with HH-X20 sgRNAs
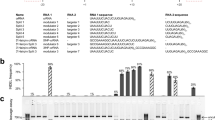
Next, we compared the specificities of the two Cas9 variants in complex with HH-X20 sgRNAs by measuring mutation frequencies at known off-target sites in HeLa cells. The CCR5-01- and EMX1-05-specific sgRNAs have no known off-target sites and were excluded from this analysis. At most of the off-target sites that differed from their respective on-target sites by one to three nucleotides, the two Cas9 variants showed much lower indel frequencies than Cas9-WT (Fig. 4). Of note, Cas9-HF1 was able to discriminate against three off-target sites (one HBB-03 off-target site and two HBB-04 off-target sites), each with a single nucleotide mismatch. These results show that attenuated Cas9 variants retain their high specificities when combined with HH-X20 sgRNAs. eCas9-1.1 and Cas9-HF1 also showed high specificities with HH-X20 sgRNAs in HEK293T cells (Additional file 1: Figure S4).
Specificities of high-fidelity Cas9 variants in combination with HH-X20 sgRNA. The HH-X20 sgRNA expression plasmid was co-transfected with the plasmid encoding Cas9-WT or a Cas9 variant into HeLa cells. Indel frequencies at on- and off-target sites were measured by targeted deep sequencing. PAM sequences are shown in blue. Mismatched bases are shown in red. The specificity ratio indicates the fold difference between the ratio of on-target indel frequencies to off-target indel frequencies obtained using Cas9 variants and that obtained using Cas9-WT. Error bars, s.e.m. of three biological replicates. Indel frequencies significantly above those of the mock transfected sample are shown by asterisks (* P < 0.05, ** P < 0.01)
Discussion and conclusions
In summary, we showed here that newly developed, high-specificity Cas9 variants, unlike the wild-type (WT) protein, are often inefficient at target sites with a mismatch at the 5’ terminus, unequivocally demonstrating the contribution of the 5’ nucleotide to the high specificity of CRISPR-Cas9 in human cells for the first time. Of note, a single 5’-end mismatch between the sgRNA and target DNA is largely tolerated by Cas9-WT. The two attenuated Cas9 variants, however, contain several alanine substitutions to weaken ionic interactions between the protein and target DNA, which can make them sensitive to a single mismatch even at the 5’ terminus. By matching the first nucleotide of sgRNAs to target DNA via the self-cleaving activity of a HH-ribozyme fusion, highly specific genome editing was achieved without sacrificing on-target editing efficiency. As an alternative to using a HH-ribozyme fusion, sgRNAs with matched 5’ non-G nucleotides could be created via tRNA fusion [14] or chemical synthesis [15] and combined with the two high-fidelity Cas9 variants. Delivery of pre-assembled Cas9 variant ribonucleoproteins [16] rather than Cas9- and sgRNA-encoding plasmids may further improve genome-wide target specificities of CRISPR genome editing. Our method expands targetable sites for high-specificity Cas9 variants, allowing broad applications in research and medicine.
Methods
Construction of high-fidelity Cas9 variant-encoding plasmids and the HH-ribozyme-fused sgRNA-encoding plasmid
eCas9-1.1- and Cas9-HF1-encoding plasmids (p3s-eCas9-1.1, Addgene #104172; p3s-Cas9-HF1, Addgene #104173) were created via site-directed mutagenesis of a WT Cas9 construct (p3s-Cas9-HN, Addgene #104171). HH-ribozyme sgRNA constructs were cloned via ligation of annealed oligonucleotides that included a HH-ribozyme sequence and a protospacer sequence into a plasmid (pRG2, Addgene #104174) in which sgRNA expression is under the control of the U6 promoter.
Cell culture and transfection
HeLa cells (ATCC, CCL-2) and HEK 293 T/17 cells (ATCC, CRL-11268) were maintained in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 100 units/mL penicillin, 100 μg/mL streptomycin, 0.1 mM non-essential amino acids, and 10% fetal bovine serum (FBS). 0.8 × 105 HeLa cells and 2 × 105 HEK293T/17 cells were transfected with the Cas9-encoding plasmid (0.5 μg) and sgRNA expression plasmid (0.5 μg) using Lipofectamine 2000 (Invitrogen) according to the manufacturer’s protocol.
Western blotting
The Cas9-WT and Cas9 variant proteins expressed in HeLa cells after transfection were detected using western blotting. Cas9 and GAPDH were detected using anti-Cas9 (Abcam, ab191468) and anti-GAPDH (Santa Cruz Biotechnology, sc-32233) primary antibodies. Goat anti-mouse IgG-HRP antibody (Santa Cruz Biotechnology, sc-2005) was used for signal detection. ImageQuant LAS4000 (GE healthcare) was used for digital imaging.
Targeted deep sequencing
The on-target and off-target regions were polymerase chain reaction (PCR) amplified for NGS library construction. Pooled PCR amplicons were sequenced using MiniSeq with a TruSeq HT Dual Index system (Illumina). Indel frequencies were obtained using Cas-Analyzer [17].
Abbreviations
- Cas9-HF1:
-
Cas9 high-fidelity variant 1
- Cas9-WT:
-
Wild-type Cas9
- DSBs:
-
DNA double strand breaks
- eCas9-1.1:
-
Enhanced Cas9-1.1
- HH:
-
Hammerhead
- Indels:
-
Insertions or deletions
- NHEJ:
-
Non-homologous end-joining
- sgRNAs:
-
Single guide RNAs
References
Jinek M, Chylinski K, Fonfara I, Hauer M, Doudna JA, Charpentier E. A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science. 2012;337:816–21.
Mali P, Yang L, Esvelt KM, Aach J, Guell M, DiCarlo JE, et al. RNA-guided human genome engineering via Cas9. Science. 2013;339:823–6.
Cong L, Ran FA, Cox D, Lin S, Barretto R, Habib N, et al. Multiplex genome engineering using CRISPR/Cas systems. Science. 2013;339:819–23.
Cho SW, Kim S, Kim JM, Kim JS. Targeted genome engineering in human cells with the Cas9 RNA-guided endonuclease. Nat Biotechnol. 2013;31:230–2.
Hsu PD, Scott DA, Weinstein JA, Ran FA, Konermann S, Agarwala V, et al. DNA targeting specificity of RNA-guided Cas9 nucleases. Nat Biotechnol. 2013;31:827–32.
Cho SW, Kim S, Kim Y, Kweon J, Kim HS, Bae S, et al. Analysis of off-target effects of CRISPR/Cas-derived RNA-guided endonucleases and nickases. Genome Res. 2014;24:132–41.
Lee HJ, Kim E, Kim JS. Targeted chromosomal deletions in human cells using zinc finger nucleases. Genome Res. 2010;20:81–9.
Lee HJ, Kweon J, Kim E, Kim S, Kim JS. Targeted chromosomal duplications and inversions in the human genome using zinc finger nucleases. Genome Res. 2012;22:539–48.
Slaymaker IM, Gao L, Zetsche B, Scott DA, Yan WX, Zhang F. Rationally engineered Cas9 nucleases with improved specificity. Science. 2016;351:84–8.
Kleinstiver BP, Pattanayak V, Prew MS, Tsai SQ, Nguyen NT, Zheng Z, et al. High-fidelity CRISPR-Cas9 nucleases with no detectable genome-wide off-target effects. Nature. 2016;529:490–5.
Fu Y, Sander JD, Reyon D, Cascio VM, Joung JK. Improving CRISPR-Cas nuclease specificity using truncated guide RNAs. Nat Biotechnol. 2014;32:279–84.
Kim D, Bae S, Park J, Kim E, Kim S, Yu HR, et al. Digenome-seq: genome-wide profiling of CRISPR-Cas9 off-target effects in human cells. Nat Methods. 2015;12:237–43. 231 p following 243.
Gao Y, Zhao Y. Self-processing of ribozyme-flanked RNAs into guide RNAs in vitro and in vivo for CRISPR-mediated genome editing. J Integr Plant Biol. 2014;56:343–9.
Port F, Bullock SL. Augmenting CRISPR applications in Drosophila with tRNA-flanked sgRNAs. Nat Methods. 2016;13:852–4.
Hendel A, Bak RO, Clark JT, Kennedy AB, Ryan DE, Roy S, et al. Chemically modified guide RNAs enhance CRISPR-Cas genome editing in human primary cells. Nat Biotechnol. 2015;33:985–9.
Kim S, Kim D, Cho SW, Kim J, Kim JS. Highly efficient RNA-guided genome editing in human cells via delivery of purified Cas9 ribonucleoproteins. Genome Res. 2014;24:1012–9.
Park J, Lim K, Kim JS, Bae S. Cas-analyzer: an online tool for assessing genome editing results using NGS data. Bioinformatics. 2017;33:286–8.
Kim S, Bae T, Hwang J, Kim JS. Rescue of high-specificity Cas9 variants using sgRNAs with matched 5’ nucleotides. NCBI Sequence Read Archive (SRA). https://www.ncbi.nlm.nih.gov/sra/?term=SRP118952.
Funding
This work was supported by the Institute for Basic Science (IBS-R021-D1).
Availability of data and materials
The deep sequencing data are available at the NCBI Sequence Read Archive (SRA) under accession number SRP118952 [18].
Author information
Authors and Affiliations
Contributions
SK, TB, and JH performed the experiments. SK and J-SK wrote the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
No ethics approval was required for this study.
Competing interests
J-SK is a co-founder of and holds stocks in ToolGen, Inc.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Additional file
Additional file 1: Figure S1.
Comparison of editing efficiencies of Cas9-WT and high-fidelity Cas9 variants using gX19 and gX20 sgRNAs in HEK293T cells. Figure S2. Comparison of relative indel frequencies of Cas9 variants normalized to that of Cas9-WT in HeLa cells. Figure S3. Comparison of indel frequencies of HH-X20, HH-gX19, and gX19 sgRNAs in combination with Cas9-WT and Cas9 variants in HEK293T cells. Figure S4. Specificities of high-fidelity Cas9 variants in combination with HH-X20 sgRNA in HEK293T cells. Table S1. Indel frequencies of Cas9-WT, eCas9-1.1, and Cas9-HF1 combined with gX19 sgRNAs at 26 target sites with an HX19 sequence. Table S2. Comparison of Cas9-WT and Cas9 variants using gX19 sgRNAs at 26 target sites with an HX19 sequence. Table S3. Comparison of indel frequencies of HH-X20, HH-gX19, and gX19 sgRNAs in combination with Cas9-WT and Cas9 variants in HeLa cells. Table S4. Comparison of indel frequencies of HH-X20, HH-gX19, and gX19 sgRNAs in combination with Cas9-WT and Cas9 variants in HEK293T cells. (PDF 497 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Kim, S., Bae, T., Hwang, J. et al. Rescue of high-specificity Cas9 variants using sgRNAs with matched 5’ nucleotides. Genome Biol 18, 218 (2017). https://doi.org/10.1186/s13059-017-1355-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13059-017-1355-3