Abstract
Background
Triple negative breast cancer (TNBC) is an aggressive subtype of invasive breast cancer that disproportionately affects Black women and contributes to racial disparities in breast cancer mortality. Prior research has suggested that neighborhood effects may contribute to this disparity beyond individual risk factors.
Methods
The sample included a cohort of 3316 breast cancer cases diagnosed between 2012 and 2020 in New Castle County, Delaware, a geographic region of the US with elevated rates of TNBC. Multilevel methods and geospatial mapping evaluated whether the race, income, and race/income versions of the neighborhood Index of Concentration at the Extremes (ICE) metric could efficiently identify census tracts (CT) with higher odds of TNBC relative to other forms of invasive breast cancer. Odds ratios (OR) and 95% confidence intervals (CI) were reported; p-values < 0.05 were significant. Additional analyses examined area-level differences in exposure to metabolic risk factors, including unhealthy alcohol use and obesity.
Results
The ICE-Race, -Income-, and Race/Income metrics were each associated with greater census tract odds of TNBC on a bivariate basis. However, only ICE-Race was significantly associated with higher odds of TNBC after adjustment for patient-level age and race (most disadvantaged CT: OR = 2.09; 95% CI 1.40–3.13), providing support for neighborhood effects. Higher counts of alcohol and fast-food retailers, and correspondingly higher rates of unhealthy alcohol use and obesity, were observed in CTs that were classified into the most disadvantaged ICE-Race quintile and had the highest odds of TNBC.
Conclusion
The use of ICE can facilitate the monitoring of cancer inequities and advance the study of racial disparities in breast cancer.
Similar content being viewed by others
Introduction
Breast cancer mortality rates are 40% higher for Black than White women in the US (28.2 vs. 20.1 per 100,000) despite similar incidence rates (127.3 vs. 131.6 per 100,000) [1]. Multiple risk factors are thought to drive this Black–White disparity [2, 3], including racial differences in insurance status, tumor characteristics, comorbidities, and treatment quality [4, 5]. However, traditional risk factor approaches typically do not consider the larger context within which these risk factors operate (e.g., neighborhood effects). For example, insurance status predicts metabolic outcomes (e.g., obesity) [6, 7] and, in turn, metabolic outcomes have been found to place Black women at a greater risk for more aggressive subtypes of breast cancer [8] and higher breast cancer mortality rates [9]—all of which can be exacerbated by residing in neighborhoods with limited healthy retail food options [10]. These findings call into question the validity of investigating risk factors separate from neighborhood circumstances.
The neighborhood context is particularly relevant in the context of triple negative breast cancer (TNBC). TNBC is an aggressive subtype of invasive breast cancer with twice the incidence rates for Black relative to White women [11, 12]. Compared to other invasive breast cancer subtypes, TNBC is more likely to present at a younger age (often before screening mammography is recommended), between screening mammograms (i.e., interval cancers), and at a more advanced stage [13], underscoring the critical need for improved prevention and early detection. As reviewed elsewhere, several potential patient-level risk factors for TNBC have been identified, with varying levels of supporting evidence, including reproductive (age at menarche and menopause, parity, breastfeeding), metabolic (obesity, type 2 diabetes, alcohol use), and genetic (BRCA1, BRCA2) factors [11, 13, 14]. More recent studies have found that area-level measures of socioeconomic status (SES) are inversely associated with TNBC risk, even after adjusting for patient characteristics, providing support for neighborhood effects [15,16,17]. Further, neighborhood effects have been found to aid in improving the targeting of prevention and early detection interventions by identifying areas with a high cancer burden that could be attributable to potentially modifiable risk factors. [18]
Viewed through a causal architecture framework, neighborhood effects can be conceptualized as a system of exposure that contributes to variations in TNBC risk across residentially segregated populations [19, 20]. In contrast to a traditional risk factor approach, a causal architecture approach would aim to clarify the network of causes that contribute to breast cancer disparities [20]. That is, rather than attempting to estimate the population-wide effects of individual risk factors, a greater emphasis would be placed on understanding how multiple, co-occurring exposures work together to produce different rates of disease between populations. Furthermore, more attention would be paid to underlying structures that explain systems of exposure [19]. For example, structural racism contributes to residential segregation and areas of disinvestment, yielding quite different systems of exposure between neighborhoods (e.g., access to employer-based insurance, high-quality health care, healthy food, etc.). [21]
New approaches are needed to efficiently identify systems of exposure that account for racial disparities in TNBC. Toward that end, the primary objective of this study was to test whether the Index of Concentration at the Extremes (ICE) metric could identify neighborhood-level systems of exposure associated with risk for TNBC in New Castle County, Delaware. Analyses were focused on this geographic region because Delaware has among the highest TNBC incidence rates in the US [22], with more cases concentrated in New Castle County relative to the other two counties in the state [23]. The ICE metric quantifies the degree to which residents within a geographic unit (e.g., census tracts) are concentrated into segregated groups of extreme disadvantage and advantage [24, 25]. Three versions of ICE can be calculated based on income, race, and both race and income. Krieger and colleagues have observed that the ICE-Race/Income metric generally outperforms the other ICE metrics when predicting health disparities [25, 26]. The ICE-Race/Income metric differs from other commonly employed indices, such as the Yost index or Area Deprivation Index (ADI) [27, 28], in at least two respects. First, the ICE metric represents a measure of social inequality by incorporating information on both disadvantage and advantage, rather than disadvantage alone. Second, the ICE-Race/Income metric operationalizes social inequality with both race and SES data, rather than SES data alone. The ICE-Race/Income metric offers the added benefit of being robust to multicollinearity, a statistical challenge frequently encountered in studies that included measures of segregation for both income and race [29]. Prior research has observed a link between ICE-Income and overall breast cancer survival [30] and ICE-Income, -Race, and -Income/Race and the odds of estrogen receptor status [29], but has not been investigated in the context of TNBC. Given the higher TNBC incidence rate observed for Black women and the relationship between TNBC and spatial measures of SES, we hypothesized that all three ICE metrics would be associated with the spatial odds of TNBC, with the greatest odds observed for the ICE-Race/Income metric.
The secondary objective of this study was to test for cross-level interactions between patient-level race and the ICE-Race metric. Prior findings that have suggested Black women living in low-SES but predominantly White neighborhoods experienced a greater risk of TNBC relative to Black women in low-SES predominantly Black neighborhoods [15]. We hypothesized that higher rates of social inequality, as measured by the ICE metrics, would be associated with greater odds of TNBC.
The tertiary objective of this study was to conduct a sensitivity check on the utility of the ICE metrics to efficiently identify neighborhoods with systems of exposure relevant to breast cancer risk. Specifically, we evaluated whether the ICE metrics were associated with metabolic risk factors, including census tract measures of alcohol and fast-food retailers, unhealthy alcohol use, and obesity. While alcohol is an established risk factor for certain breast cancer subtypes [31], its link with TNBC specifically is less clear [32]. When investigated in cohorts stratified by race, however, alcohol use has been shown to be positively associated with TNBC risk in Black but not White women [33, 34]. This would suggest that alcohol is not necessarily a ubiquitous risk factor for TNBC but that the presence of additional factors that covary with race, such as neighborhood characteristics, moderate the relationship between alcohol use and TNBC risk. Compared to White women, Black women are more likely to be exposed to racial discrimination [35], interpersonal abuse [36], and neighborhoods with elevated alcohol retailer density [37,38,39], which have all been associated with binge drinking and other patterns of unhealthy alcohol use [40]. Binge drinking predicts increased breast cancer risk even after adjusting for lifetime alcohol intake [41]. Unhealthy alcohol use may also interact with other neighborhood exposures that disproportionately affect Black women, such as limited healthy food options and its connection to obesity and metabolic syndrome [42, 43]. Metabolic syndrome has been shown to mediate nearly half the racial disparity in TNBC incidence [44]. Therefore, we hypothesized that the ICE metrics would be associated with greater exposure to metabolic risk factors.
Methods
Setting
Patient records came from the Helen F. Graham Cancer Center and Research Institute (HFGCCRI) cancer registry, a part of the Christiana Care Health System and based in New Castle County, Delaware. The HFGCCRI provides care to an average of more than 600 breast cancer cases annually. As detailed elsewhere, the HFGCCRI breast cancer population accounts for 85% of all cases from the surrounding county and are representative of the county population of cases in terms of age, race, receptor status, and stage [45].
Study population
This study population consisted of 3316 adult female New Castle County residents who were diagnosed with invasive breast cancer between the years of 2012 and 2020. To better understand Black–White disparities, the population was limited to women who self-reported as either Black (n = 776) or White (n = 2540), regardless of ethnicity. The time frame was selected to maximize the number of breast cancer cases where the subtype markers necessary for classifying patients with TNBC were routinely documented in the cancer registry. Patient residential address, demographic, insurance payer, and clinical data were abstracted from the registry. Patient addresses were manually cleaned and geocoded using ArcGIS 10.8 [46], yielding a match rate of 95% (3316/3484). Of the 168 unmatched records, 114 geocoded to another county, two geocoded to out of state, 47 had PO box addresses, three had missing address information, and two could not be located. Unmatched patients did not significantly differ from matched patients by age, race, ethnicity, stage, subtype, or insurance payer.
Patient measures
Demographic measures included age at diagnosis, race, and insurance payer status, which were all directly abstracted from the HFGCCRI cancer registry. Insurance payer status (private/commercial, Medicaid, Medicare, none, or unknown) was used as a proxy for access to health care and socioeconomic status [47]. Clinical measures included breast cancer stage and receptor status. Cases were classified into ‘TNBC’ when the receptors for estrogen (ER), progesterone (PR), and human epidermal growth factor 2 (HER2) were all known negative; all other invasive cases were classified as ‘Non-TNBC.’
Census tract measures
New Castle County is subdivided into 130 census tracts, which provide stable geographic units for reporting population statistics [48]. All census tract sociodemographic data were obtained from the US Census Bureau’s American Community Survey 2014–2018 5-year estimates [49]. ICE-Income, -Race, and -Income/Race metrics were calculated for all New Castle County census tracts according to the following general formula [24, 25]:
where Ai is the number of advantaged persons in a census tract, Di is the number of disadvantaged persons in a census tract, and Ti is the total population in the census tract i. For ICE-Income, advantaged and disadvantaged were defined as households with income ≥ $125,000 or < $20,000. For ICE-Race, advantaged and disadvantaged were defined as non-Hispanic White and non-Hispanic Black. For ICE-Race/Income, advantaged and disadvantaged were defined as non-Hispanic White households with income ≥ $125,000 and non-Hispanic Black households with income < $20,000. ICE values for geographic units range from − 1, indicating that 100% of the population can be classified into the most disadvantaged group, to + 1, indicating that 100% of the population can be classified into the most advantaged group. All ICE measures were classified into quintiles based on their distribution within New Castle County, setting Q5 (most advantaged) as the reference group.
Area-level measures were used to estimate the potential impact of environmental or neighborhood factors on rates of obesity and unhealthy alcohol use, similar to the conceptualization of ‘obesogenic’ environments [50]. Census tract prevalence measures of obesity and disordered alcohol use were generated from Christiana Care Health System electronic health record (EHR) data for 20,310 unique adult New Castle County residents who were admitted to an inpatient unit between July 1, 2018 and June 30, 2019, regardless of admitting diagnosis or demographics. Previous work has shown that such measures generated from inpatient data are generally representative of risk factor prevalence among New Castle County census tracts [51]. International Classification of Diseases (ICD) diagnosis codes abstracted from the EHR for obesity and alcohol use disorder (AUD) were used to categorize patients into ‘obese’ or ‘not obese’ and ‘AUD’ or ‘no AUD’ categories. Consistent with clinical guidelines [52], obesity was defined as a BMI of ≥ 30. AUD diagnoses were made by treating physicians and based on the Diagnostic and Statistical Manual of Mental Disorders (5th edition) [53] criteria, which assess clinically significant, unhealthy patterns of use (e.g., large quantities, cravings, tolerance, withdrawal). Patient addresses were manually cleaned and geocoded using ArcGIS 10.6, yielding a match rate of 98% (20,310/20,706). Patient-level data on obesity and alcohol use were not available for the breast cancer study population.
Census tract measures of fast-food restaurants and alcohol retailers in New Castle County were produced from commercial data and publicly available records. Fast-food retailer data were obtained from SICCODE.com, utilizing the North American Industry Classification System (NAICS) code 722513, [54] consistent with established approaches [55]. Alcohol retailer data were drawn from a public state business license database that was current as of April 17, 2019 [56]. Guided by studies that have more reliably observed a relationship between disordered alcohol use and residential exposure to off-premise alcohol retailers (e.g., liquor stores), but not on-premise alcohol retailers (e.g., bars) [57], we included only off-premise retailers. All retail locations were geocoded using ArcGIS 10.8 [46] with a match rate of 100% (fast-food retailer N = 221, alcohol retailer N = 160).
Statistical analyses
Spatial data management and statistical analyses were performed in the R Statistical Computing Environment using various packages [58,59,60,61,62,63]. Descriptive and bivariate statistics, and post hoc tests with Bonferroni-adjusted p-values, were used to compare TNBC versus Non-TNBC patient groups by the sociodemographic, clinical, and ICE variables derived from patient and census tract measures.
Multilevel logistic regression models were used to examine the odds of TNBC (vs. Non-TNBC) before and after adjusting for patient (level-1) and census tract (level-2) variables. The multilevel logistic regression model included a census tract-level random effect to account for the clustering of patients within tracts. Patient-level variables included age at diagnosis, race (Black, White), and insurance (commercial, Medicaid/none). Tract-level variables included the ICE-Race, -Income, and -Race/Income quintiles.
Three univariate and multivariate models tested each ICE measure separately, with all models adjusting for patient-level age at diagnosis and race. Additional multivariate models tested cross-level interactions between patient-level race and tract-level ICE quintiles. Based on the results of these models, details of which are provided in the results, multivariate logistic regression models were stratified by Black and White race to examine differential effects of tract-level ICE-Race on odds of TNBC after adjustment for age of diagnosis. Odds ratios and 95% confidence intervals were reported; p-values less than 0.05 were significant.
The spatial covariation of TNBC and ICE measures were visualized using bivariate choropleth maps. First, breast cancer patients were aggregated to their census tract of residence to create tract-level measures of the percentage of patients with TNBC. The % TNBC and ICE values were separated into quintiles based on their respective tract-level distributions within New Castle County. For the ICE measures, quintiles were coded such that lower ICE values (representing greater disadvantage) correspond to higher quintiles representing greater relative disadvantage. The quintiles of % TNBC and ICE were combined to create 5 × 5 classification systems that denote whether census tracts are relatively low, moderate, or high in each value. The resulting 25 classification values were symbolized using color and saturation to simultaneously show variation in both measures. For ease of visualization, only the highest/lowest quintile extremes of the classification system (low/low, low/high, high/low, and high/high) were colored in the maps. Geocoding and final map preparations were conducted in ArcMap 10.8 [46].
To begin to characterize place-based systems of exposure related to metabolic risk factors for TNBC, descriptive tables were created where census tracts were classified according to their quintiles of TNBC and ICE-Race (low/low, low/high, high/low, and high/high) that were visualized in the bivariate choropleth map. Population data from the American Community Survey were used to describe race, poverty, and education levels for the census tract groups [49]. Tract-level data on systems of exposure included alcohol and fast-food retailers, as well as prevalence of AUD and obesity. Supplemental bar charts show the variation of alcohol retailers, fast-food retailers, AUD prevalence, and obesity prevalence by census tract ICE quintiles.
Results
TNBC cases accounted for 14% of the invasive breast cancer cases in the study population (Table 1). Compared to those without TNBC, TNBC cases were significantly younger at diagnosis (mean age 60.2 vs. 63.0), twice as likely to be Black (39.5% vs. 20.9%), more likely to have Medicaid or no insurance (8.2% vs. 5.4%), less likely to have Medicare (35.8% vs. 42.2%), and twice as likely to present with a late-stage cancer (14.8% vs. 7.2%). Comparing ICE measures by census tract of residence, TNBC cases were significantly overrepresented among Q1 (the most disadvantaged quintile) tracts for ICE-Race (23.0% vs. 12.8%), ICE-Income (17.7% vs. 12.5%), and ICE-Race/Income (18.1% vs. 11.5%). TNBC cases were similarly underrepresented among Q5 (the most advantaged quintile) tracts across all ICE measures.
See Table 2 for descriptive statistics on TNBC cases stratified by race. Compared to White TNBC cases, Black TNBC cases were significantly younger (mean age 56.9 vs. 62.4) and more likely to have private insurance (63.7% vs. 50.0%) but less likely to have Medicare (24.0% vs. 43.4%). Differences in insurance status can likely be attributed to mean age differences between Black and White TNBC cases. No significant differences were observed for stage of diagnosis. Comparing ICE measures by census tract of residence, Black TNBC cases were significantly overrepresented among Q1 (the most disadvantaged quintile) tracts for ICE-Race (43.6% vs. 9.5%), ICE-Income (31.3% vs. 8.8%), and ICE-Race/Income (36.3% vs. 6.2%). Black TNBC cases were similarly underrepresented among Q5 (the most advantaged quintile) tracts across all ICE measures.
Multivariate and univariate regression analyses for both fixed and mixed effects models (with a random tract-level intercept) were tested. For both ICE-Income and ICE-Race/Income, the fixed effects only and mixed effects models produced similar coefficient results with no change in inference. However, the ICE-Race mixed effects model resulted in a singular fit and coefficients could not be estimated. This was likely due to insufficient between tract variation to support estimation of a tract-level random effect. With no covariates in the model, the variance of the random effect (measuring between-tract variance) was significant but small (var = 0.092, p = 0.014), which was reduced and became non-significant in most models once covariates were included (see Additional file 1: Table S1). Correspondingly, the intraclass correlation coefficient (ICC) for the census tract random effect was 0.027, indicating that only 2.7% of the total variance was attributed to between-tract variability. Given the similarity of results across fixed effects only and mixed effects models for ICE-Income and ICE-Race/Income and the singular fit for the ICE-Race random effects model, and the similarity of results between the fixed effects univariate and multivariate models for ICE-Race, results from the multilevel fixed effects only models are presented here for ease of interpretation (see Additional file 1: Table S2 for the full set of available random effects model results).
Table 3 shows the results of multilevel fixed-effects only models that separately test each ICE measure. In univariate models, increasing age of diagnosis was associated with lower odds of TNBC (OR: 0.93, 95% CI 0.89, 0.96), while Black race was associated with more than double the odds of TNBC relative to White race (OR: 2.48, 95% CI 2.01, 3.05). Models run with the insurance variable excluded patients age 65 and older and those with Medicare or unknown insurance to better model insurance as a proxy for health care access and SES (i.e., patients are eligible for Medicare beginning at age 65 regardless of SES). Insurance type was not significantly associated with TNBC, and therefore, the multivariate models were run for the full study population (i.e., including patients age 65 and older) and without the insurance status covariate. Across multivariate models for each ICE measure, increasing age of diagnosis and Black race were significantly associated with lower and greater odds of TNBC, respectively (p-values < 0.05). Quintiles Q1–Q4 of ICE-Race (corresponding to greater disadvantage relative to Q5) were associated with significantly higher odds of TNBC, even after adjustment for patient-level race and age of diagnosis, ranging from Q1 (AOR: 2.09) to Q4 (AOR: 1.76). Neither ICE-Income nor ICE-Race/Income quintiles were significantly associated with TNBC after covariate adjustment, though their adjusted odds ratios suggested positive associations with TNBC. No significant interactions were observed in models that included cross-level interaction terms between patient-level race and tract-level ICE (Additional file 1: Table S3).
The multilevel fixed-effects only model of age and ICE-Race was stratified by patient-level race to further characterize the relationship between patient race, area-level segregation, and TNBC (Table 4). Increasing age of diagnosis was associated with decreased odds of TNBC for Black patients (AOR: 0.89, 95% CI 0.83, 0.95) but not White patients (AOR: 0.97, 95% CI 0.92, 1.01). Among Black patients, ICE-Race quintiles were no longer associated with TNBC (p-values > 0.05). The magnitude of the adjusted odds ratios suggested a positive association, which the relatively small Black patient sample (N = 776) may be underpowered to detect. ICE-Income and -Race/Income quintiles were also not associated with TNBC for Black patients (p-values > 0.05). Among White patients, ICE-Race quintiles Q1, Q2, and Q4 were associated with significantly greater odds of TNBC (p-values < 0.05). This result would suggest that White women living in predominantly Black census tracts were more likely to be diagnosed with TNBC, relative to other forms of invasive breast cancer, compared to White women living in predominantly White census tracts. For ICE-Income, only quintile Q2 was significantly associated with TNBC (AOR: 1.52, 95% CI 1.05, 2.20), with a non-significant trend in the expected direction observed for Q1 (AOR: 1.25, 95% CI 0.76, 2.01). This would suggest that White women living in low-income census tracts are at an elevated risk for TNBC, relative to other forms of invasive breast cancer among White women living in higher-income census tracts. No significant associations with TNBC were observed for ICE-Race/Income among White women.
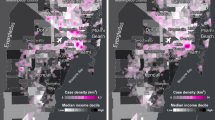
Figure 1 shows the spatial covariation of TNBC prevalence and ICE measures across county census tracts. Tracts are symbolized as follows: (1) light gray—lower% TNBC and lower ICE-measured disadvantage; (2) magenta—higher% TNBC and lower ICE-measured disadvantage; (3) teal—lower% TNBC and higher ICE-measured disadvantage, and (4) blue—higher% TNBC and higher ICE-measured disadvantage. TNBC prevalence appears to correlate most strongly with ICE-Race, represented by a greater number of dark blue census tracts in Fig. 1A relative to ICE-Income and ICE-Race/Income shown in Fig. 1B, C, respectively. Across all maps, higher TNBC prevalence and higher ICE-measured disadvantage overlap in the greater Wilmington area, extending southwest and in the northeastern-most corner of the county. Figure 1B (ICE-Income) and Fig. 1C (ICE-Race/Income) each depict two census tracts with higher TNBC prevalence but lower ICE-measured disadvantage in the southern part of the county. Each of the maps depict two census tracts with lower TNBC prevalence but higher ICE-measured disadvantage in the north-central part of the county.
Spatial covariation of triple negative breast cancer (TNBC) prevalence and ICE measures, New Castle County, DE. A–C depict quintiles of TNBC prevalence and ICE-measured disadvantage (by race, income, and race/income) at the census tract level in New Castle County, DE. The extremes of the classification system in light gray, magenta, teal, and blue represent the spatial covariation of both measures, ranging from lower in both to higher in both. Across all maps, higher TNBC prevalence and higher ICE-measured disadvantage overlap in the City of Wilmington, as shown by the blue tracts. ICE-Race (A) appears to correlate with TNBC more strongly than ICE-Income (B) or ICE-Race/Income (C), as map A has more tracts classified as low–low (light gray) or high–high (blue) in both measures. ICE-Race (A) correlates with higher TNBC prevalence in additional census tracts south of the City of Wilmington, which correspond to tracts that have relatively large Black populations but relatively less income deprivation measured by ICE (B, C)
The tract classifications based on quintiles of TNBC prevalence and ICE-Race appear to differentiate the affected populations and area-level systems of exposure (Table 5). As expected, the ten census tracts characterized as high in both TNBC and ICE-Race disadvantage (“high/high”) had a 28.8% prevalence of TNBC among breast cancer patients, and 63.9% of their general population from these census tracts were Black. Compared to the 12 tracts that were characterized as both low in TNBC and ICE-Race disadvantage (“low/low”), residents living in the high/high tracts had greater rates of poverty (23.3% vs. 5.0%) and higher rates of completing less than a high school education (13.0% vs. 5.6%). Comparing high/high and low/low tracts, the former had more than double the count (19 vs. 8) and density (0.48 vs. 0.20) of alcohol retailers. Similar but weaker patterns were observed for fast-food retailer counts (14 vs. 12) and density (0.35 vs. 0.30). High/high tracts also had greater prevalence of AUD (25.4% vs. 14.9%) and obesity (43.4% vs. 33.9%). Additional file 1: Tables S4 and S5 present these descriptive statistics according to classifications based on ICE-Income and ICE-Race/Income, respectively, which were similar to those based on ICE-Race. Additional file 2: Figure S1 shows the distribution of alcohol and fast-food retailers and AUD and obesity prevalence by tract ICE quintiles.
Discussion
In a cohort of breast cancer patients from New Castle County, Delaware, a geographic area with among the highest rates of TNBC in the US, we tested whether different versions of the ICE metric could efficiently identify census tracts with greater odds of TNBC relative to other invasive subtypes of breast cancer. Consistent with prior epidemiological findings [13], women with TNBC were younger, twice as likely to be Black, more likely to have Medicaid or no insurance, and twice as likely to present with a late-stage cancer. As hypothesized, ICE-Race, -Income, and ICE-Race/Income metrics were associated with the odds of TNBC on a bivariate basis. However, contrary to our hypotheses, only the ICE-Race metric was significantly associated with higher odds of TNBC in multilevel models that adjusted for patient-level age and race. To our knowledge, this is the first multilevel study that evaluated the use of ICE metrics in the context of TNBC.
By including both patient- and census tract-level measures of race, our results help to clarify the extent to which the higher rates of TNBC observed in predominantly Black neighborhoods can be attributed to compositional or contextual effects. That is, if the relationship between ICE-Race and census tract odds of TNBC became nonsignificant when adjusting for patient-level race, we might infer that the apparent neighborhood effects were likely an artifact of the neighborhood composition. By contrast, our results suggest that the relationship between TNBC and area-level measures of race are a function of both composition and context. Stated differently, compared to predominantly White neighborhoods, predominantly Black neighborhoods may differ in some important ways that have relevance for TNBC risk. This interpretation is bolstered by the multilevel results stratified by patient-level race, which showed that White patients who lived in predominantly Black census tracts were also at greater odds of TNBC than White patients living in predominantly White census tracts.
These results are in partial disagreement with the findings from two prior studies, which found higher odds of TNBC for Black women living in lower-SES, predominantly White neighborhoods [15, 64]. The authors of these reports reasoned that Black women who reside in predominantly Black neighborhoods may have social support systems that mitigate the effects of living in a low-SES environment. However, the potentially protective effects of social support derived from racial/ethnic enclaves on cancer outcomes has not been well-studied in Black populations compared to Hispanic or Asian populations. Not only did we find that census tracts with a higher proportion of Black residents were associated with higher odds of TNBC, we also did not observe any significant patient-level race by ICE-Income cross-level interactions. Our results are more consistent with a segregation-based disparate exposure hypothesis. That is, through a series of historical laws and policies (e.g., “redlining”), the US, state, and local governments forcibly segregated communities by race and denied these marginalized communities access to financing and other forms of investment, a residential pattern that largely persists through present day [65,66,67]. The effect of these segregationist policies can be measured in terms of disparate exposures (e.g., increased concentration of alcohol retailers) [68, 69], poorer access to healthy food [70], and ultimately worse cancer and other health outcomes [71,72,73]. Indeed, we observed higher counts of alcohol and fast-food retailers, and correspondingly higher rates of AUD and obesity, in census tracts that were categorized into the most disadvantaged ICE-Race quintile and had the highest odds of TNBC.
This study was limited by its single-site, cross-sectional design. Findings may not be generalizable to populations from other geographic areas. Nevertheless, given the notably elevated rates of TNBC in Delaware, the results of this study can help to inform local cancer control and prevention efforts while providing a methodological proof of concept that can be replicated for other geographic areas. Without patient residential histories, it is unclear to what degree prior neighborhood exposures may have been associated with TNBC risk. Furthermore, while we did have access to patient-level measures of race and insurance status, we did not have patient-level measures of other relevant exposures (e.g., alcohol use) and were not able to determine ethnicity (Hispanic vs. non-Hispanic) at the patient level. Further, we limited our investigation of neighborhood to include ICE measures, given there is no standard set of measures used to measure neighborhood deprivation. It is possible other socioeconomic indices (e.g., Yost index, ADI) could also provide additional insights into the impact of neighborhood on TNBC [74]. Future research should be conducted on cohorts from a range of geographic areas, with more detailed patient- and area-level measures of exposure, to further characterize the multilevel relationships between race, SES, and TNBC.
Conclusions
This study provides preliminary evidence to suggest that the ICE-Race metric can efficiently identify census tracts with higher odds of TNBC due to both compositional and contextual effects. Preliminary evidence also suggests that the contextual effects may be driven, at least in part, by potentially modifiable metabolic exposures, such as alcohol use and obesity. Krieger and colleagues have called for including ICE metrics in cancer registries to facilitate the monitoring of cancer inequities [29]. Going further, the use of ICE metrics can help to advance the study of racial disparities in breast cancer from a methodology based on traditional risk factors to one grounded in a causal architecture framework. Rather than studying individual risk factors in isolation without considering neighborhood effects, the use of large and representative pooled patient cohorts can be employed to evaluate the multilevel, multifactorial relationships between exposures and TNBC. Such efforts could be complemented by basic and translational research designed to delineate mechanisms of pathophysiology and facilitate biomarker discovery. Together, these lines of research could inform risk stratification approaches to improve early detection, more effectively target risk factor modification interventions to the communities at greatest risk, and advance health equity [75, 76].
Availability of data and materials
Datasets generated for this study from the HFGCCRI cancer registry or the Christiana Care Health System EHR are not publicly available because they contain protected health information but may be made available in a deidentified format from the corresponding author on reasonable request. Census tract datasets generated for this study based on publicly available sources, including ICE metrics and the alcohol and fast-food retailer locations, are available from the corresponding author on reasonable request.
References
Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2021. CA Cancer J Clin. 2021;71(1):7–33. https://doi.org/10.3322/caac.21654.
Hill HE, Schiemann WP, Varadan V. Understanding breast cancer disparities—a multi-scale challenge. Ann Transl Med. 2020;8(14):906–906. https://doi.org/10.21037/atm.2020.04.37.
Daly B, Olopade OI. A perfect storm: how tumor biology, genomics, and health care delivery patterns collide to create a racial survival disparity in breast cancer and proposed interventions for change. CA Cancer J Clin. 2015;65(3):221–38. https://doi.org/10.3322/caac.21271.
Silber JH, Rosenbaum PR, Ross RN, et al. Disparities in breast cancer survival by socioeconomic status despite medicare and medicaid insurance. Milbank Q. 2018;96(4):706–54. https://doi.org/10.1111/1468-0009.12355.
Jemal A, Robbins AS, Lin CC, et al. Factors that contributed to black-white disparities in survival among nonelderly women with breast cancer between 2004 and 2013. J Clin Oncol. 2018;36(1):14–24. https://doi.org/10.1200/JCO.2017.73.7932.
Brown AGM, Kressin N, Terrin N, et al. The influence of health insurance stability on racial/ethnic differences in diabetes control and management. Ethn Dis. 2021;31(1):149–58. https://doi.org/10.18865/ED.31.1.149.
Mylona EK, Benitez G, Shehadeh F, et al. The association of obesity with health insurance coverage and demographic characteristics: a statewide cross-sectional study. Medicine (Baltimore). 2020;99(27):e21016. https://doi.org/10.1097/MD.0000000000021016.
Palmer JR, Castro-Webb N, Bertrand K, Bethea TN, Denis GV. Type II diabetes and incidence of estrogen receptor negative breast cancer in African American women. Cancer Res. 2017;77(22):6462–9. https://doi.org/10.1158/0008-5472.CAN-17-1903.
Charlot M, Castro-Webb N, Bethea TN, et al. Diabetes and breast cancer mortality in Black women. Cancer Causes Control. 2017;28(1):61–7. https://doi.org/10.1007/s10552-016-0837-z.
Phillips AZ, Rodriguez HP. Adults with diabetes residing in “food swamps” have higher hospitalization rates. Health Serv Res. 2019;54(January):217–25. https://doi.org/10.1111/1475-6773.13102.
Newman LA, Kaljee LM. Health disparities and triple-negative breast cancer in African American women. JAMA Surg. 2017;152(5):485. https://doi.org/10.1001/jamasurg.2017.0005.
Scott LC, Mobley LR, Kuo TM, Il’yasova D,. Update on triple-negative breast cancer disparities for the United States: a population-based study from the United States Cancer Statistics database, 2010 through 2014. Cancer. 2019;125(19):3412–7. https://doi.org/10.1002/cncr.32207.
Howard FM, Olopade OI. Epidemiology of triple-negative breast cancer. Cancer J. 2021;27(1):8–16. https://doi.org/10.1097/PPO.0000000000000500.
Prakash O, Hossain F, Danos D, Lassak A, Scribner R, Miele L. Racial disparities in triple negative breast cancer: a review of the role of biologic and non-biologic factors. Front Public Heal. 2020;8:1–14. https://doi.org/10.3389/fpubh.2020.576964.
Qin B, Babel RA, Plascak JJ, et al. Neighborhood social environmental factors and breast cancer subtypes among black women. Cancer Epidemiol Prev Biomark. 2021;30:344–51. https://doi.org/10.1158/1055-9965.EPI-20-1055.
Aoki RLF, Uong SP, Gomez SL, et al. Individual- and neighborhood-level socioeconomic status and risk of aggressive breast cancer subtypes in a pooled cohort of women from Kaiser Permanente Northern California. Cancer. 2021;127:4602–12. https://doi.org/10.1002/cncr.33861.
Barber LE, Zirpoli GR, Cozier YC, et al. Neighborhood disadvantage and individual-level life stressors in relation to breast cancer incidence in US Black women. Breast Cancer Res. 2021;23(1):108. https://doi.org/10.1186/s13058-021-01483-y.
Lynch SM, Wiese D, Ortiz A, et al. Towards precision public health: geospatial analytics and sensitivity/specificity assessments to inform liver cancer prevention. SSM Popul Health. 2020;12:100640. https://doi.org/10.1016/j.ssmph.2020.100640.
Riley AR. Advancing the study of health inequality: fundamental causes as systems of exposure. SSM Popul Health. 2020;10:100555. https://doi.org/10.1016/j.ssmph.2020.100555.
Keyes KM, Galea S. The limits of risk factors revisited: is it time for a causal architecture approach? Epidemiology. 2017;28(1):1–5. https://doi.org/10.1097/EDE.0000000000000578.
Bailey ZD, Krieger N, Agénor M, Graves J, Linos N, Bassett MT. Structural racism and health inequities in the USA: evidence and interventions. Lancet. 2017;389(10077):1453–63. https://doi.org/10.1016/S0140-6736(17)30569-X.
Kohler BA, Sherman RL, Howlader N, et al. Annual report to the nation on the status of cancer, 1975–2011, featuring incidence of breast cancer subtypes by race/ethnicity, poverty, and state. J Natl Cancer Inst. 2015. https://doi.org/10.1093/jnci/djv048.
Moss JL, Tatalovich Z, Zhu L, Morgan C, Cronin KA. Triple-negative breast cancer incidence in the United States: ecological correlations with area-level sociodemographics, healthcare, and health behaviors. Breast Cancer. 2020. https://doi.org/10.1007/s12282-020-01132-w.
Massey DS. The prodigal paradigm returns: ecology comes back to sociology. In: Booth A, Crouter A, editors. Does it take a village? Community effects on children, adolescents, and families. Mahwah: Lawrence Erlbaum Associates; 2001. p. 41–8.
Krieger N, Waterman PD, Spasojevic J, Li W, Maduro G, Van Wye G. Public health monitoring of privilege and deprivation with the index of concentration at the extremes. Am J Public Health. 2016;106(2):256–63. https://doi.org/10.2105/AJPH.2015.302955.
Krieger N, Kim R, Feldman J, Waterman PD. Using the Index of Concentration at the Extremes at multiple geographical levels to monitor health inequities in an era of growing spatial social polarization: Massachusetts, USA (2010–14). Int J Epidemiol. 2018;47:788–819. https://doi.org/10.1093/ije/dyy004.
Yost K, Perkins C, Cohen R, Morris C, Wright W. Socioeconomic status and breast cancer incidence in California for different race/ethnic groups. Cancer Causes Control. 2001;12(8):703–11. https://doi.org/10.1023/A:1011240019516.
Kind AJH, Buckingham WR. Making neighborhood-disadvantage metrics accessible—the neighborhood Atlas. N Engl J Med. 2018;378(26):2456–8. https://doi.org/10.1056/NEJMp1802313.
Krieger N, Singh N, Waterman PD. Metrics for monitoring cancer inequities: residential segregation, the Index of Concentration at the Extremes (ICE), and breast cancer estrogen receptor status (USA, 1992–2012). Cancer Causes Control. 2016;27(9):1139–51. https://doi.org/10.1007/s10552-016-0793-7.
Wiese D, Stroup AM, Crosbie A, Lynch SM, Henry KA. The impact of neighborhood economic and racial inequalities on the spatial variation of breast cancer survival in New Jersey. Cancer Epidemiol Biomark Prev. 2019;28(12):1958–67. https://doi.org/10.1158/1055-9965.EPI-19-0416.
Zuckerman AJ. IARC Monographs on the evaluation of carcinogenic risks to humans. J Clin Pathol. 1995;48(7):691–691. https://doi.org/10.1136/jcp.48.7.691-a.
Gaudet MM, Gierach GL, Carter BD, et al. Pooled analysis of nine cohorts reveals breast cancer risk factors by tumor molecular subtype. Cancer Res. 2018;78(20):6011–21. https://doi.org/10.1158/0008-5472.CAN-18-0502.
Williams LA, Olshan AF, Tse CK, Bell ME, Troester MA. Alcohol intake and invasive breast cancer risk by molecular subtype and race in the Carolina Breast Cancer Study. Cancer Causes Control. 2016;27(2):259–69. https://doi.org/10.1007/s10552-015-0703-4.
Williams LA, Olshan AF, Hong CC, et al. Alcohol intake and breast cancer risk in african American women from the AMBER consortium. Cancer Epidemiol Biomark Prev. 2017;26(5):787–94. https://doi.org/10.1158/1055-9965.EPI-16-0792.
Desalu JM, Goodhines PA, Park A. Racial discrimination and alcohol use and negative drinking consequences among Black Americans: a meta-analytical review. Addiction. 2019;114(6):957–67. https://doi.org/10.1111/add.14578.
Hitch AE, Brown JL, DiClemente RJ. Interpersonal abuse and alcohol use among African American young women: the mediating role of emotion dysregulation. J Ethn Subst Abuse. 2021. https://doi.org/10.1080/15332640.2021.1952130.
Fairman BJ, Goldstein RB, Simons-Morton BG, et al. Neighbourhood context and binge drinking from adolescence into early adulthood in a US national cohort. Int J Epidemiol. 2020;49(1):103–12. https://doi.org/10.1093/ije/dyz133.
Rhew IC, Kosterman R, Lee JO. Neighborhood typologies associated with alcohol use among adults in their 30s: a finite mixture modeling approach. J Urban Health. 2017;94(4):542–8. https://doi.org/10.1007/s11524-017-0161-2.
Siegel SD, Brooks M, Ragozine-Bush HE, Schnoll RA, Curriero FC. The co-occurrence of smoking and alcohol use disorder in a hospital-based population: applying a multimorbidity framework using geographic information system methods. Addict Behav. 2021;118:106883. https://doi.org/10.1016/j.addbeh.2021.106883.
Zapolski TCB, Baldwin P, Banks DE, Stump TE. Does a crossover age effect exist for African American and hispanic binge drinkers? Findings from the 2010 to 2013 national study on drug use and health. Alcohol Clin Exp Res. 2017;41(6):1129–36. https://doi.org/10.1111/acer.13380.
White AJ, DeRoo LA, Weinberg CR, Sandler DP. Lifetime alcohol intake, binge drinking behaviors, and breast cancer risk. Am J Epidemiol. 2017;186(5):541–9. https://doi.org/10.1093/aje/kwx118.
Gallagher EJ, Fei K, Feldman SM, et al. Insulin resistance contributes to racial disparities in breast cancer prognosis in US women. Breast Cancer Res. 2020;22(1):1–10. https://doi.org/10.1186/s13058-020-01281-y.
Lindtner C, Scherer T, Zielinski E, et al. Binge drinking induces whole-body insulin resistance by impairing hypothalamic insulin action. Sci Transl Med. 2013. https://doi.org/10.1126/scitranslmed.3005123.
Luo J, Kroenke CH, Hendryx M, et al. Mediation analysis of racial disparities in triple-negative breast cancer incidence among postmenopausal women. Breast Cancer Res Treat. 2021. https://doi.org/10.1007/s10549-021-06158-y.
Siegel SD, Brooks MM, Sims-Mourtada J, et al. A population health assessment in a community cancer center catchment area: triple-negative breast cancer, alcohol use, and obesity in New Castle County, Delaware. Cancer Epidemiol Biomark Prev. 2021. https://doi.org/10.1158/1055-9965.EPI-21-1031.
ArcGIS Desktop. 2021.
Casey JA, Pollak J, Glymour MM, Mayeda ER, Hirsch AG, Schwartz BS. Measures of SES for electronic health record-based research. Am J Prev Med. 2018;54(3):430–9. https://doi.org/10.1016/j.amepre.2017.10.004.
U.S. Census Bureau. Census tracts and block numbering areas. Geogr Areas Ref Man. http://www.census.gov/geo/reference/garm.htm (1994).
U.S. Census Bureau. 2014–2018 American Community Survey 5-year estimates. https://www.census.gov/programs-surveys/acs/technical-documentation/table-and-geography-changes/2018/5-year.html (2020).
Folkvord F, Hermans RCJ. Food marketing in an obesogenic environment: a narrative overview of the potential of healthy food promotion to children and adults. Curr Addict Rep. 2020;7(4):431–6. https://doi.org/10.1007/s40429-020-00338-4.
Siegel SD, Brooks M, Curriero FC. Operationalizing the population health framework: clinical characteristics, social context, and the built environment. Popul Health Manag. 2020. https://doi.org/10.1089/pop.2020.0170.
Donato KA. Executive summary of the clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults. Arch Intern Med. 1998;158(17):1855–67. https://doi.org/10.1001/archinte.158.17.1855.
American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5th ed. Arlinton: American Psychiatric Association; 2013. https://doi.org/10.1176/appi.books.9780890425596.
SIC Code & NAICS Code Business Lists. NAICS food retailer data [Data file]. SICCODE.com.
Centers for Disease Control and Prevention. Census tract level state maps of the modified retail food environment index (MREFI). https://www.cdc.gov/obesity/downloads/census-tract-level-state-maps-mrfei_TAG508.pdf (2011). Accessed 9 July 2021.
Delaware Division of Revenue. Delaware business licenses. Delaware open data. https://revenue.delaware.gov/business-license-search/ (2019). Accessed 17 April 2019.
Gmel G, Holmes J, Studer J. Are alcohol outlet densities strongly associated with alcohol-related outcomes? A critical review of recent evidence. Drug Alcohol Rev. 2016;35(1):40–54. https://doi.org/10.1111/dar.12304.
R Core Team. R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. http://wwwR-project.org/ (2013). https://doi.org/10.1348/000712608X366867
Pebesma E. Simple features for R: standardized support for spatial vector data. R J. 2018;10(1):439. https://doi.org/10.32614/RJ-2018-009.
Walker K, MH. Tidycensus: load US census boundary and attribute data as “tidyverse” and ‘sf’-read data frames. R package version 0.10.2 (2020).
Prener C, Grossenbacher T, Zehr A. Biscale: tools and palettes for bivariate thematic mapping. R package version 0.2.0 (2020).
Bates D, Mächler M, Bolker B, Walker S. Fitting linear mixed-effects models using lme4. J Stat Softw. 2015. https://doi.org/10.18637/jss.v067.i01.
Banghart M. pgnm: parametric bootstrap test of nexted models. R Package version 0.3.0.9003 (2015).
Linnenbringer E, Geronimus AT, Davis KL, Bound J, Ellis L, Gomez SL. Associations between breast cancer subtype and neighborhood socioeconomic and racial composition among Black and White women. Breast Cancer Res Treat. 2020;180(2):437–47. https://doi.org/10.1007/s10549-020-05545-1.
Rothstein R. The color of law. New York: Liverright Publishing Corporation; 2018.
An B, Orlando AW, Rodnyansky S. The physical legacy of racism: how redlining cemented the modern built environment. SSRN Electron J. 2019. https://doi.org/10.2139/ssrn.3500612.
Purifoy DM, Seamster L. Creative extraction: black towns in white space. Environ Plan D Soc Sp. 2020. https://doi.org/10.1177/0263775820968563.
Scott J, Danos D, Collins R, et al. Structural racism in the built environment: segregation and the overconcentration of alcohol outlets. Health Place. 2020;64:102385. https://doi.org/10.1016/j.healthplace.2020.102385.
Lee JP, Ponicki W, Mair C, Gruenewald P, Ghanem L. What explains the concentration of off-premise alcohol outlets in black neighborhoods? SSM Popul Health. 2020;12:100669. https://doi.org/10.1016/j.ssmph.2020.100669.
Sadler RC, Bilal U, Furr-Holden CD. Linking historical discriminatory housing patterns to the contemporary food environment in Baltimore. Spat Spatiotemporal Epidemiol. 2021;36:100387. https://doi.org/10.1016/j.sste.2020.100387.
Collin LJ, Gaglioti AH, Beyer KM, et al. Neighborhood-level redlining and lending bias are associated with breast cancer mortality in a large and diverse metropolitan area. Cancer Epidemiol Biomark Prev. 2020. https://doi.org/10.1158/1055-9965.epi-20-1038.
Krieger N, Wright E, Chen JT, Waterman PD, Huntley ER, Arcaya M. Cancer stage at diagnosis, historical redlining, and current neighborhood characteristics: breast, cervical, lung, and colorectal cancers, Massachusetts, 2001–2015. Am J Epidemiol. 2020;189(10):1065–75. https://doi.org/10.1093/aje/kwaa045.
Beyer KMM, Zhou Y, Matthews K, Bemanian A, Laud PW, Nattinger AB. New spatially continuous indices of redlining and racial bias in mortgage lending: links to survival after breast cancer diagnosis and implications for health disparities research. Health Place. 2016;40:34–43. https://doi.org/10.1016/j.healthplace.2016.04.014.
Boscoe FP, Liu B, Lee F. A comparison of two neighborhood-level socioeconomic indexes in the United States. Spat Spatiotemporal Epidemiol. 2021;37:100412. https://doi.org/10.1016/j.sste.2021.100412.
Lynch SM, Rebbeck TR. Bridging the gap between biologic, individual, and macroenvironmental factors in cancer: a multilevel approach. Cancer Epidemiol Biomark Prev. 2013;22(4):485–95. https://doi.org/10.1158/1055-9965.EPI-13-0010.
Boggs DA, Rosenberg L, Adams-Campbell LL, Palmer JR. Prospective approach to breast cancer risk prediction in African American women: the black women’s health study model. J Clin Oncol. 2015;33(9):1038–44. https://doi.org/10.1200/JCO.2014.57.2750.
Acknowledgements
We would like to acknowledge the HFGCCRI Community Research Advisory Board (CRAB) for consulting with us on the design of our TNBC research projects.
Funding
This project was supported by NIGMS (P20 GM103446) from the NIH and the State of Delaware (to S.D. Siegel).
Author information
Authors and Affiliations
Contributions
SDS, FCC, MMB, JS-M, and ZTS contributed to the conception of the study. SDS, FCC, and MMB contributed to the design. JS-M coordinated the abstraction/acquisition of data. MMB conducted data management, statistical analyses, and visualizations. FCC oversaw the statistical analysis. SDS and MMB drafted the manuscript. SML conducted a review of the manuscript, including making critical revisions for conceptual content and methods. All authors reviewed, revised, and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was reviewed and approved by the Christiana Care Health System Institutional Review Board (CCC# 35082), which provided a waiver of informed consent (45 CFR 46.116), and was conducted in accordance with the US Common Rule.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Tables S1
. Variance of census tract random effect for breast cancer subtype (triple negative breast cancer vs. not), before and after covariate adjustment. Table S2. Comparison of fixed- and mixed-effects models for odds of triple negative breast cancer by age and census tract race-, income-, and race/income ICE. Table S3. Odds of triple negative breast cancer by race and census tract ICE interactions. Table S4. Census tract characteristics by TNBC prevalence and income-ICE quintiles. Table S5. Census tract characteristics by TNBC prevalence and ICE-Race/Income quintiles.
Additional file 2: Figure S1
. Retail exposures and comorbidity prevalence by ICE quintiles, New Castle County, DE. Shows place-based systems of exposure related to metabolic risk factors for triple negative breast cancer (TNBC) by census tract ICE quintiles in New Castle County, DE. Alcohol retailers (A) and alcohol use disorder prevalence (C) show a graded relationship with ICE, with both measures highest in tracts classified as Q1 (greatest ICE-measured disadvantage). Fast-food retailers (B) are most prevalent in Q3–Q4 ICE tracts, while obesity prevalence (D) varies little by ICE. All measures show similar variation by race-, income-, and race/income-ICE.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Siegel, S.D., Brooks, M.M., Lynch, S.M. et al. Racial disparities in triple negative breast cancer: toward a causal architecture approach. Breast Cancer Res 24, 37 (2022). https://doi.org/10.1186/s13058-022-01533-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13058-022-01533-z