Abstract
Background
Non-ventilator-associated ICU-acquired pneumonia (NV-ICU-AP), a nosocomial pneumonia that is not related to invasive mechanical ventilation (IMV), has been less studied than ventilator-associated pneumonia, and never in the context of patients in an ICU for severe acute exacerbation of chronic obstructive pulmonary disease (AECOPD), a common cause of ICU admission. This study aimed to determine the factors associated with NV-ICU-AP occurrence and assess the association between NV-ICU-AP and the outcomes of these patients.
Methods
Data were extracted from the French ICU database, OutcomeRea™. Using survival analyses with competing risk management, we sought the factors associated with the occurrence of NV-ICU-AP. Then we assessed the association between NV-ICU-AP and mortality, intubation rates, and length of stay in the ICU.
Results
Of the 844 COPD exacerbations managed in ICUs without immediate IMV, NV-ICU-AP occurred in 42 patients (5%) with an incidence density of 10.8 per 1,000 patient-days. In multivariate analysis, prescription of antibiotics at ICU admission (sHR, 0.45 [0.23; 0.86], p = 0.02) and no decrease in consciousness (sHR, 0.35 [0.16; 0.76]; p < 0.01) were associated with a lower risk of NV-ICU-AP. After adjusting for confounders, NV-ICU-AP was associated with increased 28-day mortality (HR = 3.03 [1.36; 6.73]; p < 0.01), an increased risk of intubation (csHR, 5.00 [2.54; 9.85]; p < 0.01) and with a 10-day increase in ICU length of stay (p < 0.01).
Conclusion
We found that NV-ICU-AP incidence reached 10.8/1000 patient-days and was associated with increased risks of intubation, 28-day mortality, and longer stay for patients admitted with AECOPD.
Similar content being viewed by others
Introduction
Severe acute exacerbation of COPD (AECOPD) is a common cause of ICU admission [1] and nosocomial pneumonia is the most frequently reported nosocomial infection in intensive care units (ICUs) [2, 3]. While ventilator-associated pneumonia (VAP) acquired in ICUs has been widely studied [4], there are less consistent data on nosocomial ICU-acquired pneumonia in patients without invasive mechanical ventilation (IMV) (Non-ventilator-associated ICU-acquired pneumonia (NV-ICU-AP)) [5,6,7,8,9].
Non-ventilator hospital-acquired pneumonia (NV-HAP) in or outside the ICU, is associated with a similar or higher risk of mortality than VAP, significant morbidity, and high associated costs [8,9,10,11,12,13,14], but NV-HAPs are currently less tracked, reported, and prevented than VAPs [15, 16]. Nevertheless, a growing interest in NV-ICU-APs has recently been observed, and as remarked by Vallecoccia et al., nosocomial pneumonia is a multifaced disease with NV-ICU-AP being one of them [7,8,9, 16, 17]. As pointed out by Bergin et al., evaluating the risks of NV-ICU-AP and its outcomes is essential to identify those patients at the highest risk of and from NV-ICU-AP. In addition, studies are needed on diagnostic criteria, new treatments, and prevention strategies focused on the patients who are most likely to benefit [16].
Studies of hospitalised patients suggest that the prevalence of NV-HAP is high in patients with COPD [18,19,20,21]. However, NV-ICU-APs have been poorly studied in COPD patients [22, 23] and never in patients admitted to an ICU for severe AECOPD.
NV-ICU-AP is a major concern in patients with COPD exacerbation because of its potentially adverse impact in terms of medical and/or economic burden; particularly in the context of reducing the need for invasive mechanical ventilation and switching to management using non-invasive ventilation (NIV) [24].
The aim of this study was to investigate, using the prospective French OutcomeRea™ database, the factors associated with the occurrence of NV-ICU-AP in ICU patients with severe AECOPD and the association between NV-ICU-AP and the outcomes of these patients.
METHODS
Study population
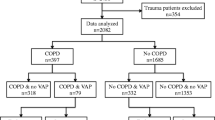
All adults admitted to one of the 32 ICUs participating in the prospective national OutcomeRea™ database between 1st January, 1997 and 31st December, 2018, with a diagnosis of COPD exacerbation or acute respiratory failure with a medical history of COPD were included in the analysis. The exclusion criteria are summarised in Fig. 1.
Population Flowchart. AECOPD Acute exacerbation of chronic obstructive pulmonary decease; COPD chronic obstructive pulmonary disease, OUTCOMEREA database multicenter longitudinal database fuelled by ICUs contributing to the OUTCOMEREA Network, ICU intensive care unit, NV-Hospital-AP non-ventilator hospital-acquired pneumonia, NV-ICU-AP non-ventilator-associated intensive care unit acquired pneumonia
Ethics
The French Advisory Committee on Data Processing in Health Research, the French Commission on Informatics and Liberty, and the Ethics Committee of the University of Clermont-Ferrand, France, (IRB No. 5891) approved this data collection (ref. 2007–16). Patients were informed of the inclusion of their de-identified data in the database and could oppose this if they so wished.
OutcomeRea™ database
OutcomeRea™ is an ongoing, prospective, observational, collaborative, and multicentre database. All codes and definitions used to describe diseases, comorbidities, and outcomes were established before the study began and have been described previously [25]. Clinical and outcome data, treatments, and prescribed medications are prospectively entered into the database daily for a random sample of patients admitted to 32 French ICUs. Of these 32 participating ICUs (including 18 university hospitals), 16 were polyvalent or surgical ICUs and 16 were primarily medical ICUs.
Methods and measurements
Patient characteristics, prescriptions, and use and type of ventilatory support were extracted from the database. The diagnosis of “very severe COPD” was defined as previous long-term treatment with home oxygen therapy, home NIV, or documented forced expiratory volume in 1 s < 30% predicted value (GOLD classification, stage 4) [1]. Patients who did not meet these criteria, with limited follow-up data, without recent spirometry or without available data were classified as having “not very severe COPD or unknown COPD severity.”
NV-ICU-AP definition
The risk of NV-ICU-AP was considered from the end of the first 48 h in ICU (without IMV) until ICU discharge, death, or need for at least 48 h of IMV later during their ICU stay. The period considered as risk of VAP ranged from 48 h after intubation to weaning from the invasive ventilation. Pulmonary infection was suspected in patients with a new or persistent infiltrate on the chest radiograph that was associated with any of the following criteria: (1) purulent tracheal secretions, (2) fever ≥ 38.5 °C or hypothermia ≤ 36.5 °C, and (3) leucocytosis > 109 G/L or leucopenia < 4.108 G/L. The diagnosis of NV-ICU-AP was confirmed by bacteriological tests or by a senior investigator in cases of strong clinical suspicion and impossibility of sampling /and or bacteriological tests. The diagnosis of VAP was confirmed by bacteriological tests.
The bacteriological samples considered were a sputum culture (threshold, > 105 cfu/mL of a good-quality sample), bronchoalveolar lavage fluid (threshold, ≥ 104 cfu/mL), plugged telescopic catheter (threshold, ≥ 103 cfu/mL), or a quantitative endotracheal aspirate (threshold, ≥ 105 cfu/mL), received within the first 24 h after suspicion of NV-ICU-AP or VAP. When bacteriological examinations yielded only coagulase-negative staphylococci or Enterococcus species, a nosocomial pneumonia diagnosis was confirmed only after checking the patient’s data by a senior investigator.
Definitions
Appropriate antibiotic therapy (AT) required a positive culture result of bacteriological tests, received within the first 24 h after suspicion of NV-ICU-AP. The time to administration of appropriate AT was the number of days before the patient received appropriate AT (in case of inadequate AT or delay to AT prescription) after suspicion of NV-ICU-AP. The presence of multidrug-resistant (MDR) bacteria required documented evidence of at least one of the four classes of MDR: methicillin-resistant Staphylococcus aureus, extended-spectrum β-lactamase–producing Enterobacteriaceae, AmpC-producing Enterobacteriaceae, and Pseudomonas aeruginosa resistant to ticarcillin and/or imipenem and/or ceftazidime in the bacteriological samples.
Statistical analysis
Categorical parameters are expressed as numbers and percentages, and continuous parameters are given as median and interquartile range [IQR]. For missing data concerning body mass index (BMI), multiple imputations were performed using a fully conditional specification method with linear regression and twenty imputed datasets were created to consider this variable with less than 20% of missing values [26]. Factors associated with the occurrence of NV-ICU-AP were identified using the Fine and Gray competing risk model [27], with 2 days of IMV, discharge from the ICU, and death as competing risks. An adjusted analysis was performed including variables collected at ICU admission, with p < 0.2 in the univariate analysis and considered potentially collinear. The association between NV-ICU-AP and survival on Day-28 was assessed by a Cox model with NV-ICU-AP as a time-dependent variable. To assess the association between NV-ICU-AP and the length of stay in the ICU (using the daily instantaneous risk of alive discharge from the ICU), and association between NV-ICU-AP and intubation, we used a cause-specific regression model controlling for the competing risks of death or of discharge from the ICU with NV-ICU-AP as a time-dependent variable. The association between inadequate antibiotic treatment, time to appropriate antibiotic treatment, and documented presence of MDR bacteria on mortality and intubation, were assessed using the same models. For all the multivariate regression models, variables with p < 0.2 in the univariate models were introduced using a stepwise selection method. In cases of multiple episodes of NV-ICU-AP, only the first episode was considered. All models were stratified by centre, and a 2-sided alpha threshold of 0.05 was considered statistically significant. Statistical analyses were performed using SAS 9.4 software (SAS Institute, Cary, NC, USA).
Results
Study flow
Among the 23,249 patients registered in the OutcomeRea™ database between 1st January 1997 and 31st December 2018, 1,816 patients had a diagnosis of acute exacerbation of COPD or acute respiratory failure with a medical history of COPD. Of these 844 were not initially given IMV and were thus were exposed to the risk of NV-ICU-AP. Fifty episodes of NV-ICU-APs occurred in 42 patients (six patients presented two episodes of NV-ICU-AP, and one patient had 3 episodes) with an incidence density of 10.8 per 1,000 patients-days exposed to the risk of NV-ICU-AP (Figs. 1 and 2). The first episode of NV-ICU-AP occurred after a median delay of 6 [4; 11] days after ICU admission, and 32 patients with NV-ICU-AP (76.2%) were intubated within 48 h after diagnosis of NV-ICU-AP (Table 1). The baseline characteristics and outcomes of patients are presented in Table 2. The main microorganisms found were S. aureus, P. aeruginosa, and Escherichia Coli. Six (14.29%) NV-ICU-AP cases were diagnosed without bacteriological documentation (Table 1). MDR bacteria were found in 13 of 36 patients (36%) with a first episode of NV-ICU-AP (with bacteriological documentation) and mainly concerned S. aureus and P. aeruginosa (Table 1). 219 patients were intubated and 59 episodes of VAP occurred in 39 patients with an incidence density of 23.1 per 1,000 patients-days at risk of VAP (Fig. 2). The first episode of VAP occurred after a median delay of 11 [8; 14] days after ICU admission and after 9 [5; 11] days of invasive mechanical ventilation. Baseline characteristics and outcomes of patients according to the occurrence of VAP are shown in Additional file 1: Tables S1 and S2.
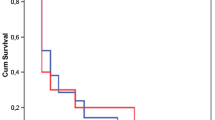
Cumulative incidences of non-ventilator-associated ICU-acquired pneumonia and ventilator-associated pneumonia in patients admitted in ICU for a severe acute exacerbation of COPD. A Cumulative incidence of non-ventilator-associated ICU-acquired pneumonia from ICU admission in patients admitted to an ICU for a severe acute exacerbation of COPD. B Cumulative incidence of ventilator-associated pneumonia from intubation in ICU for a severe acute exacerbation of COPD (n = 219). ICU intensive care unit, NV-ICU-AP non-ventilator-associated intensive care unit acquired pneumonia, VAP ventilator-associated pneumonia
Factors associated with NV-ICU-AP occurrence
In the adjusted analysis, the prescription of empirical antibiotic therapy at ICU admission (Sub-Distribution Hazard Ratio (sdHR) = 0.45 [0.23; 0.86], p = 0.02) and no decrease in consciousness (Glasgow Coma Scale = 15) during the two first days in the ICU (sdHR = 0.35 [0.16; 0.76], p < 0.01) were associated with a lower risk of NV-ICU-AP. Age, sex, severity of COPD, corticosteroid therapy, or non-invasive ventilation (NIV) at admission were not associated with an increased risk of NV-ICU-AP (Additional file 1: Table S3). Data and outcomes by the level of consciousness are summarised in the Additional file 1: Table S4.
NV-ICU-AP and mortality, intubation, and length of stay in ICU.
At Day 28, 40 patients had undergone at least one episode of NV-ICU-AP (two patients had NV-ICU-AP after D28). For the survival analysis, we excluded 66 patients with limitation of therapeutic effort (LTE) at admission to the ICU, among whom only three developed NV-ICU-AP at before D28. NV-ICU-AP was an independent risk factor for mortality after adjustment for other risk factors for in-hospital death (Hazard Ratio (HR) = 3.03 [1.36; 6.73], p < 0.01) (Additional file 1: Table S5). The negative effect of NV-ICU-AP on mortality was even greater if the patient was intubated shortly after the diagnosis of NV-ICU-AP (for 48 h maximum) (HR = 4.23 [1.88; 9.55], p < 0.01).
The occurrence of NV-ICU-AP was strongly associated with an increased risk of intubation (Cause-specific hazard ratios (csHR) = 4.98 [2.53; 9.80], p < 0.01) for 48 h maximum after the diagnosis of NV-ICU-AP (Additional file 1: Tables S6 and S7), and with an increase in the length of stay in the ICU of 9.8 [8.2; 16.3] days (p < 0.01) (Additional file 1: Tables S8 and S9).
Antibiotic treatment
Among the 36 patients with a microbiologically documented first occurrence of NV-ICU-AP, 20 patients received appropriate antibiotic therapy within the first 24 h after developing NV-ICU-AP. For the 16 patients with a delay in receiving appropriate AT, the median delay was 2 [1.0; 3.0] days. After adjustment, inadequate AT, time to appropriate AT, and the implication of an MDR bacterium in the pneumonia was not associated with survival or intubation (Additional file 1: Tables S10 to S14).
Discussion
To our knowledge, this is the first study addressing the impact of NV-ICU-AP on the outcomes of patients with COPD admitted to an ICU for AECOPD. Prescription of empirical antibiotic therapy at ICU admission and a good level of consciousness were associated with a lower risk of NV-ICU-AP. In this specific population, NV-ICU-AP is an independent risk factor for 28-day mortality, intubation, and increased ICU length of stay. The early prescription of antibiotics, close to ICU admission, and a good level of consciousness were associated with a lower risk of NV-ICU-AP.
In our observational study, the incidence density (10.8 per 1,000 patient-days) and incidence (5%) of NV-ICU-AP are higher than those reported in the literature (0.6 to 4.5 per 1000 patient-days and 1.6–2.5%, respectively [5, 8, 13, 14]) in unselected ICU populations. They are closer to the incidence of nosocomial pneumonia of 3.1% (corresponding to 4.5 per 1000 NIV-days) in ICU patients treated by NIV [23]. This higher incidence of NV-ICU-AP in COPD patients is consistent with the results of studies of NV-HAP outside of ICUs, where chronic bronchitis/emphysema was a risk factor for NV-HAP [8, 18,19,20,21].
Other risk factors for NV-HAP identified in previous studies were male sex, older age, comorbidities, immunosuppression [6, 8, 17, 19], and ICU admission [17, 19]. In our population, we found the risk of NV-ICU-AP decreased with better levels of consciousness (as rated by the Glasgow score). Diminished consciousness has already been identified as a risk factor for HAP outside of ICUs, owing to a reduced ability to protect the airways, thus increasing the risk of aspiration of pathogens [8, 28, 29]. NV-ICU-AP was less frequent in patients who started a course of antibiotics (empirical then appropriate) upon ICU admission. However, our data alone are not sufficient to promote systematic antibiotics, especially until the effect of this antibiotic therapy on the acquisition of MDROs in this setting has not been evaluated. The patients with very severe COPD showed a trend towards to a protective effect on the occurrence of NV-ICU-AP. Therefore, the ICU practitioners need to carefully monitor the occurrence of NV-ICU-AP, also in patients with less severe COPD. However, an important limitation of this result is the presence of a group of patients with unknown COPD severity. In fact, some patients had limited follow-up data or no recent spirometry.
The hospital mortality of patients with NV-ICU-AP reported in the literature is 22 to 36%, increasing to 48% when NV-ICU-AP results in intubation, and is significantly higher than the mortality of patients without NV-ICU-AP [5, 13, 14, 22]. The in-hospital mortality of patients in our population who developed NV-ICU-AP was 43%, higher than in previous studies of unselected ICU populations. Nevertheless, it remained lower than the in-hospital mortality rate of 75% reported by Zhang et al. [23] in patients with NV-ICU-PA treated with NIV; most of whom suffered from COPD. We found the occurrence of NV-ICU-AP to be independently associated with an increased risk of death up to D 28 (HR = 3.03 [1.36; 6.73]), which was higher than in the general ICU population (HR 30-day mortality = 1.82 [1.35; 2.45] according to Saied et al. [14]). We found an even higher risk in cases requiring intubation after the diagnosis of NV-ICU-AP (HR = 4.23 [1.88; 9.55]), and Vallecoccia et al. reported that HAs requiring mechanical ventilation had the greatest 28-day mortality rate among all types of nosocomial pneumonia, including VAPs [9]. Therefore, the need for intubation should alert the ICU teams to the high severity and the poor prognosis of the patients with NV-ICU-AP.
We found that 76% of patients with NV-ICU-AP were intubated shortly after NV-ICU-AP diagnosis and that NV-ICU-AP was associated with an independent risk of intubation (csHR, 4.98 [2.53; 9.80]), similar to the intubation rate (63 to 75%) [16, 23] and risk of intubation in patients in the ICU on NIV or oxygen therapy (OR: 6.74; 95% CI: 2.24–20.28) [23, 30].
Given these high rates of intubation and mortality, we investigated the possibility that delayed intubation was responsible for these poor outcomes. Only 4 patients had criteria for the use of invasive mechanical ventilation the day before intubation, and they were alive at ICU discharge (Additional file 1: Table S15). Therefore, a possible delay in intubation cannot explain the poor outcome of the patients in our study.
In accordance with the recommendations of the Surviving Sepsis Campaign [31] and the European treatment guidelines for HAPs and VAPs [3], patients with pneumonia suspected as being due to bacterial infection should be given empirical antibiotic treatment as early as possible, and switched to the appropriate antibiotic once the results of bacteriological tests are known. We found no association between outcomes and inappropriate antibiotic therapy in patients with NV-ICU-AP. This finding should be interpreted with great caution given the small number of events in our study. Several observational studies and a meta-analysis have examined the effect of inappropriate initial antibiotic therapy in cases of nosocomial pneumonia in the ICU, mainly VAP. Inappropriate initial antibiotic therapy has been associated with worse outcomes in terms of duration of mechanical ventilation, lengths of stay, and mortality [32,33,34,35].
To summarise the specificities of patients admitted to an ICU with AECOPD, (compared to the few studies of NV-ICU-AP among the general ICU population [5, 8, 14, 16]), we note the higher incidence of NV-ICU-AP with a strong association with survival, intubation, and long length of ICU stay. The high incidence of NV-ICU-AP among patients admitted with AECOPD was not explained by the use of NIV on ICU admission (Additional file 1: Table S3), although it has been suggested that NIV could promote the risk of pneumonia due to oesophageal or stomach distension [36]. COPD has previously been described as a risk factor for HAP outside of ICUs [18] and this could be explained by mucus production in patients with chronic bronchitis, the presence of pathogenic bacteria in the airways, increased inflammation, and the host’s immune response. These hypotheses might also be proposed for ICU patients.
Consistent with our results, Zhang et al. [23] and Rinaudo et al. [22] reported that 90-day mortality rates of patients with vs. without COPD who developed NV-ICU-AP tended to be significantly to be different (22/41 [54%] vs. 29/82 [36%], respectively; p = 0.06); COPD patients seem to be a population at risk of poor outcomes in case of NV-ICU-AP.
Patients in our study were frequently intubated in the event of NV-ICU-AP, had a long duration of ICU stay (25 days) and 75% mortality. The length of stay compares to 14–20 days for Esperatti et al. [5] and Saied et al. [14], 11 days for Zhang et al. [23]. This long duration of ICU stay in our population of COPD patients could be explained by the fact that COPD is associated with an increase in the length of ICU stay and the duration of invasive ventilation [37], with a longer weaning period from invasive ventilation [38].
Strengths of our study are that we used the well-established high-quality OutcomeRea™ database, and statistical analyses were controlled for competing risks. There were several limitations. It was possible for clinicians to declare NV-ICU-AP without a bacteriological diagnosis (recorded in the database) in cases of strong clinical suspicion and impossibility of bacteriological sampling. Unsurprisingly, antibiotic therapy at ICU admission was a protective factor against the occurrence of NV-ICU-AP, but we did not have data on previous antibiotic treatments received at home or outside the ICU during the weeks preceding ICU admission. Such data could help us understand the bacterial environment of patients and influence the risk of NV-ICU-AP, as has been previously demonstrated [16], and may be crucial when multidrug-resistant organisms (MDRO) are implicated [9]. The European guidelines for nosocomial pneumonia [3] include the following risk factors for MDRO infection: a hospital environment with high levels of MDROs, prior use of antibiotics, recent hospitalisation (> 5 days), and prior infection by an MDRO.
A major concern at ICU admission must be the better identification of patients at high risk of developing NV-ICU-AP, as patients with COPD seem to be. Clinical trials are needed to determine the optimal preventive strategies, diagnostic tools (including fast reliable molecular diagnostic techniques) and ways to improve the management of NV-ICU-AP (including new therapeutic agents or strategies) [8, 9, 16, 39]. Indeed, insufficient knowledge of NV-ICU-AP and insufficient codified diagnostic methods in these non-ventilated patients may lead to underdiagnosis, diagnostic delay, and poor prognosis [7, 40].
Conclusion
The occurrence of NV-ICU-AP was high in this large population of patients admitted to French ICUs with severe AECOPD. Prescription of empirical antibiotic therapy on ICU admission and a good level of consciousness were associated with a lower risk of NV-ICU-AP. NV-ICU-AP was associated with an increased risk of death before day 28, intubation, and prolonged ICU stay. The prognosis of these patients could be improved by the implementation of preventive measures and greater efficiency in the diagnosis of NV-ICU-AP.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Change history
09 April 2024
A Correction to this paper has been published: https://doi.org/10.1186/s13054-024-04864-9
Abbreviations
- COPD:
-
Chronic obstructive pulmonary disease
- AECOPD:
-
Acute exacerbation of chronic obstructive pulmonary disease
- ICU:
-
Intensive care unit
- VAP:
-
Ventilator-associated pneumonia
- IMV:
-
Invasive mechanical ventilation
- NV-ICU-AP:
-
Non-ventilator-associated ICU-acquired pneumonia
- NV-HAP:
-
Non-ventilator hospital-acquired pneumonia
- HAP:
-
Hospital-acquired pneumonia
- NIV:
-
Non-invasive ventilation
- GOLD:
-
Global Initiative for Chronic Obstructive Lung Disease
- Cfu/mL:
-
Colony-forming unit/millilitre
- AT:
-
Antibiotic therapy
- MDR:
-
Multidrug-resistant
- IQR:
-
Interquartile range
- BMI:
-
Body mass index
- sdHR:
-
Sub-distribution hazard ratio
- HR:
-
Hazard Ratio
- csHR:
-
Cause-specific hazard ratio
- OR:
-
Odds ratio
- MDRO:
-
Multidrug-resistant organisms
References
Halpin DMG, Criner GJ, Papi A, Singh D, Anzueto A, Martinez FJ, et al. Global Initiative for the Diagnosis, Management, and Prevention of Chronic Obstructive Lung Disease@ The 2020 GOLD Science Committee Report on COVID-19 and Chronic Obstructive Pulmonary Disease. Am J Respir Crit Care Med. 2021;203(1):24–36.
AC Kalil ML Metersky M Klompas J Muscedere DA Sweeney LB Palmer 2016 Management of adults with hospital-acquired and ventilator-associated Pneumonia: 2016 clinical practice guidelines by the infectious diseases Society of America and the American Thoracic Society Clin Infect Dis Off Publ Infect Dis Soc Am 63 5 e61 111
A Torres MS Niederman J Chastre S Ewig P Fernandez-Vandellos H Hanberger 2017 International ERS/ESICM/ESCMID/ALAT guidelines for the management of hospital-acquired pneumonia and ventilator-associated pneumonia: Guidelines for the management of hospital-acquired pneumonia (HAP)/ventilator-associated pneumonia (VAP) of the European Respiratory Society (ERS), European Society of Intensive Care Medicine (ESICM), European Society of Clinical Microbiology and Infectious Diseases (ESCMID) and Asociación Latinoamericana del Tórax (ALAT) Eur Respir J 50 3 1700582
L Papazian M Klompas CE Luyt 2020 Ventilator-associated pneumonia in adults: a narrative review Intensive Care Med 46 5 888 906
M Esperatti M Ferrer A Theessen A Liapikou M Valencia LM Saucedo 2010 Nosocomial pneumonia in the intensive care unit acquired by mechanically ventilated versus nonventilated patients Am J Respir Crit Care Med 182 12 1533 1539
ST Micek B Chew N Hampton MH Kollef 2016 A case-control study assessing the impact of nonventilated hospital-acquired pneumonia on patient Outcomes Chest 150 5 1008 1014
W Ibn-Saied I Martin-Loeches JF Timsit 2020 What is new in non-ventilated ICU-acquired pneumonia? Intensive Care Med 46 3 488 491
Zaragoza R, Vidal-Cortés P, Aguilar G, Borges M, Diaz E, Ferrer R, et al. Update of the treatment of nosocomial pneumonia in the ICU. Crit Care. 2020; 24. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7322703/
MS Vallecoccia C Dominedò SL Cutuli I Martin-Loeches A Torres G Pascale De 2020 Is ventilated hospital-acquired pneumonia a worse entity than ventilator-associated pneumonia? Eur Respir Rev Off J Eur Respir Soc 29 157 200023
MD Zilberberg BH Nathanson LA Puzniak AF Shorr 2022 Descriptive epidemiology and outcomes of nonventilated hospital-acquired, ventilated hospital-acquired, and ventilator-associated bacterial pneumonia in the United States, 2012–2019 Crit Care Med 50 3 460 468
GH Talbot A Das S Cush A Dane M Wible R Echols 2019 Evidence-based study design for hospital-acquired bacterial pneumonia and ventilator-associated bacterial pneumonia J Infect Dis 219 10 1536 1544
D Baker B Quinn 2018 Hospital acquired pneumonia prevention initiative-2: incidence of nonventilator hospital-acquired pneumonia in the United States Am J Infect Control 46 1 2 7
A Kohlenberg F Schwab M Behnke C Geffers P Gastmeier 2010 Pneumonia associated with invasive and noninvasive ventilation: an analysis of the German nosocomial infection surveillance system database Intensive Care Med 36 6 971 978
W Ibn-Saied B Mourvillier Y Cohen S Ruckly J Reignier G Marcotte 2019 A comparison of the mortality risk associated with ventilator-acquired bacterial pneumonia and nonventilator ICU-acquired bacterial pneumonia Crit Care Med 47 3 345 352
Carey E, Blankenhorn R, Chen P, Munro S. Non-ventilator associated hospital acquired pneumonia incidence and health outcomes among U.S. veterans from 2016–2020. Am J Infect Control. 2022;50(1):116–9.
SP Bergin A Coles SB Calvert J Farley JH Powers MJ Zervos 2020 PROPHETIC Chest 158 6 2370 2380
SA Lukasewicz Ferreira C Hubner Dalmora F Anziliero KR Souza de ZP Klarmann 2022 Factors predicting non-ventilated hospital-acquired pneumonia: systematic review and meta-analysis J Hosp Infect 119 64 76
de-Miguel-Diez J, Jimenez-Garcia R, Hernandez-Barrera V, de-Miguel-Yanes JM, Carabantes-Alarcon D, Lopez-de-Andres A. Assessing the Impact of Gender and COPD on the Incidence and Mortality of Hospital-Acquired Pneumonia. A Retrospective Cohort Study Using the Spanish National Discharge Database (2016–2019). J Clin Med. 2021;10(22):5453.
PD Strassle EE Sickbert-Bennett M Klompas JL Lund PW Stewart AH Marx 2020 Incidence and risk factors of non-device-associated pneumonia in an acute-care hospital Infect Control Hosp Epidemiol 41 1 73 79
J Miguel-Diez de R Albaladejo-Vicente V Hernández-Barrera Z Ji M Lopez-Herranz R Jimenez-Garcia 2020 Hospital admissions for community-acquired, ventilator-associated and nonventilator hospital-acquired pneumonia in COPD patients in Spain (2016–2017) Eur J Intern Med 79 93 100
M Vignari 2020 Non-ventilator health care-associated pneumonia (NV-HAP): NV-HAP risk factors Am J Infect Control 48 5S A10 A13
M Rinaudo M Ferrer S Terraneo F Rosa De R Peralta L Fernández-Barat 2015 Impact of COPD in the outcome of ICU-acquired pneumonia with and without previous intubation Chest 147 6 1530 1538
Z Zhang J Duan 2015 Nosocomial pneumonia in non-invasive ventilation patients: incidence, characteristics, and outcomes J Hosp Infect 91 2 153 157
Galerneau LM, Bailly S, Terzi N, Ruckly S, Garrouste-Orgeas M, Cohen Y, et al. Management of acute exacerbations of chronic obstructive pulmonary disease in the ICU: an observational study from the OUTCOMEREA Database, 1997–2018. Crit Care Med. 2023
A Lautrette M Garrouste-Orgeas PM Bertrand D Goldgran-Toledano S Jamali V Laurent 2015 Respective impact of no escalation of treatment, withholding and withdrawal of life-sustaining treatment on ICU patients’ prognosis: a multicenter study of the Outcomerea Research Group Intensive Care Med 41 10 1763 1772
A Vesin E Azoulay S Ruckly L Vignoud K Rusinovà D Benoit 2013 Reporting and handling missing values in clinical studies in intensive care units Intensive Care Med 39 8 1396 1404
JP Fine RJ Gray 1999 A proportional hazards model for the subdistribution of a competing risk J Am Stat Assoc 94 446 496 509
M Pasquale Di S Aliberti M Mantero S Bianchini F Blasi 2016 Non-intensive care unit acquired pneumonia: a new clinical entity? Int J Mol Sci 17 3 287
N Sopena E Heras I Casas J Bechini I Guasch ML Pedro-Botet 2014 Risk factors for hospital-acquired pneumonia outside the intensive care unit: a case-control study Am J Infect Control 42 1 38 42
E Girou F Schortgen C Delclaux C Brun-Buisson F Blot Y Lefort 2000 Association of noninvasive ventilation with nosocomial infections and survival in critically ill patients JAMA 284 18 2361 2367
L Evans A Rhodes W Alhazzani M Antonelli CM Coopersmith C French 2021 Surviving sepsis campaign: international guidelines for management of sepsis and septic shock 2021 Intensive Care Med 47 11 1181 1247
H Dupont H Mentec JP Sollet G Bleichner 2001 Impact of appropriateness of initial antibiotic therapy on the outcome of ventilator-associated pneumonia Intensive Care Med 27 2 355 362
PJZ Teixeira R Seligman FT Hertz DB Cruz JMG Fachel 2007 Inadequate treatment of ventilator-associated pneumonia: risk factors and impact on outcomes J Hosp Infect 65 4 361 367
Muscedere JG, Shorr AF, Jiang X, Day A, Heyland DK, Canadian Critical Care Trials Group. The adequacy of timely empiric antibiotic therapy for ventilator-associated pneumonia: an important determinant of outcome. J Crit Care. 2012 ;27(3) :322.e7–14.
EL Kuti AA Patel CI Coleman 2008 Impact of inappropriate antibiotic therapy on mortality in patients with ventilator-associated pneumonia and blood stream infection: a meta-analysis J Crit Care 23 1 91 100
Gay PC. Complications of noninvasive ventilation in acute care. Respir Care. 2009;54(2):246–57; discussion 257–258.
GC Funk P Bauer OC Burghuber A Fazekas S Hartl H Hochrieser 2013 Prevalence and prognosis of COPD in critically ill patients between 1998 and 2008 Eur Respir J 41 4 792 799
A Demoule L Brochard M Dres L Heunks A Jubran F Laghi 2020 How to ventilate obstructive and asthmatic patients Intensive Care Med 46 12 2436 2449
A Roquilly A Torres JA Villadangos MG Netea R Dickson B Becher 2019 Pathophysiological role of respiratory dysbiosis in hospital-acquired pneumonia Lancet Respir Med 7 8 710 720
PH Wicky I Martin-Loeches JF Timsit 2022 HAP and VAP after Guidelines Semin Respir Crit Care Med 43 2 248 254
Acknowledgements
We thank all the medical and research teams members of the OutcomeReaTM Network (listed in the supplementary material).
We thank Alison Foote (an independent medical writer based in Grenoble, France) for writing assistance, technical editing, language editing, and proofreading.
Funding
SB and JLP. are supported by the French National Research Agency in the framework of the ‘Investissements d’avenir’ program (ANR-15-IDEX-02) and Grenoble Alpes University Foundation Chairs of excellence: “e-health and integrated care and trajectories medicine and MIAI artificial intelligence” This work has been partially supported by MIAI @ Grenoble Alpes (ANR-19-P3IA-0003).
Author information
Authors and Affiliations
Consortia
Contributions
LMG, SB, NT, SR, MGO, JO, VHTH, MG, SS, CD, JMF, AD, JD, CA, DGT, VL, LA, JR, JLP, MD, and JFT were helped in acquisition of clinical data and patient’s diagnosis and treatment, and final approval of the article. LMG, SB, SR, NT, JLP, and JFT were helped in designing the presented idea, interpretation of data, and drafting the article. LMG performed statistical analysis. LMG, SB, SR, and JFT were done data management. The article was written on behalf of the OUTCOMES network listed in supplementary material.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All studies were approved by the national ethics committees.
Consent for publication
Not applicable.
Competing interests
LMG is supported by Pfizer for attending meetings and/or travel. NT is supported by Pfizer for attending meetings and/or travel and non-financial supports from Gilead outside this work. The other authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised: the authors identified an error in Table 1. The results were inverted between for variable No decrease in consciousness Day 1-Day 2.
The members of the Outcomerea study group are listed in Additional file 1.
Supplementary Information
Additional file 1
. Members of the OutcomeRea Network.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Galerneau, LM., Bailly, S., Terzi, N. et al. Non-ventilator-associated ICU-acquired pneumonia (NV-ICU-AP) in patients with acute exacerbation of COPD: From the French OUTCOMEREA cohort. Crit Care 27, 359 (2023). https://doi.org/10.1186/s13054-023-04631-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13054-023-04631-2