Abstract
Purpose
A hallmark of acute respiratory distress syndrome (ARDS) is hypoxaemic respiratory failure due to pulmonary vascular hyperpermeability. The tyrosine kinase inhibitor imatinib reversed pulmonary capillary leak in preclinical studies and improved clinical outcomes in hospitalized COVID-19 patients. We investigated the effect of intravenous (IV) imatinib on pulmonary edema in COVID-19 ARDS.
Methods
This was a multicenter, randomized, double-blind, placebo-controlled trial. Invasively ventilated patients with moderate-to-severe COVID-19 ARDS were randomized to 200 mg IV imatinib or placebo twice daily for a maximum of seven days. The primary outcome was the change in extravascular lung water index (∆EVLWi) between days 1 and 4. Secondary outcomes included safety, duration of invasive ventilation, ventilator-free days (VFD) and 28-day mortality. Posthoc analyses were performed in previously identified biological subphenotypes.
Results
66 patients were randomized to imatinib (n = 33) or placebo (n = 33). There was no difference in ∆EVLWi between the groups (0.19 ml/kg, 95% CI − 3.16 to 2.77, p = 0.89). Imatinib treatment did not affect duration of invasive ventilation (p = 0.29), VFD (p = 0.29) or 28-day mortality (p = 0.79). IV imatinib was well-tolerated and appeared safe. In a subgroup of patients characterized by high IL-6, TNFR1 and SP-D levels (n = 20), imatinib significantly decreased EVLWi per treatment day (− 1.17 ml/kg, 95% CI − 1.87 to − 0.44).
Conclusions
IV imatinib did not reduce pulmonary edema or improve clinical outcomes in invasively ventilated COVID-19 patients. While this trial does not support the use of imatinib in the general COVID-19 ARDS population, imatinib reduced pulmonary edema in a subgroup of patients, underscoring the potential value of predictive enrichment in ARDS trials.
Trial registration NCT04794088, registered 11 March 2021. European Clinical Trials Database (EudraCT number: 2020-005447-23).
Similar content being viewed by others
Introduction
Coronavirus disease 2019 (COVID-19) can lead to the development of acute respiratory distress syndrome (ARDS) [1]. Diffuse inflammatory damage of the alveolocapillary membrane [2] and subsequent alveolar-capillary hyperpermeability results in protein-rich pulmonary edema, eventually causing acute hypoxaemic respiratory failure [3, 4]. Patients with COVID-19 ARDS benefit from immune modulation by dexamethasone [5], interleukin-6 (IL-6) receptor inhibitors [6], Janus kinase (JAK) inhibitors [7] and monoclonal antibodies [8, 9]. However, there is no effective treatment that directly targets increased alveolar-capillary hyperpermeability [10].
The tyrosine kinase inhibitor imatinib attenuates vascular hyperpermeability and pulmonary edema in various preclinical in vitro and animal models of vascular leak [11-14]. Imatinib protects endothelial barrier integrity under inflammatory conditions by inhibiting the tyrosine kinase Arg/Abl2. Arg/Abl2 mediates endothelial barrier disruption by increasing integrin turnover and reducing endothelial cell–matrix interaction [12, 15]. Translating these findings into the clinical setting, the randomized, placebo-controlled, double-blind CounterCOVID trial examined the efficacy and safety of oral imatinib in hospitalized COVID-19 patients requiring supplemental oxygen therapy [16]. While the primary endpoint, time to discontinuation of supplemental oxygen and mechanical ventilation for more than 48 consecutive hours, was negative, oral imatinib reduced duration of invasive ventilation and length of intensive care unit (ICU) admission at 28-days and additionally reduced mortality in 90-day follow up [16, 17].
Clinical benefits of imatinib were predominantly observed in patients admitted to the intensive care unit (ICU). In a secondary analysis of the CounterCOVID trial, patients were characterized using plasma biomarkers for hierarchical clustering [18]. Three subphenotypes were identified. Notably, only patients classified into subphenotype 3, characterized by elevated levels of inflammatory cytokines and endothelial and epithelial injury biomarkers, showed a mortality benefit from imatinib treatment. These findings suggested moderation of the beneficial effect of imatinib through biological subphenotypes [18].
In this trial, a newly developed intravenous (IV) formulation was used to bypass gastrointestinal dysfunction often observed in critically ill patients. Critically ill patients often suffer from gastrointestinal dysfunction, which includes delayed gastric emptying and intestinal edema, which may delay drug uptake and drug exposure [19, 20]. The dosing scheme was based on preclinical evidence of the optimal protective effect of oral imatinib on the endothelial barrier (plasma concentrations of 2–10 μM) [11] and pharmacokinetic studies of IV imatinib [21]. The CounterCOVID trial supported the above-mentioned dosing, showing that treatment with 400 mg/day was sufficient to obtain target plasma levels [22].
The InventCOVID trial evaluated the efficacy and safety of IV imatinib in patients receiving invasive ventilation for moderate-to-severe COVID-19 ARDS. We hypothesized that IV imatinib would stabilize the alveolocapillary barrier and thereby reduce pulmonary edema, quantified by extravascular lung water index (EVLWi), a validated measure of pulmonary edema [23-25]. To our knowledge, this is the first translational trial to directly target and measure pulmonary vascular leak in patients with ARDS.
METHODS
Study design and population
This phase IIb, randomized, double-blind, placebo-controlled clinical trial was performed in four academic and non-academic ICUs in the Netherlands between March 2021 and March 2022 (Fig. 1, Additional file 1: Figure S1). The trial protocol was approved by the Medical Ethics Committee of the Amsterdam UMC (location VUMC, IRB number NL75871.029.20, approved on 22-01-2021) and has been published [26]. Written informed consent was obtained from the patient’s legal representative. The trial was conducted according to the principles of the World Medical Association’s Declaration of Helsinki.
Patients were eligible if aged ≥ 18 years, intubated for invasive ventilation and had moderate-to-severe ARDS due to COVID-19. ARDS was classified according to Berlin criteria [27]. The patients were included in the trial as soon as possible after intubation and were excluded from participation if the anticipated start of study medication was > 48 h after the start of invasive ventilation. Other exclusion criteria included a history of severe chronic pulmonary disease, ejection fraction of < 40% and participation in another clinical trial. A complete overview of all exclusion criteria can be found in Additional file 1 (p. 2).
Randomization and blinding
Patients were randomized 1:1 to receive IV imatinib or placebo for 7 days. Randomization was done in the web-based application Castor electronic data capture using variable block sizes (4–6 patients per block) and stratification per participating center. Allocation of randomization group was only visible to the pharmacy staff preparing the treatment. The patients, clinical staff, investigators and statisticians remained blinded to randomization allocation during the entire study. Blinding was guaranteed by distribution of the study drug in amber-colored syringes and lines to conceal any color differences. The local pharmacies were responsible for the preparation of the study drug and, if necessary, for unblinding.
Study procedures
At baseline, all patients received a Pulse Contour Cardiac Output (PiCCO; Pulsion Medical Systems, Munich, Germany) catheter. PiCCO catheter placement was performed as part of a deferred consent procedure and replaced the standard care arterial line. Extravascular lung water (EVLW) measurements were performed daily for seven days or until transfer to the ward, as previously described [28, 29]. EVLW was indexed to predicted body weight.
After randomization and PiCCO measurement, the study drug was administered twice daily as a 25 ml, two-hour infusion for seven days or until ICU discharge. The study drug consisted of a 9.6 mg/mL imatinib mesylate solution (Exvastat Ltd, Cambridge, United Kingdom), corresponding to 200 mg imatinib per dose, or placebo. Clinical and ventilation parameters were recorded on days 1,2,4,7,10 and 28. Ventilation parameters were recorded once per hour, and partial oxygen pressure (PaO2) at least once per shift (i.e. every 4 h) or more frequently, if clinically indicated. Ventilation parameters were collected at the time of the lowest ratio of PaO2 to the fraction of inspired oxygen (FiO2). Blood samples were collected for plasma biomarker analyses (Additional file 1: Table S1).
Adverse events (AEs) and serious adverse events (SAEs) were recorded until day 28. Due to the high incidence of adverse events in the ICU, only prespecified events were recorded (Additional file 2, pp. 26–29). Reporting was conducted according to the Council for International Organizations of Medical Sciences (CIOMS) reporting guidelines. Safety was assessed using clinical laboratory tests and electrocardiograms (ECG) at baseline and on days 1,2,4,7 and 10. Details on predefined stopping criteria, are described in the protocol (Additional file 2, pp. 24–25). Patients discharged before day 28 were contacted by telephone on day 28 to evaluate their clinical status.
Prespecified outcomes
The primary endpoint was the change (Δ) in EVLWi between days 1 and 4. We chose this period to capture the effect of imatinib during the exudative phase of ARDS, characterized by vascular leak and alveolar flooding [3, 30, 31]. We hypothesized that this period would be the window-of-opportunity in which imatinib could best exert its vasculoprotective effect.
The following key secondary outcomes were analyzed in hierarchical order: change in PaO2/FiO2 ratio, number of ventilator-free days (VFD), length of ICU, hospital length of stay and 28-day mortality. Other explorative secondary endpoints included the duration of invasive ventilation, change in the pulmonary vascular permeability index (PVPi) and plasma biomarker concentrations (Additional file 1: Table S1), ventilation parameters, Sequential Organ Failure Assessment (SOFA) score and the 9-point World Health Organization (WHO) ordinal scale for clinical improvement (all specified in Additional file 1).
Sample size calculation
A sample size of 90 patients was determined to demonstrate a 25% reduction in EVLWi, with 80% power and a type 1 error rate of 0.05. The calculation was based on an anticipated baseline EVLWi of 17 ml/kg with a standard deviation (SD) of 7 ml/kg. This was based on previously performed EVLW measurements in patients with moderate to severe ARDS [32-34]. The expected EVLWi reduction of 25% was based on preclinical data [11].
The study protocol (Additional file 2) allowed for a sample size re-estimation in case of falling recruitment rates. Revision of the power calculation was supported by a lower variation in EVLWi than initially anticipated based on data in non-COVID-19 ARDS (i.e. SD of 4.9 instead of 7.0). Re-estimation was done by updating the assumptions using pre-randomization data from the 66 patients included at the time of re-estimation. The estimated difference between groups (µ1 – µ2) was left unchanged, as we were not able to re-estimate the treatment effect without unblinding. Based on the observed mean EVLWi at n = 66 of 16.5 ml/kg with an SD of 4.9 and assuming an alpha of 5%, 19 patients per allocated group were considered sufficient to detect a 25% reduction in EVLWi between the groups at 80% power. Therefore, recruitment was halted after 67 patients.
Statistical analysis
Statistical analyses were performed in the intention-to-treat (ITT) population of 66 randomized patients. An overview of predefined statistical tests is provided in the Statistical Analysis Plan (Additional file 3). In summary, normal distribution was tested using the Shapiro–Wilk test. Baseline imbalances were defined as a difference of ≥ 5% between the placebo and imatinib groups, and baseline imbalances deemed clinically relevant to the primary outcome by consensus were adjusted for in subsequent analyses. Categorical data were expressed as numbers and percentages, and differences between categorical variables were tested using a Chi-square test. Continuous data were expressed as mean ± SD or median with interquartile range [IQR]. Differences between continuous variables were analyzed depending on parametric or non-parametric distribution using a two-tailed t-test, or Mann–Whitney-U-test, respectively.
The primary endpoint was presented as mean ± SD and analyzed using a two-tailed t-test. In addition, an analysis of covariance (ANCOVA) was performed. For the primary endpoint, data imputation was performed in case of missing EVLWi values. In case of missing values on day 1, the data from day 2 was carried backwards. In case of missing values at day 4, the value was imputed by carrying the day 3 value forward, or, in case of no day 3 measurement, carrying the day 5 value backwards. For sensitivity analysis, the primary endpoint was analyzed in the ITT population without data imputation. In addition, a per-protocol analysis of the primary endpoint was performed in all patients missing ≤ 1 study drug dose in the first four study days and who had no missing EVLWi measurements on days 1 and 4.
28-day mortality was analyzed using a Cox proportional hazards model and visualized using a Kaplan–Meier curve. EVLWi, PVPi, ventilation parameters, plasma biomarkers and clinical laboratory outcomes over the first seven days were analyzed using linear mixed effect (LME) models, with time point, randomization group and their interaction term as fixed effects, study ID as random intercept and, in case of baseline imbalances, relevant covariates. For LME analyses, logarithmic transformation was applied to non-normally distributed data.
We performed a posthoc analysis applying the three biomarker-derived subphenotypes identified in the CounterCOVID trial [18]. Using the nnet package [35], a multinomial logistic regression model was trained using data from the CounterCOVID trial with plasma levels of interleukin (IL)-6, tumor necrosis factor receptor 1 (TNFR1), surfactant protein (SP)-D and angiopoietin (Ang)-2 to Ang-1 ratio measured at baseline as predictors of cluster allocation. Based on pre-treatment biomarker data obtained from the InventCOVID patients and using the predict() function in R studio [36], this model was used to classify patients into three subphenotypes: i.e. subphenotype 1 (high IL-6, high TNFR1, low SP-D), subphenotype 2 (low IL-6, low TNFR1, low SP-D) and subphenotype 3 (high IL-6, high TNFR1, high SP-D). LME modelling of ΔEVLWi per treatment day was repeated in the subphenotypes. Statistical analyses were performed using R, version 4.1.3 and RStudio, version 2022.02.1.
Results
Patient characteristics
Between March 2021 and March 2022, 434 patients admitted to the ICU were screened for trial participation. Of these, 67 patients underwent randomization. One patient withdrew consent shortly after randomization, before receiving the first dose of study medication, and was excluded from further analysis. The study medication was not administered in two patients because of a contraindication to study drug administration and transferal to a non-participating hospital before administration of the first dose. Altogether, the ITT population contained 33 patients per arm (Fig. 1).
Baseline patient characteristics were largely balanced between the two groups. Imbalances in age, BMI and d-dimer levels were deemed clinically relevant (Table 1) and adjusted for in the analysis of primary and secondary outcomes. 53 patients (80%) were classified as moderate ARDS and 13 patients (20%) as severe ARDS. All patients received dexamethasone treatment and 91% received IL-6 receptor inhibitors (Table 1). Additionally, 15 patients (23%) received monoclonal antibodies (Additional file 1: Table S2). The median duration of study treatment was 7 days [4, 7]. The most common reasons for study drug discontinuation were transferal to a non-participating hospital and discharge to the ward before study day 7 (Additional file 1: Table S3).
Primary outcome
No significant difference in mean ∆EVLWi between days 1 and 4 was found between the two groups (difference 0.19 ml/kg, 95% CI − 3.16 to 2.77, p = 0.90, Table 2, Fig. 2A). This finding was confirmed by ANCOVA using the randomization group and individual patients as covariates (p = 0.39, Table 2). Four patients (6%) did not have a day 1 EVLWi measurement and 10 patients (15%) did not have a day 4 measurement. These values could be imputed from a previous or subsequent day in 7 of the 14 patients (50%). Sensitivity analysis without imputation of missing EVLWi data comprising 52 patients (78%) and the per-protocol analysis containing 48 patients (72%) both did not reveal significant differences in ∆EVLWi (Table 2).
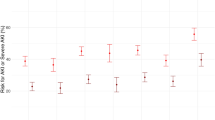
The primary endpoint and extravascular lung water index (EVLWi) over time, stratified by randomization group. Boxplot (A) depicting the distribution of the change in EVLWi in the imatinib group versus the placebo group between baseline (day 1) and day 4. Scatterplot (B) depicting the dynamic changes of the EVLWi per treatment day in the imatinib group versus the placebo group from day 1 to day 7
Secondary outcomes
The median number of VFDs was 14 days [0, 23] in the imatinib group versus 19 days [0, 24] in the placebo group (p = 0.29) (Table 3). There was no significant difference in the median duration of invasive ventilation, ICU stay and hospital stay (Table 3). The unadjusted hazard ratio for mortality was 0.89 (95% CI 0.39–2.07, p = 0.79, Fig. 3). No difference in PaO2/FiO2 ratio, EVLWi, PVPi, oxygenation index, driving pressure, compliance, mechanical power, SOFA score or 9-point WHO ordinal scale for clinical improvement was observed between groups (Table 4, Fig. 2B; Additional file 1: Figure S2, S3 and S6).
Safety
The reported AEs and SAEs are listed in Table 5. AEs and SAEs were recorded in 18 imatinib-treated patients and 17 placebo-treated patients. 16 patients (50%) in the imatinib group and 11 patients (34%) in the placebo group had at least one SAE. The most frequently reported AEs were pulmonary embolism and bacteremia. The most frequently reported SAE was respiratory failure. Respiratory failure events were reported more frequently in the imatinib group (11 versus four patients, respectively). Four cases of respiratory failure in the imatinib group occurred after study day 28 but were reported in adherence to the European Commission’s CT3 guideline. In 18 patients, the final outcome of a reported SAE was death, with 10 deaths in the imatinib group and eight deaths in the placebo group. No infusion reactions due to imatinib administrations were observed and no AEs or SAEs were attributed to imatinib treatment.
Laboratory test results over time are displayed in Table S4 and Figure S4 (Additional file 1). There was no difference in hemoglobin, liver function and kidney function between the groups. Patients receiving imatinib showed a larger increase in leucocyte count over time. No difference in the course of NT-proBNP or QTc time was observed between the groups.
Posthoc analysis
Biomarker analyses are provided in Table S5 and Figure S5 (Additional file 1). Based on the three subphenotypes identified in the CounterCOVID trial, 43 patients were classified into subphenotype 1, 20 patients into subphenotype 3 and none in the milder subphenotype 2 Three patients had no baseline plasma biomarker measurements and could not be assigned. Subphenotype characteristics and biomarker levels can be found in Table S6, S7 and S8 (Additional file 1). In the LME analysis modelling EVLWi over the 7 treatment days, a significant difference was found in patients classified into subphenotype 3 receiving imatinib (∆EVLWi per treatment day, with placebo as the reference group: − 1.17 ml/kg, 95% CI − 1.87 to − 0.44, Fig. 4B). In subphenotype 1, no significantly different change in EVLWi over time was observed between the two groups (Fig. 4A).
The change in extravascular lung water index over time, stratified by treatment group in subphenotypes 1 and 3. Scatterplots depicting the dynamic changes of the extravascular lung water index (EVLWi) per treatment day in the imatinib group versus the placebo group from day 1 to day 7 in subphenotype 1 and subphenotype 3
Discussion
Administration of IV imatinib in invasively ventilated patients with COVID-19 ARDS had no significant effect on ΔEVLWi between days 1 and 4, indicating that imatinib did not reduce pulmonary edema. Imatinib did not improve clinical outcomes such as oxygenation, duration of invasive ventilation or 28-day mortality. IV imatinib was well-tolerated, with no adverse events attributed to imatinib administration. A posthoc analysis in previously identified subphenotypes suggests that IV imatinib reduced EVLWi in a subgroup of patients characterized by high levels of IL-6, TNFR1 and SP-D.
This trial follows up on the CounterCOVID trial, which showed that oral imatinib, in hospitalized COVID-19 patients, resulted in a shorter duration of invasive ventilation and a lower 90-day mortality [16, 17]. There are several possible explanations why imatinib did not reduce pulmonary edema and did not improve clinical outcomes in the current study. The first explanation comprises the underlying heterogeneity of COVID-19 ARDS [37]. By applying previously identified subphenotypes [18], we aimed to investigate whether the biological heterogeneity found in the CounterCOVID trial may explain the absence of an effect observed in the current trial. While the sample size of the subphenotype groups is limited, subgroup analysis revealed a significant reduction in EVLWi one biological subphenotype, suggesting that predictive enrichment using ARDS severity classification was insufficient. Notably, a minority of patients were classified into this subphenotype, which may explain the overall neutral finding of the trial.
Second, a notable difference with the CounterCOVID trial was that in the current trial, virtually all patients received IL-6 receptor inhibitors before starting imatinib treatment. Although attenuation of pulmonary endothelial barrier disruption was the hypothesized working mechanism of imatinib, an immune-modulating effect was previously observed [12, 18, 38, 39]. Potentially, this beneficial effect was already achieved by concomitant treatment with immunomodulatory drugs, making it more challenging to find an additional effect of imatinib. An argument against this is that imatinib treatment did not result in a decrease in IL-6 levels, providing no direct evidence for immune modulation through IL-6 reduction. It is unlikely that a potential difference in IL-6 levels was masked by the use of IL-6 receptor blockers, as these tend to cause an increase in IL-6 concentrations [40, 41].
Third, the timing of treatment may have been too late. Imatinib may not be able to effectively reverse vascular leak and pulmonary edema once the alveolocapillary barrier has suffered excessive damage. The high baseline EVLWi and PVPi levels suggest that the peak in pulmonary edema formation likely occurred before intubation. This could explain the discrepancy with the beneficial clinical effect seen in the CounterCOVID ICU population, in whom imatinib treatment was started within 24 h after hospital admission [16]. We thus suggest that optimal start of alveolar endothelial barrier-enhancing drugs is before intubation.
Fourth, autopsy studies in COVID-19 patients have revealed extensive endothelial injury [42], the relative contribution of endothelial dysfunction to pulmonary edema development and clinical outcome may be outweighed by extensive inflammation and alveolar epithelial cell injury [43, 44]. Lastly, although the daily dosing in the current study was similar to that in the CounterCOVID trial (400 mg/day), pharmacokinetic analysis of the CounterCOVID data demonstrated that the free fraction of imatinib inversely correlates with acute-phase proteins [22]. The pharmacokinetics of imatinib may thus be perturbed in conditions of critical illness, potentially affecting the dose–response relationship in the current study population.
This was the first trial to intravenously administer imatinib and evaluate its safety. While no clear safety concerns were observed, a critical view of the data could indicate a trend towards more (serious) adverse events in the imatinib group. However, these differences were not statistically significant, and none of the AEs could be attributed to the treatment of imatinib. A larger trial is necessary to provide a definitive answer to the question of safety of IV imatinib in critically ill patients.
A strength of this trial was that by using EVLWi as a measure of pulmonary edema, we could directly translate the preclinical hypothesis of the effect of imatinib on vascular leak to measurement in the clinical setting. EVLWi is a validated measure of pulmonary edema and an independent prognostic factor in patients with ARDS [29, 45]. Moreover, COVID-19 ARDS is characterized by higher EVLWi than non-COVID ARDS [46]. Thus, despite the neutral findings of this trial, we remain confident that it was a well-chosen endpoint for a phase IIb study investigating the efficacy of a drug targeting pulmonary vascular hyperpermeability. Another strength of the study design is the collection of a comprehensive array of parameters to study vascular leak in ARDS, such as transpulmonary thermodilution, ventilation parameters and plasma biomarker data. This provides an unprecedented biological and clinical characterization of vascular leak in ARDS patients. Moreover, this trial was conducted in a period in which dexamethasone [5], IL-6 receptor inhibitors [6] and monoclonal antibodies [9] had already been implemented for the management of COVID-19, thus reflecting current pharmacotherapeutic practice.
Some limitations should be acknowledged. EVLW measurements can be affected by external and patient factors, such as ventilator settings [47] and rhythm disturbances [47], potentially causing inaccuracies. Moreover, imputation of missing EVLWi data may have introduced over-or underestimation. Attrition due to transferal to non-participating centers in the context of the pandemic lead to a relatively high rate of patients in whom the study drug had to be prematurely discontinued. Lastly, concomitant treatment with IL-6 receptor inhibitors may have affected subphenotype attribution in the posthoc analysis, as clustering was based on data from the CounterCOVID trial, which was conducted before the introduction of tocilizumab.
The study’s findings have several implications. The neutral results of this study question the efficacy of targeting endothelial barrier disruption as a strategy in ARDS. Although preventing or attenuating endothelial barrier disruption may form a suitable strategy to prevent reaching the state of ARDS, the temporal dynamics of vascular leak in ARDS are still insufficiently understood. This precludes the current implementation of imatinib for the general COVID-19 ARDS population or translation to all-cause ARDS. A second implication is that a certain subpopulation may benefit from imatinib. Further clinical and biological characterization of subphenotypes is necessary here. Finally, the trial results underscore the importance of what is taking an increasingly central role in ARDS research: the characterization of biologically distinct subphenotypes for improved predictive enrichment in trials that examine potential treatments.
In conclusion, IV imatinib did not reduce EVLWi in invasively ventilated patients with COVID-19 ARDS. The administration of IV imatinib was well-tolerated and did not result in major safety concerns. Possible explanations for the lack of observed benefit include the biological heterogeneity of COVID-19 ARDS, concomitant use of immune-modulating medication and/or timing of imatinib administration. Further characterization of the imatinib-responsive subphenotype in future studies may help identify patients who benefit from vascular barrier-enhancing drugs.
Availability of data and materials
All anonymized patient data will be available after the publication of the article. The data can be requested from the corresponding author (j.aman@amsterdamumc.nl) by other researchers when reuse conditions are met.
Abbreviations
- ARDS:
-
Acute respiratory distress syndrome
- COVID-19:
-
Coronavirus disease 2019
- EVLW:
-
Extravascular lung water
- EVLWi:
-
Extravascular lung water index
- ICU:
-
Intensive care unit
- LUS:
-
Lung ultrasound
- PiCCO:
-
Pulse contour cardiac output monitoring
- VFD:
-
Ventilator-free days
References
Botta M, Tsonas AM, Pillay J, et al. Ventilation management and clinical outcomes in invasively ventilated patients with COVID-19 (PRoVENT-COVID): a national, multicentre, observational cohort study. Lancet Respir Med. 2021;9:139–48. https://doi.org/10.1016/S2213-2600(20)30459-8.
Caramaschi S, Kapp ME, Miller SE, et al. Histopathological findings and clinicopathologic correlation in COVID-19: a systematic review. Mod Pathol. 2021;34:1614–33. https://doi.org/10.1038/s41379-021-00814-w.
Matthay MA, Ware LB, Zimmerman GA. The acute respiratory distress syndrome. J Clin Invest. 2012;122:2731–40. https://doi.org/10.1172/JCI60331.
Bos LDJ, Ware LB. Acute respiratory distress syndrome: causes, pathophysiology, and phenotypes. Lancet Lond Engl. 2022;S0140–6736(22):01485–94. https://doi.org/10.1016/S0140-6736(22)01485-4.
RECOVERY Collaborative Group, Horby P, Lim WS, et al. Dexamethasone in hospitalized patients with covid-19. N Engl J Med. 2021;384:693–704. https://doi.org/10.1056/NEJMoa2021436.
Investigators REMAP-CAP, Gordon AC, Mouncey PR, et al. Interleukin-6 receptor antagonists in critically Ill patients with covid-19. N Engl J Med. 2021;384:1491–502. https://doi.org/10.1056/NEJMoa2100433.
Kalil AC, Patterson TF, Mehta AK, et al. Baricitinib plus remdesivir for hospitalized adults with covid-19. N Engl J Med. 2021;384:795–807. https://doi.org/10.1056/NEJMoa2031994.
Gupta A, Gonzalez-Rojas Y, Juarez E, et al. Early treatment for covid-19 with SARS-CoV-2 neutralizing antibody sotrovimab. N Engl J Med. 2021;385:1941–50. https://doi.org/10.1056/NEJMoa2107934.
Weinreich DM, Sivapalasingam S, Norton T, et al. REGEN-COV antibody combination and outcomes in outpatients with covid-19. N Engl J Med. 2021;385:e81–e81. https://doi.org/10.1056/NEJMoa2108163.
Matthay MA, McAuley DF, Ware LB. Clinical trials in acute respiratory distress syndrome: challenges and opportunities. Lancet Respir Med. 2017;5:524–34. https://doi.org/10.1016/S2213-2600(17)30188-1.
Aman J, van Bezu J, Damanafshan A, et al. Effective treatment of edema and endothelial barrier dysfunction with imatinib. Circulation. 2012;126:2728–38. https://doi.org/10.1161/CIRCULATIONAHA.112.134304.
Rizzo AN, Aman J, van Nieuw Amerongen GP, Dudek SM. Targeting Abl kinases to regulate vascular leak during sepsis and acute respiratory distress syndrome. Arterioscler Thromb Vasc Biol. 2015;35:1071–9. https://doi.org/10.1161/ATVBAHA.115.305085.
Rizzo AN, Sammani S, Esquinca AE, et al. Imatinib attenuates inflammation and vascular leak in a clinically relevant two-hit model of acute lung injury. Am J Physiol Lung Cell Mol Physiol. 2015;309:L1294–304. https://doi.org/10.1152/ajplung.00031.2015.
Xin Y, Cereda M, Yehya N, et al. Imatinib alleviates lung injury and prolongs survival in ventilated rats. Am J Physiol Lung Cell Mol Physiol. 2022;322:L866–72. https://doi.org/10.1152/ajplung.00006.2022.
Amado-Azevedo J, van Stalborch A-MD, Valent ET, et al. Depletion of Arg/Abl2 improves endothelial cell adhesion and prevents vascular leak during inflammation. Angiogenesis. 2021;24:677–93. https://doi.org/10.1007/s10456-021-09781-x.
Aman J, Duijvelaar E, Botros L, et al. Imatinib in patients with severe COVID-19: a randomised, double-blind, placebo-controlled, clinical trial. Lancet Respir Med. 2021;9:957–68. https://doi.org/10.1016/S2213-2600(21)00237-X.
Duijvelaar E, Schippers JR, Smeele PJ, et al. Long-term clinical outcomes of COVID-19 patients treated with imatinib. Lancet Respir Med. 2022;10:e34–5. https://doi.org/10.1016/S2213-2600(22)00052-2.
de Brabander J, Duijvelaar E, Schippers JR, et al. Immunomodulation and endothelial barrier protection mediate the association between oral imatinib and mortality in hospitalised COVID-19 patients. Eur Respir J. 2022. https://doi.org/10.1183/13993003.00780-2022.
Reintam Blaser A, Preiser J-C, Fruhwald S, et al. Gastrointestinal dysfunction in the critically ill: a systematic scoping review and research agenda proposed by the section of metabolism, endocrinology and nutrition of the european society of intensive care medicine. Crit Care Lond Engl. 2020;24:224. https://doi.org/10.1186/s13054-020-02889-4.
Ladopoulos T, Giannaki M, Alexopoulou C, et al. Gastrointestinal dysmotility in critically ill patients. Ann Gastroenterol. 2018;31:273–81. https://doi.org/10.20524/aog.2018.0250.
Peng B, Dutreix C, Mehring G, et al. Absolute bioavailability of imatinib (Glivec) orally versus intravenous infusion. J Clin Pharmacol. 2004;44:158–62. https://doi.org/10.1177/0091270003262101.
Bartelink IH, Bet PM, Widmer N, et al. Elevated acute phase proteins affect pharmacokinetics in COVID-19 trials: lessons from the counter COVID—imatinib study. CPT Pharmacomet Syst Pharmacol. 2021;10:1497–511. https://doi.org/10.1002/psp4.12718.
Tagami T, Kushimoto S, Yamamoto Y, et al. Validation of extravascular lung water measurement by single transpulmonary thermodilution: human autopsy study. Crit Care Lond Engl. 2010;14:R162–R162. https://doi.org/10.1186/cc9250.
Venkateswaran RV, Dronavalli V, Patchell V, et al. Measurement of extravascular lung water following human brain death: implications for lung donor assessment and transplantation. Eur J Cardio Thorac Surg Off J Eur Assoc Cardio Thorac Surg. 2013;43:1227–32. https://doi.org/10.1093/ejcts/ezs657.
Katzenelson R, Perel A, Berkenstadt H, et al. Accuracy of transpulmonary thermodilution versus gravimetric measurement of extravascular lung water. Crit Care Med. 2004;32:1550–4. https://doi.org/10.1097/01.ccm.0000130995.18334.8b.
Atmowihardjo L, Schippers JR, Bartelink IH, et al. The INVENT COVID trial: a structured protocol for a randomized controlled trial investigating the efficacy and safety of intravenous imatinib mesylate (Impentri®) in subjects with acute respiratory distress syndrome induced by COVID-19. Trials. 2022;23:158. https://doi.org/10.1186/s13063-022-06055-9.
The ARDS Definition Task Force*. Acute respiratory distress syndrome: the Berlin definition. JAMA. 2012;307:2526–33. https://doi.org/10.1001/jama.2012.5669.
Monnet X, Persichini R, Ktari M, et al. Precision of the transpulmonary thermodilution measurements. Crit Care Lond Engl. 2011;15:R204. https://doi.org/10.1186/cc10421.
Jozwiak M, Silva S, Persichini R, et al. Extravascular lung water is an independent prognostic factor in patients with acute respiratory distress syndrome. Crit Care Med. 2013;41:472–80. https://doi.org/10.1097/CCM.0b013e31826ab377.
Tomashefski JFJ. Pulmonary pathology of acute respiratory distress syndrome. Clin Chest Med. 2000;21:435–66. https://doi.org/10.1016/s0272-5231(05)70158-1.
Huppert LA, Matthay MA, Ware LB. Pathogenesis of acute respiratory distress syndrome. Semin Respir Crit Care Med. 2019;40:31–9. https://doi.org/10.1055/s-0039-1683996.
Kushimoto S, Taira Y, Kitazawa Y, et al. The clinical usefulness of extravascular lung water and pulmonary vascular permeability index to diagnose and characterize pulmonary edema: a prospective multicenter study on the quantitative differential diagnostic definition for acute lung injury/acute respiratory distress syndrome. Crit Care Lond Engl. 2012;16:R232. https://doi.org/10.1186/cc11898.
Kaneko T, Kawamura Y, Maekawa T, et al. Global end-diastolic volume is an important contributor to increased extravascular lung water in patients with acute lung injury and acuterespiratory distress syndrome: a multicenter observational study. J Intensive Care. 2014;2:25–25. https://doi.org/10.1186/2052-0492-2-25.
Craig TR, Duffy MJ, Shyamsundar M, et al. A randomized clinical trial of hydroxymethylglutaryl—coenzyme a reductase inhibition for acute lung injury (The HARP Study). Am J Respir Crit Care Med. 2011;183:620–6. https://doi.org/10.1164/rccm.201003-0423OC.
Venables WN, Ripley BD. Modern Applied Statistics with S, Fourth. New York: Springer; 2002.
R Core Team. R: a language and environment for statistical computing. Vienna: R Foundation for Statistical Computing; 2022.
Sinha P, Furfaro D, Cummings MJ, et al. Latent class analysis reveals COVID-19–related acute respiratory distress syndrome subgroups with differential responses to corticosteroids. Am J Respir Crit Care Med. 2021;204:1274–85. https://doi.org/10.1164/rccm.202105-1302OC.
Stephens RS, Johnston L, Servinsky L, et al. The tyrosine kinase inhibitor imatinib prevents lung injury and death after intravenous LPS in mice. Physiol Rep. 2015;3:e12589. https://doi.org/10.14814/phy2.12589.
Letsiou E, Rizzo AN, Sammani S, et al. Differential and opposing effects of imatinib on LPS- and ventilator-induced lung injury. Am J Physiol Lung Cell Mol Physiol. 2015;308:L259-269. https://doi.org/10.1152/ajplung.00323.2014.
Shimamoto K, Ito T, Ozaki Y, et al. Serum interleukin 6 before and after therapy with tocilizumab is a principal biomarker in patients with rheumatoid arthritis. J Rheumatol. 2013;40:1074–81. https://doi.org/10.3899/jrheum.121389.
Zhang J, Hao Y, Ou W, et al. Serum interleukin-6 is an indicator for severity in 901 patients with SARS-CoV-2 infection: a cohort study. J Transl Med. 2020;18:406. https://doi.org/10.1186/s12967-020-02571-x.
Ackermann M, Verleden SE, Kuehnel M, et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in covid-19. N Engl J Med. 2020;383:120–8. https://doi.org/10.1056/NEJMoa2015432.
Leisman DE, Mehta A, Thompson BT, et al. Alveolar, endothelial, and organ injury marker dynamics in severe COVID-19. Am J Respir Crit Care Med. 2021. https://doi.org/10.1164/rccm.202106-1514OC.
Tojo K, Yamamoto N, Mihara T, et al. Distinct temporal characteristics of circulating alveolar epithelial and endothelial injury markers in ARDS with COVID-19. Crit Care. 2021;25:169. https://doi.org/10.1186/s13054-021-03596-4.
Gavelli F, Shi R, Teboul J-L, et al. Extravascular lung water levels are associated with mortality: a systematic review and meta-analysis. Crit Care Lond Engl. 2022;26:202. https://doi.org/10.1186/s13054-022-04061-6.
Shi R, Lai C, Teboul J-L, et al. COVID-19 ARDS is characterized by higher extravascular lung water than non-COVID-19 ARDS: the PiCCOVID study. Crit Care Lond Engl. 2021;25:186. https://doi.org/10.1186/s13054-021-03594-6.
Monnet X, Teboul J-L. Transpulmonary thermodilution: advantages and limits. Crit Care Lond Engl. 2017;21:147–147. https://doi.org/10.1186/s13054-017-1739-5.
Acknowledgements
We thank the research organization Simbec-Orion for their assistance with data management and pharmacovigilance, which greatly contributed to the overall quality of the trial, as well as for their comments to improve the manuscript. Furthermore, we would like to acknowledge the contribution of the members of the data safety monitoring board, Prof. Dr. H.R. Buller (Amsterdam University Medical Center, Amsterdam, The Netherlands), Prof. DR. L.B. Ware (Vanderbilt University, Nashville, Unites States), Dr. R.M. van Hest (Amsterdam University Medical Center, Amsterdam, The Netherlands). We are grateful for their efforts in reviewing the safety throughout the duration of the trial.
Funding
This project has received funding from the Innovative Medicines Initiative 2 Joint Undertaking under grant agreement number 101005142. This Joint Undertaking receives support from the European Union’s Horizon 2020 research and innovation program and EFPIA. For more information, see http://www.imi.europa.eu. The funder of this trial had no role in the trial design, data collection, data interpretation or writing of the scientific report.
Author information
Authors and Affiliations
Contributions
JA, JS, LA and LB had full access to all data and guarantee data integrity. AVN, HJB, JA, LB, LH, MS, NJ and PRT were responsible for the trial design and for creating the study protocol. Patient enrolment and data collection were done by JS, LA and ED. Data management was performed by Simbec-Orion, JS and LA. Statistical analyses, interpretation of the data and drafting of the manuscript were done by JA, JS, LA and LB. All authors revised the manuscript. JA, JS, LA and LB were responsible for the decision to submit for publication. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The medical ethics review committee of the Amsterdam UMC, location VUMC (reference number 2020.0752, approved on 21 January 2021) approved the InventCOVID trial and written informed consent for the use of clinical data, PiCCO measurements and blood samples was obtained from the patient or their legal representatives. The trial was conducted according to the principles of the World Medical Association’s Declaration of Helsinki.
Consent for publication
Not applicable.
Competing interests
JA and AVN are inventors of a patent (WO2012150857A1; 2011) covering protection against endothelial barrier dysfunction through inhibition of the tyrosine kinase abl-related gene (arg). JA reports serving as a non-compensated scientific advisor for Exvastat. All other authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1.
Supplementray figures and tables.
Additional file 2.
The study protocol.
Additional file 3.
Statistical Analysis Plan.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Atmowihardjo, L.N., Schippers, J.R., Duijvelaar, E. et al. Efficacy and safety of intravenous imatinib in COVID-19 ARDS: a randomized, double-blind, placebo-controlled clinical trial. Crit Care 27, 226 (2023). https://doi.org/10.1186/s13054-023-04516-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13054-023-04516-4