Abstract
Background
The Clinical Frailty Scale (CFS) is the most commonly used frailty measure in intensive care unit (ICU) patients. The hospital frailty risk score (HFRS) was recently proposed for the quantification of frailty. We aimed to compare the HFRS with the CFS in critically ill patients in predicting long-term survival up to one year following ICU admission.
Methods
In this retrospective multicentre cohort study from 16 public ICUs in the state of Victoria, Australia between 1st January 2017 and 30th June 2018, ICU admission episodes listed in the Australian and New Zealand Intensive Care Society Adult Patient Database registry with a documented CFS, which had been linked with the Victorian Admitted Episode Dataset and the Victorian Death Index were examined. The HFRS was calculated for each patient using the International Statistical Classification of Diseases and Related Health Problems, Tenth Revision (ICD-10) codes that represented pre-existing conditions at the time of index hospital admission. Descriptive methods, Cox proportional hazards and area under the receiver operating characteristic (AUROC) were used to investigate the association between each frailty score and long-term survival up to 1 year, after adjusting for confounders including sex and baseline severity of illness on admission to ICU (Australia New Zealand risk-of-death, ANZROD).
Results
7001 ICU patients with both frailty measures were analysed. The overall median (IQR) age was 63.7 (49.1–74.0) years; 59.5% (n = 4166) were male; the median (IQR) APACHE II score 14 (10–20). Almost half (46.7%, n = 3266) were mechanically ventilated. The hospital mortality was 9.5% (n = 642) and 1-year mortality was 14.4% (n = 1005). HFRS correlated weakly with CFS (Spearman’s rho 0.13 (95% CI 0.10–0.15) and had a poor agreement (kappa = 0.12, 95% CI 0.10–0.15). Both frailty measures predicted 1-year survival after adjusting for confounders, CFS (HR 1.26, 95% CI 1.21–1.31) and HFRS (HR 1.08, 95% CI 1.02–1.15). The CFS had better discrimination of 1-year mortality than HFRS (AUROC 0.66 vs 0.63 p < 0.0001).
Conclusion
Both HFRS and CFS independently predicted up to 1-year survival following an ICU admission with moderate discrimination. The CFS was a better predictor of 1-year survival than the HFRS.
Similar content being viewed by others
Background
Clinical frailty describes a state of decline in physical, physiologic and cognitive reserve [1]. Frailty increases with age and is characterised by poor mobility, weakness, reduced muscle mass, poor nutritional status and diminished cognitive function.1 Frailty has been associated with falls, prolonged hospitalisation, and delayed recovery from illness and surgery. Frail individuals require more support with activities of daily living and are more susceptible to adverse events and death when compared to age-stratified non-frail individuals [2,3]. Frailty, across the adult age spectrum, is common in patients that are admitted to intensive care units (ICU) [2,4]. Patients with frailty generally have poorer hospital and 1-year outcomes, poorer quality of life, disability and functional dependence [[[[[2,3,4,5,6]]]]].
In ICU, frailty has been most commonly assessed [3,7,8] using the clinical frailty scale (CFS) [9], and the frailty index [10]. The ease of use, with clinical descriptors and pictographs, has made the CFS the most commonly used frailty measure [9]. Despite having some limitations, including subjectivity in assessment and using a judgement-based score, the CFS is validated to stratify older adults according to the level of vulnerability [9] and reliably predict poor short and longer-term outcomes in critically ill patients [4,5,11,12].
Hospital frailty risk score (HFRS) is a novel administrative frailty measure to identify patients at risk of frailty [13]. This is a validated tool to assess frailty in hospitalised patients [14]. HFRS is based on the International Statistical Classification of Diseases and Related Health Problems, Tenth Revision (ICD-10) coding system obtained from the Australian-refined Diagnosis-related groups (AR-DRG). The resulting score is categorised as low-risk (< 5), intermediate-risk (5–15) and high-risk (> 15). Patients with intermediate-risk and high-risk categories were classified as being frail [13]. However, there is conflicting evidence when it comes to the validity in ICU patients, especially in older patients [15,16,17].
A frailty tool that can predict long-term survival and other clinically relevant outcomes is more likely to be valid in assessing at-risk patients with frailty. The primary aim of the study was to compare the HFRS with the CFS in critically ill patients in predicting long-term survival up to 1 year following ICU admission.
Methods
Ethics approval
This study was approved by The Research Governance of Peninsula Health Ethics Committee (reference number HREC/47502/PH-2018, DHHS/RQ907) with a waiver of informed consent.
Study design, setting and patients
We conducted a retrospective multicentre observational study from 1st April 2017 to 30th June 2018 including consecutive critically ill patients admitted to 16 public ICUs in the state of Victoria, Australia with a clinical documented CFS score. The censor date for survival follow-up was 31st July 2018 to ensure that there was at least one-month follow up for all patients. We only included the first hospital admission during the study period.
Data sources and measurement
Australia and New Zealand Intensive Care Society (ANZICS) Adult Patient Database (APD) This bi-national clinical quality registry dataset collects de-identified information on all admissions to contributing adult ICUs in Australia and New Zealand. Trained staff working in each ICU collect this data. All ICUs within public hospitals in Victoria contributed throughout the study period. Apart from each patient’s demographic details, the data also captured their diagnostic, biochemical, physiological, and chronic health parameters from the first 24 h of ICU admission as required to calculate illness severity scores. The definitions are described in the ANZICS-APD data dictionary [18].
Victorian Admitted Episodes Dataset (VAED) All Victorian public hospitals submit data to the Victorian Department of Health and Human Services. This administrative dataset contains the ICD-10 coded diagnostic information, demographic data, and outcomes for all hospitalisations.
Victorian Death Index (VDI) This administrative dataset records the date and cause of all deaths that occur in Victoria, based on the issued death certificates. Any deaths that occur outside Victoria are not included in the registry. Information was available up to 31st July 2018.
Probabilistic methods were used to match de-identified ICU admission episodes listed in the ANZICS registry to their equivalent administrative data by the Centre for Victorian Data Linkage.
Definitions of Frailty
The CFS is voluntarily collected as part of the ANZICS-APD at the time of ICU admission, based on the patient’s level of physical function in the two months before ICU admission by sixteen out of twenty-three hospitals. The CFS (range 1–8) categorises patients as non-frail (1 = very fit; 2 = well; 3 = managing well; 4 = vulnerable) or frail (5 = mildly frail; 6 = moderately frail; 7 = severely frail; 8 = very severely frail). Patients with a score ≥ 5 were considered frail. CFS was assigned by data collectors in participating ICUs based on the patient’s level of physical function in the two months preceding ICU admission [18].
The HFRS (range 0 to 45) was estimated using routine data based on the ICD-10 coding system obtained from the AR-DRG [13,19]. ICD-10 codes used to estimate HFRS, and the respective points awarded for each diagnosis are summarised in Additional file 1: Table S1. For this study, we categorised patients with HFRS score > 5 as frail and those with score < 5 as non-frail [13]. We only used the ICD-10 codes that represented pre-existing conditions at the time of index hospital admission, rather than those that were developed during the hospitalisation. A complete list of ICD-10 variables extracted from the VAED database is listed in Additional file 1: Table S2. VDI database is summarised in Additional file 1: Table S3. The patients were categorised as non-frail and frail for both CFS and HFRS.
Study aims and outcomes
The primary aim was to assess the use of HFRS as a frailty screening tool in ICU patients by comparing its performance with the CFS as a predictor of one-year survival following ICU admission. The secondary aims were to compare the performance of the CFS and the HFRS as predictors of ICU, hospital, 28-day, 90-day, 6-month and 1-year mortality. The pre-defined subgroup analyses included prediction of 1-year mortality for patients ≥ 75 years of age and those needing mechanical ventilation.
Statistical analysis
Categorical comparisons between frail and non-frail patients were performed using Chi-square, two-sample t-tests for normally distributed data and Wilcoxon rank-sum test otherwise, with results reported as counts (%), means [standard deviation (SD)] or median (interquartile range [IQR]), respectively. Correlation between the continuous CFS and HFRS was assessed using Spearman correlation coefficient and agreement using Kappa for dichotomous (not frail: CFS 1–4, HFRS 0–5; frail: CFS ≥ 5, HFRS > 5). Patient survival was compared using Cox proportional hazards regression adjusting for patient’s illness severity, and sex, with results reported as hazard ratios (HR, 95% CI). Time-dependent covariate analysis and log-minus-log plots were performed to assess proportionality assumptions. While HR were reported as the risk associated with a 1-unit increment for the CFS (range 1–8), to facilitate a more proportional comparison between the 2 frailty tools, HR for HFRS were reported as the risk associated with a 5-unit increase (range 0–45). The dichotomised Kaplan–Meier survival curves were performed for the two frailty measures. The performance of the CFS and the HFRS in predicting time-specific mortality rates was determined using logistic regression models with results reported as the area under the receiver operating characteristic (AUROC) plots with comparison using chi-square tests [20]. Multivariable logistic regression analysis was used to compare the performance of the CFS and the HFRS as predictors of ICU, hospital, 28-day, 90-day, 6-month and 1-year mortality. Illness severity was determined using the Australian and New Zealand Risk of death (ANZROD) which is a highly predictive mortality prediction model used for benchmarking ICU performance in Australia and New Zealand. ANZROD includes components of the APACHE III/IV scoring system, such as age, chronic illnesses, acute physiological disturbance and diagnosis, and the presence of treatment limitation on admission to ICU and provides an accurate estimate of the severity of illness in the first 24 h of ICU admission [21,22]. Hence, adjusting for sex was required as a separate variable, and not age. Additional sensitivity analyses were performed based on elective and non-elective admissions and those who were discharged home alive. The HFRS was quantified with the ICD-10 codes from the linked dataset using R software, version 3.5.0 (The R Foundation). The data analysis was performed using SPSS Version 27 (IBM). A two-sided p value of 0.05 was used to indicate statistical significance.
Results
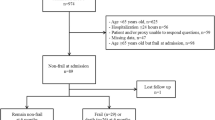
During the study period, there were a total of 20,457 hospitalisations from 16 hospitals (9 rural, 3 metropolitan and 4 tertiary) listed in the ANZICS adult patient database. Of these, 14,943 (73%) were linked with the VAED and VDI datasets. Of the linked admissions, there were 7451 patients with a documented CFS, the ICD-10 codes were available to estimate HFRS. 450 patients who were readmitted to the ICU during the same hospital stay were excluded. The final study dataset comprised 7001 patients from whom both the CFS and HFRS measures were available. Additional file 1: Table S4 illustrates the comparison between included (n = 7001) vs excluded (n = 13,457). While there are some differences between groups, there was no difference for hospital or ICU mortality, ICU length of stay or APACHE 3.
The overall median (IQR) age was 63.7 (49.1–74.0) years; 59.5% (n = 4166) were male. Overall, 2390 patients (34.1%) were ≥ 70 years of age and 35.5% of these (848/2390) were ≥ 80 years of age. The prevalence estimates of frailty measured by CFS and HFRS across different age categories are provided in Additional file 1: Figure S1. The demographic characteristics, illness severity scores and proportion requiring mechanical ventilation are presented in Table 1.
Comparison between CFS and HFRS The number of patients quantified as frail differed between the two frailty scores. Compared to the CFS, a higher proportion of patients were categorised as frail by the HFRS (18.9% for CFS [n = 1323] vs. 26.2% for HFRS [n = 1837] (Table 1). The overall hospital mortality was 9.2% (n = 642). The HFRS weakly correlated with the CFS (Spearman’s rho = 0.13 [95% CI 0.10–0.15]; p < 0.0001) (Table 2) and had poor agreement (kappa = 0.12 [95% CI 0.10–0.15]; p < 0.0001).
Primary outcome: long-term survival The categorical (frail vs. non-frail) unadjusted 1-year mortality rates were similar for the two frailty measures. The Cox proportional hazards regression (both CFS and HFRS as ordinal variables), adjusted for ANZROD, and sex, showed that both the CFS (1-unit increment; HR 1.26, 95% CI 1.21–1.31) and HFRS (5-unit increment; HR 1.08, 95% CI 1.02–1.15) were associated with long-term survival up to one year (Table 3). The categorised Kaplan–Meier survival curves for the two frailty measures demonstrated greater survival separation between non-frail and frail patients for CFS than HFRS (Additional file 1: Figure S2). When considering unadjusted models, CFS had significantly better discrimination than HFRS (AUROC 0.66 vs 0.63 p < 0.0001) for 1-year mortality. When CFS and HFRS were combined into a single model, the resulting AUROC further increased to 0.70 suggesting that both frailty measures were capturing unique variation in frailty outcome. However, after multivariable adjustment, neither frailty measurement was able to improve on the discrimination provided by patient illness severity assessed by ANZROD (Fig. 1). Additional file 1: Figure S3 illustrates the AUROC performance for CFS and HFRS for other mortalities.
Secondary outcomes The multivariable logistic regression, adjusted for ANZROD and sex, demonstrated that the CFS independently predicted mortality at hospital discharge, 28-day, 90-day, 6-month, and 1-year for frail patients. The HFRS was independently predictive of only 90-day and 1-year mortalities (Additional file 1: Table S5, Fig. 2), but the magnitude of prediction was lower than the CFS prediction.
Subgroup analysis
Patients ≥ 75 years of age There were 1,683 patients 75 years and over. The HFRS weakly correlated with the CFS (Spearman’s rho = 0.22 [95% CI 0.18–0.27]; p < 0.0001) and had a poor agreement (Kappa = 0.05; 95% CI 0.01–0.08; p = 0.004; Additional file 1: Table S5). Although the AUROC curves had moderate discrimination the scores were similar for both CFS and HFRS (Additional file 1: Figure S4). The HFRS (5-unit increment; HR 1.05, 95% CI 0.95–1.16), adjusted for ANZROD and sex, was not independently associated with 1-year survival for HFRS when compared with CFS (1-unit increment; HR 1.18, 95% CI 1.10–1.27; Additional file 1: Table S6). Although the HFRS was independently predictive of only 90-day, 6-month and 1-year mortalities (Additional file 1: Table S7), the magnitude of prediction was weaker than the CFS prediction.
Patients needing mechanical ventilation 3266 patients received mechanical ventilation. While the HFRS did not significantly correlate with the CFS (Spearman’s rho = 0.02 (95% CI − 0.05, 0.02; p = 0.40), the AUROC curves indicated moderate discrimination with CFS AUROC lower than HFRS (0.60 vs 0.63 p < 0.0001; Additional file 1: Figure S4). After adjustment, both CFS (1-unit increment; HR 1.14; 95% CI 1.08–1.20) and HFRS (5-unit increment; HR 1.17, 95% CI 1.08–1.28) were independently associated with survival up to one year (Additional file 1: Table S6). While the CFS was predictive of both short- and long-term mortalities, HFRS was not (Additional file 1: Table S7).
Sensitivity analysis
Elective versus non-elective admissions 1740 patients were electively admitted, while 5261 were non-elective admissions. There were no differences in correlation, agreement, or 1-year survival based on their admission type (Additional file 1: Tables S5 and S6). The HFRS was independently predictive only for 90-day mortality for electively admitted patients, while the CFS was independently predictive only for 6-month and 1-year mortalities (Additional file 1: Table S7). Contrarily, the HFRS was not predictive of both short- and long-term mortalities for non-elective admissions, whereas the CFS was independently predictive of both short- and long-term mortalities (Additional file 1: Table S7).
Outcomes of patients who survived to hospital discharge Of the 6359 patients who were discharged home alive, 364 patients (5.7%) subsequently died (13.8% [154/1114] were frail and 4.0% [210/5245] were non-frail, based on CFS). The HFRS weakly correlated with the CFS (Spearman’s rho = 0.12 [95% CI 0.09–0.15]; p < 0.001) and had a poor agreement (Kappa = 0.12; 95% CI 0.07–0.14; p < 0.001; Additional file 1: Table S5). Both HFRS (5-unit increment; HR 1.13, 95% CI 1.02–1.25) and CFS (1-unit increment; HR 1.51, 95% CI 1.42–1.61), adjusted for ANZROD and sex, were independently associated with 1-year survival, but the magnitude of prediction for HFRS was weaker than the CFS prediction (Additional file 1: Table S6). Although the HFRS was independently predictive of only 90-day, 6-month and 1-year mortalities (Additional file 1: Table S7), the magnitude of prediction was weaker than the CFS prediction.
Discussion
Key findings
This large multicentre retrospective cohort study demonstrated both CFS and HFRS independently predicted 1-year survival with moderate discrimination. The CFS had better prediction than the HFRS. Both frailty measures were independently predictive and moderately discriminatory in differentiating long-term survivors from patients who died up to one year after ICU admission. The use of CFS to categorise patients as frail resulted in greater separation of Kaplan Meier curves. Neither CFS nor HFRS provided additional discriminatory value over and above that provided by the acute assessment of illness severity measured using ANZROD, however, the increased area under the curve for the combination of the two measures suggested they may both be measuring different aspects of frailty. Finally, frailty diagnosed using the CFS was predictive of both short- and long-term mortality in mechanically ventilated patients and those who were admitted non-electively, but HFRS was not.
Relationship with previous studies
There is no gold standard for frailty measure in critically ill patients. Diagnostic confirmation of frailty requires a comprehensive assessment across a spectrum of contributing domains, which are impractical and challenging in ICU settings. The validated HFRS, on the contrary, is estimated using administrative data. Both CFS and HFRS are readily interpreted by non-geriatric specialists and hence well suited for screening at-risk patients with frailty [23]. However, there is controversy of the performance of these two frailty measures.
The lower prevalence of HFRS-categorized frailty in our study compares with the reported literature (58–67% in hospitalised patients [13,24], and 59% in an ICU cohort [25]). The higher published prevalence is likely because the HFRS heavily depends on simple counts of co-morbidities for frailty classification generally without being able to differentiate pre-existing conditions from those which develop or are identified de-novo during the hospitalisation. By only including the pre-existing ICD-10 codes to determine HFRS, we observed that the prevalence of frailty was comparable to CFS-categorized frailty (26.2% and 18.9%, respectively) in our study. This was also relative to the recent meta-analysis that pooled ten observational studies that estimated a frailty prevalence of 30% among patients in ICU settings.
Higher age, comorbidity burden and primary diagnosis have previously been related to decreased survival in all patient groups following ICU admission [26,27]. CFS has been validated to predict short-term and long-term mortality in critically ill patients [4,6,11,28,29,30,31]. HFRS however relies on patient records. ICD-10 codes cannot reflect disease severity; they are normally used for reimbursement purposes. We observed that the CFS had greater predictive validity of long-term survival than the HFRS, further strengthening the argument that the CFS is a better and more reliable frailty screening measure in critically ill patients. The CFS meets all the criteria: i.e., being multi-dimensional, time-efficient, accurate and also simple. While the HFRS might be useful to assess morbidity, however, frailty is more than just the sum of comorbidities. Although the HFRS has been shown to predict ICU readmission [25,32], it relies on patient records, which are prone to be incomplete or possibly incorrect. As previously observed by Flaatten and colleagues [2], our study found that it significantly fell short in evaluating the true burden of frailty.
The HFRS, created for hospitalised but not ICU patients, was originally developed as low-risk, medium-risk, and high-risk (0–5, 5–15, > 15) and not dichotomised. The ICD-10 codes were arbitrarily selected. Furthermore, HFRS uses weights (i.e., points for each diagnosis) based on the coefficients of likelihood ratios in UK cohorts; thus, it may not be appropriate to use exactly the same weight in Australian ICU populations. For instance, 2.3 points for "Other disorders of fluid, electrolyte and acid–base balance" (observed in 33% of our cohort). Similarly, 3.2 points for "Delirium, not induced by alcohol and other psychoactive substances" (observed in 12% of our cohort). Future studies should focus on re-calculating the weight for each diagnosis using a larger Australian cohort.
Previous evidence has exhibited the challenges in comparing results of different frailty scales, with a 2017 review of 35 different frailty scores demonstrating significantly different degrees of agreement between scales in a longitudinal study of over 5000 older community-dwelling participants [33] however, HFRS was not included in that review. Our study demonstrated poor agreements between the CFS and the HFRS. The most likely explanation is that both measures estimate frailty based on different concepts of frailty.
Study implications
Although the HFRS correlated weakly with poor agreements and a lower magnitude of independently predicting longer-term survival than the CFS, both frailty measures have some validity and may be beneficial in different situations. These two frailty tools measure different aspects: the CFS is a clinical assessment, that is more likely to be immediately available to clinicians and does have some validity for differentiating long-term survivors from those who die, even before the calculation of acute illness severity. On the other hand, the HFRS is likely to be available to administrators and health departments since it is collected in and calculable from routine coding with the data. The CFS and ANZROD are less likely to be available than administrators and health departments. However, by using only the ICD-10 codes from only the pre-existing conditions, the frailty status could be extrapolated as being recorded at the time of the indexed hospitalisation. This implies that an automated HFRS could be made available when the patient is hospitalised again or readmitted to ICU. Furthermore, the combined HFRS and CFS AUROC model demonstrated that both measures could explain differing proportions of variation associated with outcome, so both would be useful if ANZROD was not available. Furthermore, as ANZROD requires 24 h of data whereas HFRS and CFS do not, a prediction model for death using frailty could potentially be developed earlier.
Strengths and limitations
The strengths of the study included a large patient dataset along with long-term outcomes. Unlike previous evidence for HFRS, to the best of our knowledge, this was the first study to use ICD-10 codes only from the pre-existing conditions and not illnesses that might have developed (and coded) during the index hospitalisation. A few limitations need to be acknowledged. Firstly, the CFS measures patients’ frailty in the 2 months before ICU admission, however, the derived HFRS used in our study for only pre-existing conditions may pre-date index hospitalisation, and hence comparable with CFS. Secondly, there could be a possibility of inappropriate categorisation, which may be a consequence of how the data is collected. For example, historically, elective surgical patients are more likely to have comorbidities listed because they often have had a preoperative workup, while the past medical histories for emergency patients were often incomplete [34]. Although the HFRS is calculated at the time of discharge, sensitivity analysis on elective and non-elective admissions suggested that under-coding of comorbidities happened with non-elective admissions. This could have affected the HFRS scores and therefore the frailty status of patients [35]. Thirdly, with the advent of population and disease registers, more studies rely on such registries for gathering vital information; hence inadvertently making them prone to information bias due to either inaccurate measurement or documentation [36]. Fourthly, we were unable to determine inter-rater reliability for CFS in our study. However, previous studies investigating the inter-rater reliability of the CFS in critically ill patients showed that the inter-rater reliability was strong when intensive care clinicians assessed the CFS [37]. However, we were unable to determine inter-rater reliability for CFS in our study. Fifthly, not all data from the ANZICS-APD with the CFS were linked to VAED and VDI datasets. Sixthly, there was a significant variation in the CFS documentation (ranging between 7 and 100%) in the 16 hospitals that reported on the CFS with documentation of the CFS increasing over time (17% at commencement, 85% at completion; Additional file 1: Table S8). However, given there was little clinical difference between included and missing patient data (n = 13,457), our cohort was broadly representative of the larger population. Finally, the lower ICU mortality rates and short ICU length of stays are real-world outcomes for ANZ ICUs and may not be applicable to other countries.
Conclusion
This retrospective multicentre cohort study in critically ill patients found that although both frailty measures independently predicted 1-year survival with moderate discrimination, the CFS was a better predictor of 1-year survival than the HFRS. The findings also suggest that the CFS had greater validity, particularly in mechanically ventilated patients and those who were admitted non-electively compared to HFRS. However, neither frailty measure was able to clinically improve upon the predictability provided by baseline patient illness severity as assessed by ANZROD.
Availability of data and materials
The datasets generated and/or analysed during the current study are not publicly available as these are linked from three registries (ANZICS, VAED and VDI), but are available from the corresponding author on reasonable request.
Abbreviations
- ANZICS:
-
Australia and New Zealand Intensive Care Society
- ANZm:
-
ANZROD + male sex
- ANZROD:
-
Australia and New Zealand risk of death
- APACHE:
-
Acute Physiology and Chronic Health Evaluation
- APD:
-
Adult Patient Database
- AR-DRG:
-
Australian-refined Diagnosis-related groups
- AUROC:
-
Area under the receiver operating characteristic
- COPD:
-
Chronic obstructive pulmonary disease
- CFS:
-
Clinical Frailty Scale
- DHHS:
-
Department of Health and Human Services
- HFRS:
-
Hospital frailty risk score
- HR:
-
Hazard ratio
- HREC:
-
Hospital Research ethics committee
- ICD-10:
-
International Statistical Classification of Diseases and Related Health Problems, 10th Revision
- ICU:
-
Intensive care unit
- IQR:
-
Interquartile range
- LOS:
-
Length of stay
- n:
-
Number
- NYHA:
-
New York Heart Association
- OR:
-
Odds ratio
- RACF:
-
Residential aged care facility
- RoD:
-
Risk of death
- RRT:
-
Renal replacement therapy
- SAPS 3:
-
Simplified Acute Physiology admission score
- SD:
-
Standard deviation
- SOFA:
-
Sequential organ failure assessment
- T_COV_:
-
Time-dependent covariate
- TCP:
-
Transitional care program
- VAED:
-
Victorian admitted episode database
- VDI:
-
Victorian death index
References
Clegg A, Young J, Iliffe S, Rikkert MO, Rockwood K. Frailty in elderly people. Lancet. 2013;381(9868):752–62.
Flaatten H, De Lange DW, Morandi A, Andersen FH, Artigas A, Bertolini G, Boumendil A, Cecconi M, Christensen S, Faraldi L, et al. The impact of frailty on ICU and 30-day mortality and the level of care in very elderly patients (>/= 80 years). Intensive Care Med. 2017;43(12):1820–8.
Muscedere J, Waters B, Varambally A, Bagshaw SM, Boyd JG, Maslove D, Sibley S, Rockwood K. The impact of frailty on intensive care unit outcomes: a systematic review and meta-analysis. Intensive Care Med. 2017;43(8):1105–22.
Bagshaw SM, Stelfox HT, McDermid RC, Rolfson DB, Tsuyuki RT, Baig N, Artiuch B, Ibrahim Q, Stollery DE, Rokosh E, et al. Association between frailty and short- and long-term outcomes among critically ill patients: a multicentre prospective cohort study. CMAJ. 2014;186(2):E95-102.
Bagshaw SM, Stelfox HT, Johnson JA, McDermid RC, Rolfson DB, Tsuyuki RT, Ibrahim Q, Majumdar SR. Long-term association between frailty and health-related quality of life among survivors of critical illness: a prospective multicenter cohort study. Crit Care Med. 2015;43(5):973–82.
Brummel NE, Bell SP, Girard TD, Pandharipande PP, Jackson JC, Morandi A, Thompson JL, Chandrasekhar R, Bernard GR, Dittus RS, et al. Frailty and subsequent disability and mortality among patients with critical illness. Am J Respir Crit Care Med. 2017;196(1):64–72.
De Biasio JC, Mittel AM, Mueller AL, Ferrante LE, Kim DH, Shaefi S. Frailty in critical care medicine: a review. Anesth Analg. 2020;130(6):1462–73.
Pugh RJ, Ellison A, Pye K, Subbe CP, Thorpe CM, Lone NI, Clegg A. Feasibility and reliability of frailty assessment in the critically ill: a systematic review. Crit Care. 2018;22(1):49.
Rockwood K, Song X, MacKnight C, Bergman H, Hogan DB, McDowell I, Mitnitski A. A global clinical measure of fitness and frailty in elderly people. CMAJ. 2005;173(5):489–95.
Mitnitski AB, Mogilner AJ, Rockwood K. Accumulation of deficits as a proxy measure of aging. ScientificWorldJournal. 2001;1:323–36.
Fisher C, Karalapillai DK, Bailey M, Glassford NG, Bellomo R, Jones D. Predicting intensive care and hospital outcome with the Dalhousie Clinical Frailty Scale: a pilot assessment. Anaesth Intensive Care. 2015;43(3):361–8.
Le Maguet P, Roquilly A, Lasocki S, Asehnoune K, Carise E, Saint Martin M, Mimoz O, Le Gac G, Somme D, Cattenoz C, et al. Prevalence and impact of frailty on mortality in elderly ICU patients: a prospective, multicenter, observational study. Intensive Care Med. 2014;40(5):674–82.
Gilbert T, Neuburger J, Kraindler J, Keeble E, Smith P, Ariti C, Arora S, Street A, Parker S, Roberts HC, et al. Development and validation of a Hospital Frailty Risk Score focusing on older people in acute care settings using electronic hospital records: an observational study. Lancet. 2018;391(10132):1775–82.
Nghiem S, Sajeewani D, Henderson K, Afoakwah C, Byrnes J, Moyle W, Scuffham P. Development of frailty measurement tools using administrative health data: A systematic review. Arch Gerontol Geriatr. 2020;89: 104102.
Bruno RR, Guidet B, Wernly B, Flaatten H, Jung C: Frailty assessment in very old intensive care patients: the Hospital Frailty Risk Score answers another question. Intensive Care Med. 2020.
Bruno RR, Wernly B, Flaatten H, Scholzel F, Kelm M, Jung C. The hospital frailty risk score is of limited value in intensive care unit patients. Crit Care. 2019;23(1):239.
Redfern OC, Harford M, Gerry S, Prytherch D, Watkinson PJ. Frailty and unplanned admissions to the intensive care unit: a retrospective cohort study in the UK. Intensive Care Med. 2020;46(7):1512–3.
APD Data Dictionary ANZICS CORE - ADULT PATIENT DATABASE. https://www.anzics.com.au/wp-content/uploads/2018/08/ANZICS-APD-Dictionary.pdf
Soong J, Poots AJ, Scott S, Donald K, Woodcock T, Lovett D, Bell D. Quantifying the prevalence of frailty in English hospitals. BMJ Open. 2015;5(10):e008456.
DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44(3):837–45.
Paul E, Bailey M, Kasza J, Pilcher D. The ANZROD model: better benchmarking of ICU outcomes and detection of outliers. Crit Care Resusc. 2016;18(1):25–36.
Pilcher D, Paul E, Bailey M, Huckson S. The Australian and New Zealand Risk of Death (ANZROD) model: getting mortality prediction right for intensive care units. Crit Care Resusc. 2014;16(1):3–4.
Utino Taniguchi L, Ibrahim Q, Azevedo LCP, Stelfox HT, Bagshaw SM. Comparison of two frailty identification tools for critically ill patients: a post-hoc analysis of a multicenter prospective cohort study. J Crit Care. 2020;59:143–8.
Kundi H, Cetin EHO, Canpolat U, Aras S, Celik O, Ata N, Birinci S, Cay S, Ozeke O, Tanboga IH, et al. The role of frailty on adverse outcomes among older patients with COVID-19. J Infect. 2020;81(6):944–51.
Redfern OC, Harford M, Gerry S, Prytherch D, Watkinson PJ: Frailty assessment in very old intensive care patients: the Hospital Frailty Risk Score answers another question. Intensive Care Med. 2020.
Kristinsdottir EA, Long TE, Sigvaldason K, Karason S, Sigurdsson GH, Sigurdsson MI. Long-term survival after intensive care: a retrospective cohort study. Acta Anaesthesiol Scand. 2020;64(1):75–84.
Williams TA, Dobb GJ, Finn JC, Knuiman MW, Geelhoed E, Lee KY, Webb SA. Determinants of long-term survival after intensive care. Crit Care Med. 2008;36(5):1523–30.
Darvall JN, Greentree K, Braat MS, Story DA, Lim WK. Contributors to frailty in critical illness: multi-dimensional analysis of the Clinical Frailty Scale. J Crit Care. 2019;52:193–9.
Dent E, Lien C, Lim WS, Wong WC, Wong CH, Ng TP, Woo J, Dong B, de la Vega S, Hua Poi PJ, et al. The Asia-Pacific Clinical Practice Guidelines for the Management of Frailty. J Am Med Dir Assoc. 2017;18(7):564–75.
Gregorevic KJ, Hubbard RE, Lim WK, Katz B. The clinical frailty scale predicts functional decline and mortality when used by junior medical staff: a prospective cohort study. BMC Geriatr. 2016;16:117.
Tipping CJ, Hodgson CL, Harrold M, Chan T, Holland AE. Frailty in patients with trauma who are critically ill: a prospective observational study to determine feasibility, concordance, and construct and predictive validity of 2 frailty measures. Phys Ther. 2019;99(8):1089–97.
Street A, Maynou L, Gilbert T, Stone T, Mason S, Conroy S. The use of linked routine data to optimise calculation of the Hospital Frailty Risk Score on the basis of previous hospital admissions: a retrospective observational cohort study. Lancet Healthy Longev. 2021;2(3):e154–62.
Aguayo GA, Donneau AF, Vaillant MT, Schritz A, Franco OH, Stranges S, Malisoux L, Guillaume M, Witte DR. Agreement between 35 published frailty scores in the general population. Am J Epidemiol. 2017;186(4):420–34.
Lindner T, Slagman A, Senkin A, Mockel M, Searle J. Medical history of elderly patients in the emergency setting: not an easy point-of-care diagnostic marker. Emerg Med Int. 2015;2015: 490947.
Gani F, Canner JK, Pawlik TM. Use of the Modified Frailty Index in the American College of Surgeons National Surgical Improvement Program Database: Highlighting the Problem of Missing Data. JAMA Surg. 2017;152(2):205–7.
Kesmodel US. Information bias in epidemiological studies with a special focus on obstetrics and gynecology. Acta Obstet Gynecol Scand. 2018;97(4):417–23.
Shears M, Takaoka A, Rochwerg B, Bagshaw SM, Johnstone J, Holding A, Tharmalingam S, Millen T, Clarke F, Rockwood K, et al. Assessing frailty in the intensive care unit: A reliability and validity study. J Crit Care. 2018;45:197–203.
Acknowledgements
The authors acknowledge the contribution of Mr Cameron Green, Mr Brendan McAlister, and Dr Scott May of the Frailtrail team from the Bendigo Datathon 2018. The authors and the ANZICS CORE management committee would like to thank clinicians, data collectors and researchers at the following contributing sites: Alfred Hospital, Austin Hospital, Bendigo Health Care Group, Box Hill Hospital, Central Gippsland Health Service, Dandenong Hospital, Frankston Hospital, Goulburn Valley Health, Latrobe Regional Hospital, Monash Medical Centre-Clayton Campus, Northeast Health Wangaratta, South West Healthcare (Warrnambool), University Hospital Geelong, Western District Health Service (Hamilton), and Wimmera Health Care Group (Horsham).
Funding
No financial support, including any institutional departmental funds, was used for the study.
Author information
Authors and Affiliations
Contributions
AS: This author conceived the project idea, conducted the literature review, assisted with data analysis, wrote the initial drafts of the manuscript, created tables and figures, and finalized the manuscript. RU: This author conducted the data clean up, statistical analysis, created tables and figures and finalized the manuscript. RT: This author contributed to the concept and project design, edited, critically evaluated and finalized the manuscript. MB: This author conducted the statistical analysis, created figures, wrote the initial drafts of the manuscript, and finalized the manuscript. DP: This author contributed to the concept and project design, assisted with the linked dataset, assisted with data analysis, wrote the initial drafts of the manuscript, and finalized the manuscript. All authors critically reviewed the manuscript and approved the final version before submission. All authors have agreed both to be personally accountable for the author's own contributions and ensured that questions related to the accuracy or integrity of any part of the work. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All experimental protocols were approved by The Research Governance of Peninsula Health Ethics Committee (HREC reference number HREC/47502/PH-2018, DHHS/RQ907) with a waiver of informed consent. All methods were carried out in accordance with the relevant guidelines and regulations of the Declaration of Helsinki.
Consent for publication
Not applicable.
Competing interests
All authors declare no support from any organization for the submitted work. All authors declare no financial or non-financial competing interests with regards to the submitted work.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1.
Supplementary Table 1: ICD-10 codes used to estimate the HFRS, and the points awarded for each diagnosis. Supplementary Table 2: A complete list of ICD-10 variables extracted from the VAED database. Supplementary Table 3: A complete list of ICD-10 variables extracted from the VDI database. Supplementary Table 4: Comparison between included (n=7,001) vs excluded (n = 13,457). Supplementary Table 5: Spearman correlation and Kappa agreement between the two frailty measures. Supplementary Table 6: Subgroup analysis: Unadjusted and adjusted Cox proportional hazards regression for CFS and the HFRS as continuous variables, adjusting for ANZROD, and sex CFS and HFRS or (a) Patients ≥ 65 years of age, (b) Patients ≥75 years of age, (c) Patients needing mechanical ventilation, (d) elective patients, (e) non-elective patients and (f) patients who survived to hospital discharge. Supplementary Table 7: Multivariable Logistic Regression analysis for short-term and long-term mortalities. Unadjusted and adjusted for sex and ANZROD. The odds ratio (OR) in bold represent statistically significant results. Graphically presented in Fig. 2. Supplementary Table 8: Reduction over time in the proportion of patients without a documented CFS in the ANZICS database. Supplementary Figure 1: Prevalence of frailty measure by CFS and HFRS across different age categories. Supplementary Figure 2: Kaplan Meier curves between frail and non-frail patients (treated as a dichotomous variable) for CFS and HFRS. Supplementary Figure 3: Area under the receiver operator curve for short-term mortality. The CFS was significantly better than the HFRS. However, CFS and HFRS must both be measuring different aspects, because the combination of the two was better than either measure on its own. The bottom 2 graphs showed that while both variables may be predictive of mortality, neither provided any worthwhile improvement on top of ANZROD. Supplementary Figure 4: Area under the receiver operator curve for 1-year mortality for patients ≥ 75 years of age and those needing mechanical ventilation.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Subramaniam, A., Ueno, R., Tiruvoipati, R. et al. Comparison of the predictive ability of clinical frailty scale and hospital frailty risk score to determine long-term survival in critically ill patients: a multicentre retrospective cohort study. Crit Care 26, 121 (2022). https://doi.org/10.1186/s13054-022-03987-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13054-022-03987-1