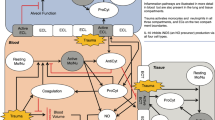
Abstract
Background
Trauma-induced coagulopathy includes thrombocytopenia and platelet dysfunction that impact patient outcome. Nevertheless, the role of platelet transfusion remains poorly defined. The aim of the study was 1/ to evaluate the impact of early platelet transfusion on 24-h all-cause mortality and 2/ to describe platelet count at admission (PCA) and its relationship with trauma severity and outcome.
Methods
Observational study carried out on a multicentre prospective trauma registry. All adult trauma patients directly admitted in participating trauma centres between May 2011 and June 2019 were included. Severe haemorrhage was defined as ≥ 4 red blood cell units within 6 h and/or death from exsanguination. The impact of PCA and early platelet transfusion (i.e. within the first 6 h) on 24-h all-cause mortality was assessed using uni- and multivariate logistic regression.
Results
Among the 19,596 included patients, PCA (229 G/L [189,271]) was associated with coagulopathy, traumatic burden, shock and bleeding severity. In a logistic regression model, 24-h all-cause mortality increased by 37% for every 50 G/L decrease in platelet count (OR 0.63 95% CI 0.57–0.70; p < 0.001). Regarding patients with severe hemorrhage, platelets were transfused early for 36% of patients. Early platelet transfusion was associated with a decrease in 24-h all-cause mortality (versus no or late platelets): OR 0.52 (95% CI 0.34–0.79; p < 0.05).
Conclusions
PCA, although mainly in normal range, was associated with trauma severity and coagulopathy and was predictive of bleeding intensity and outcome. Early platelet transfusion within 6 h was associated with a decrease in mortality in patients with severe hemorrhage. Future studies are needed to determine which doses of platelet transfusion will improve outcomes after major trauma.
Similar content being viewed by others
Background
Acute post-traumatic haemorrhage is the first cause of preventable death [1, 2]. Deaths from haemorrhage occur early, typically within the first 6 h. Trauma-induced coagulopathy (TIC) is a complex, multifactorial failure of haemostasis that occurs in 25% of severely injured patients and results in a fourfold higher mortality [3]. It appears immediately after trauma, at the scene of the accident, before any treatment [4]. It is characterized by hyperfibrinolysis, hypofibrinogenemia, systemic anticoagulation and endothelial dysfunction. It is secondarily aggravated by shock, hypothermia, acidosis, hypocalcaemia and dilution with filling solutions. More recently, the role of platelets in TIC has been highlighted, including thrombocytopenia, often mild [5] and especially platelet dysfunction [6]. Both quantitative and qualitative platelet damages are all the more marked when tissue injury is severe, transfusion is large, and hypoperfusion is intense and prolonged [7]. It is also associated with mortality. Nevertheless, the pathophysiological role of platelets in TIC remains poorly understood and is still an area of intense research.
The guidelines for the management of major bleeding and coagulopathy following trauma specify that platelet transfusion should maintain a platelet count above 50 G/L, or even above 100 G/L in the event of active bleeding or brain injury [8]. However, improved knowledge on trauma-induced platelet dysfunction and its prognostic role has led to the hypothesis that earlier and more aggressive platelet transfusion would improve the outcome of bleeding severe trauma patients. Observational studies on severe trauma patients support this hypothesis: they show an association between early platelet transfusion and mortality [9] but also between the ratio of platelet units to packed red blood cell units (RBCs) and mortality [10, 11]. The substudy of the PROPPR randomized trial showed that platelet transfusion was associated with improved haemostasis and reduced mortality in severely injured, bleeding patient [12]. Conversely, other observational data suggest that platelet transfusion may be inefficient [13], or may even paradoxically worsen platelet function [14]. However, no randomized trial has evaluated the potential benefit of early platelet transfusion in traumatic haemorrhagic shock. So, lacking high level of evidence on the subject, the role of platelet transfusion in severe trauma management remains poorly defined and recommendations are limited to expert opinions.
The aim of this study was to evaluate the impact of early platelet transfusion on 24-h all-cause mortality. Secondary objective was to analyse modalities of platelet transfusion and to describe platelet count at admission and its relationship with trauma severity and outcome.
Methods
This is a retrospective study conducted on patients whose data were prospectively collected in the Traumabase® between January 2012 and June 2019. The Traumabase® (www.traumabase.eu) is a French major trauma registry initiated in 2010. Patients included in the Traumabase® are suspected of severe trauma from the scene and admitted (primarily or secondarily) to the participating trauma centres (n = 14). The Traumabase® is in accordance with all requirements from the Advisory Committee for the processing of research information in the field of health (CCTIRS), the French National Commission on Computing and Liberty (CNIL, authorization number 911461) and meets the requirements of the local and national ethics committee (Comité de Protection des Personnes, Paris VI). The structure of the database integrates algorithms for consistency and coherence, and the data monitoring is performed by a central administrator [15].
We included all trauma patients collected in the registry during the predefined period. Patients managed after secondary transfer, aged < 15 years and those receiving antiplatelet agents were excluded from analysis. Two subpopulations were studied, referred as bleeding severity: (i) trauma patients presenting severe haemorrhage (i.e. transfusion of at least 4 RBCs within the first 6 h of management and/or death from haemorrhagic causes whatever the level of transfusion); (ii) patients with massive transfusion (i.e. transfusion of at least 10 RBCs within the first 24 h).
The course of trauma patients was structured in five stages: prehospital, resuscitation room (RR), first 6 h (H6), first 24 h (H24), and intensive care unit length of stay (ICU LOS). Collected data regarding transfusion included the number of RBCs, fresh frozen plasma (FFP) and platelet units (PUs) in RR, H6, H24 and ICU LOS. For the study purpose, we considered that one platelet concentrate was equivalent to five donor PUs. The transfusion ratios, FFP:RBC and PU:RBC were calculated. In cases where the ratio could not be obtained in the mutually exclusive cases of a numerator or denominator equal to 0, a dummy value of 0.5 was imputed to preserve the observation in the description and analysis. Of note, all participating centres followed the European guidelines on the management of major bleeding and coagulopathy following trauma, and all-centre massive transfusion protocols contained platelets every 3-to-6 RBC. Severity scores such as Simplified Acute Physiology Score (SAPS2), Injury Severity Score (ISS) and Sequential Organ Failure Assessment (SOFA) were calculated from the values of the first 24 h and recorded in the database. The 24-h and ICU all-cause mortality were used as the final outcomes.
Patients were categorized according to admission platelet count into one of the following categories: 0–49 G/L, 50–99 G/L, 100–149 G/L, 150–299 G/L and ≥ 300 G/L. Patients were further categorized into four injury severity groups according to Injury Severity Score (ISS) and admission base deficit: (1) ISS ≥ 25, base deficit ≥ −6; (2) ISS ≥ 25, base deficit < −6; (3) ISS < 25, base deficit ≥ −6; and (4) ISS < 25, base deficit < -6. These cut-offs for ISS and base deficit have been used in prior studies as reliable markers for anatomical injury burden (ISS) and shock severity (base deficit) [5].
The primary objective was to assess the association between early platelet transfusion (defined as platelet transfusion within the first 6 h) and 24-h all-cause mortality.
Secondary objectives targeted the correlation between platelet count and trauma severity (injury, coagulopathy and acidosis), the association between platelet count and outcome, including bleeding intensity and mortality (24-h all-cause and ICU mortality), the association between platelet transfusion and blood product consumption.
Statistical methods
The report follows the Strobe guidelines [16]. Categorical variables are presented as counts and percentages (%) and 95% confidence interval (95%CI, when appropriate), while continuous variables are expressed as mean (standard deviation) or median [quartile 1, 3] according to their distribution. Multimodal plotting was used to represent associations between clinical characteristics and platelet count and between transfusion modalities and time.
To assess whether early platelet transfusion was an independent risk factor of 24-h all-cause mortality, we performed first bivariate analyses using Chi-square test and Student’s t test (or Mann–Whitney test) depending on variable type, to assess crude associations and to identify confounders (i.e. covariates statistically associated with 24-h all-cause mortality and those associated with early platelet transfusion). Then, multivariate analysis was performed after imputation of missing values (all were < 20% missing) to identify independent predictors of 24-h all-cause mortality. The variables having a p value < 0.20 in the bivariate analysis were checked for collinearity and then entered in the logistic regression model, as well as well-established predictors of mortality, previously listed by a group of experts on a Delphi [17]. Calibration and discrimination of the models were computed. Statistical analysis was performed with R software version 3.3.3 for Windows (R Foundation for Statistical Computing, Vienna, Austria).
Results
During the study period, 23,751 patients were admitted in the participating trauma centres. After exclusion of patients out of defined criteria, 19,596 remained for analysis, 1622 (8%) presented with severe haemorrhage and 539 (3%) received massive transfusion (flowchart in Fig. 1). The characteristics of each subpopulation are presented in Table 1.
Platelet count and severity
Platelet count at admission varied from 2 G/L to 979 G/L, with a median of 229 G/L [189, 271]. Platelet count was associated with acute trauma coagulopathy as it was correlated with prothrombin time ratio (r = 0.31, p < 0.001). The lower the admission platelet count, the more coagulopathic the patients (prothrombin time ratio > 1.5): 18% were coagulopathic when platelet count was above 150 G/L, 52% were coagulopathic when between 100 and 150 G/L, and 67% when below 100 G/L. Platelet count was also weakly correlated with fibrinogen concentration (r = 0.27, p < 0.001). Moreover, platelet count was associated with the main drivers of coagulopathy, i.e. with ISS (as a proxy for anatomical injury and tissue damage) and BD (as a proxy for shock severity)(Fig. 2): the worst platelet counts were measured in patients admitted with the combination of the most severe injuries (ISS > 35) and highest base deficit (BD > 12).
Relationship between traumatic burden and intensity of shock (i.e. Injury Severity Score (ISS) and base deficit (BD). A/ Multivariate plot of median platelet count against both ISS and BD. The worst platelet counts were seen in patients admitted with the combination of the most severe injuries and highest BD (median Platelet count = 146 G/L for patients having ISS > 35 and BD > 12; with p < 0.05 compared with all other groups). B/ Multivariate plot of 24-h all-cause survival rate against both ISS and BD. The worst survival rates were seen in patients admitted with the combination of the most severe injuries and highest BD (66% for patients having ISS > 35 and BD > 12; with p < 0.05 compared with all other groups)
Platelet count at admission predicted bleeding severity (SH or MT): counts were lower in patients with severe haemorrhage or massive transfusion compared to the complete cohort (180 (88) G/L and 158 (77) G/L vs 231 (70) G/L, respectively, p < 0.01) (Table 1). Moreover, platelet count was correlated with haemoglobin concentration at admission (r = 0.24, p < 0.001) and with RBC requirements over 24 h: for every 50 G/L decrease in platelet count, one more unit of RBC was transfused (1, 95% CI 0.8–1.2, p < 10–3). Violin plots presented in Fig. 3 show that both RBC transfusion and platelet transfusion increased when admission platelet count decreased.
Transfusion of red blood cell units (in red) and platelet units (in yellow) at 6 h according to platelet count at admission in patients with severe haemorrhage (left) and patients with massive transfusion (right). Violin plots represent both distribution and density of data: they visually combine the plotting of descriptive data of the box-and-whisker plots (median, interquartile ranges, minimum and maximum) with the addition of a rotated kernel density plot on each side. The violin plots show the presence of different peaks, at different positions reflecting different transfusion behaviours. The x-axis presents admission platelet count categories. Transfusion requirements according to admission platelet count were compared (t-tests): *p < 0.05: transfusion requirement of the concerned admission platelet count category significantly different from the immediate previous category
Platelet count at admission was associated with mortality (Fig. 4). In the whole cohort, univariate logistic regression showed that, for every 50 G/L decrease in admission platelet count, the odds of 24-h all-cause mortality increased by 61% (OR 0.39, 95% CI 0.36–0.42, p < 10–3) and ICU mortality by 44% (OR 0.56, 95% CI 0.54–0.58, p < 10–3). Moreover, in a logistic regression model predicting 24-h all-cause mortality and adjusted on universally admitted confounders (ISS, Glasgow Coma Score and base deficit)[5], the odds of death increased by 37% for every 50 G/L decrease in platelet count (OR 0.63, 95% CI 0.57–0.70, p < 0.001) (Additional file 1: Table S1).
Platelets and transfusion
Platelet transfusion requirements during the ICU stay varied according to bleeding severity: platelets were transfused to 1335 patients of the whole cohort (7%), to 901 patients of those with severe haemorrhage (56%) and to 470 of the massively transfused patients (87%).
Similarly, platelet transfusion requirements varied according to admission platelet count. Among 233 patients presenting with minimal platelet count < 50 G/L, 159 (68%) received platelet transfusion within the first 6 h and 74 (32%) did not. Among those latter, 22 (30%) died within 24 h, and half of them during initial resuscitation. Among patients receiving early platelet transfusion, 33 (21%) died within 24 h (n = 5 during initial resuscitation).
Regarding patients with SH, 640 (40%) did not receive platelet transfusion within the first day (4% missing data), 176 patients (11%) received platelet transfusion in the resuscitation room, 590 patients (36%) received platelets thereafter but within the first 6 h and 148 (9%) patients within the following 18 h. This resulted in low platelet:plasma ratios (0.5 [0.1; 1]) and low platelet:RBC (0.3 [0.1; 0.7]) ratios within the first six hours (Fig. 3, Additional file 3: Fig. S1). Patients who received platelet transfusion within 6 h were more severely injured, had more severe shock and coagulopathy at admission with lower prothrombin time, lower fibrinogen concentration and lower platelet count than those who did not (Table 2, p < 0.001). They also required more RBC transfusion (p < 0.001).
Multivariate logistic regression analysis showed that early platelet transfusion (within 6 h) was an independent protective factor for 24-h all-cause mortality: OR 0.56, 95% CI 0.38–0.84, p = 0.004) in the SH subpopulation (Table 3). The same results were observed in the MT subgroup, including patients dying from haemorrhage without obtaining 10 RBC units in order to account for a potential survival bias: OR 0.33, 95% CI 0.20–0.55, p = 0.007) (Additional file 2: Table S2).
Discussion
This analysis of 19,596 trauma patients showed that platelet count at admission was a biomarker of trauma severity and was predictive of outcome, including bleeding intensity and mortality. Early platelet transfusion within 6 h was associated with a lower 24-h mortality.
So far, the role of platelets in trauma-induced coagulopathy remains unclear. Platelets play a central role in haemostasis, as an essential substrate of clot formation. Immediately after trauma, whereas coagulation factors, including fibrinogen, decrease and fibrinolysis, is activated, platelet count remains surprisingly essentially normal and the prevalence of thrombocytopenia at hospital admission is usually low. The conservation of rather normal counts results from a post-traumatic enhanced and compensatory release of platelets, from their storage pool in the spleen and the bone marrow [18]. As a result, admission platelet count is > 100 G/L in most trauma patients, even in those massively transfused [5, 12]. Nevertheless, variations of platelet count within “normal ranges” reflect trauma severity (Fig. 3D). Several observational studies confirmed that admission platelet count was a biomarker of trauma severity as it was associated with injury severity, shock intensity and with coagulopathy at admission [5].
Admission platelet count is also a prognostic marker. In our study, platelet count predicted bleeding severity and transfusion requirements (Fig. 3 violin plots). Similarly, in a cohort of massively transfused trauma patients, patients with a higher admission platelet count receive fewer RBCs: for every 50 G/L increase in platelet count, patients received 0.7 fewer units of blood within the first 6 h and one less unit of blood within the first 24 h (5). Several studies also reported that admission platelet count was associated with mortality. Hess et al. found a stepwise relationship between platelet count and in-hospital mortality for nine levels of injury severity [19]. Similarly, Brown et al. showed that the probability of death at 24 h decreased as the admission platelet count increased [5]. Nevertheless, as platelet count is closely related to trauma severity and coagulopathy that are well-established predictors of mortality, we performed a multivariate logistic regression model to assess the specific role of platelet count on outcome. Our study demonstrated for the first time that every 50 G/L decrease of admission platelet count was an independent predictor of early mortality (OR 0.39).
Subsequently, we hypothesized that early platelet transfusion would improve both platelet count and trauma-induced platelet dysfunction, thereby leading to a decreased mortality. Despite a strong rationale, the efficacy of platelet transfusion in trauma patients remains controversial. There is no large, randomized trial to support the benefit of platelet transfusion on patient outcome, and available data lead to conflicting conclusions. The substudy of the PROPPR trial showed that early platelet transfusion was associated with improved haemostasis and reduced mortality but was limited to severely bleeding trauma patients [12]. On the opposite, Sambasivan and colleagues reported that high ratios of platelet unit:RBC (> 1:2) administrated to non-massively transfused trauma patients had no effect on mortality compared to low ratios (≤ 1:2) but significantly decreased ventilator-free and ICU-free days [20]. In another study also including non-massively transfused blunt injury victims, platelet transfusion was found to increase the risk of acute respiratory distress syndrome without any survival benefit, but platelet transfusion was recorded over 48 h and only 488 patients were included [21]. Our study provided the opportunity to examine a large cohort of trauma patients and suggested that early platelet transfusion improved survival, not only in massively transfused patients but also in severely bleeding trauma patients. The other variables that were significant predictors of mortality were previously known and thus not discussed here [17].
The current European Guidelines on the management of major bleeding and coagulopathy following trauma recommend maintaining a platelet count greater than 50 G/L for patients with ongoing bleeding, or greater than 100 G/L if associated with traumatic brain injury. Nevertheless, such thresholds may be insufficient after severe trauma. First, platelet count, although essentially higher than 100 G/L, is a prognostic marker following trauma. Early platelet transfusion improved survival, when 96% of early platelet transfusion were performed to patients with an admission platelet count > 100 G/L. Similarly, in the PROPRR substudy that also supports transfusion of platelets as early as possible, only 2% of patients had platelet count below 100 G/L [12]. These data support platelet transfusion despite normal platelet count. Second, low thresholds expose to dilution: in the case of massive RBC and FFP transfusion without platelet transfusion, platelet count will decrease during the course of trauma resuscitation. In a model of major surgery, a blood loss of one blood volume replaced with RBC and colloids led to decreased platelet count by half, with a large interindividual variability [22, 23]. A decrease in the platelet count to 50 G/L is expected when RBC equivalent to two blood volumes has been transfused (ref). Third, the quality of evidence that supports 50 G/L as an haemostatic threshold in trauma is very low and was defined from small series of surgical patients, with isolated thrombocytopenia and not from trauma patients with complex haemostatic disorders [24, 25]. Fourth, these thresholds do not take into account trauma-induced platelet dysfunction. Platelet dysfunction has been identified as one of the mechanisms of trauma-induced coagulopathy and deeply impacts platelet aggregation and activation pathways. Platelet dysfunction occurs in approximately 50% of trauma patients and is associated with increased morbidity and mortality. This is an additional argument to increase platelet transfusion threshold [3].
This study presents some limitations. First, it is a retrospective analysis, impairing the collection of some specific confounders such as local situation or patient dynamic clinical response to treatments. Nevertheless, data collection was prospective, and the use of the Traumabase® registry secures data processing, limits missing data through responsive data-monitoring and integrates control of biases inherent to retrospective data collection [26]. Moreover, the logistic regression model that assessed the relationship between platelet transfusion and mortality was performed rigorously in order to control for confounding factors. Second, some degree of survival bias cannot be ruled out when considering platelet transfusion within 6 h of hospital admission. Third, the exact dose of transfused platelet units is unknown as the label of the recorded variable used was “platelet concentrates”. Fourth, we cannot rule out a part of a survival bias as the time period of 6 h to define early platelet transfusion is long. Nevertheless, too few patients were transfused during initial resuscitation to allow proper analysis on this subpopulation. Last, our study did not catch platelet dysfunction and thus is unable to integrate this element to explore its relationship with poor outcomes.
Conclusion
Admission platelet count, although mainly in normal range, was associated with trauma severity and coagulopathy, and was predictive of bleeding intensity and outcome. Early platelet transfusion within 6 h was associated with a decrease in mortality. A normal platelet count might be an insufficient goal after severe trauma, and severe trauma patients might benefit from an earlier and higher threshold for platelet transfusion. Future studies are needed to determine which doses of platelet transfusion will improve outcomes after major trauma.
Availability of data and materials
Data are from the Traumabase, a collaborative project that consists of trauma practitioners, from different centres all over the country, sharing the registry for research and public health issues. Even though datasets are de-identified, the National Commission for data protection (CNIL) has imposed restrictions on data sharing since they contain sensitive informations. Conventions are signed for researcher before any access to the data. For data access, interested researchers can contact the Traumabase scientific committee on reasonable request with the following email address: contact@traumabase. eu (secretary of the scientific committee- Dr. Anatole Harrois).
Abbreviations
- BD:
-
Base deficit
- CI:
-
Confidence interval
- FFP:
-
Fresh-frozen plasma
- ICU:
-
Intensive care unit
- ISS:
-
Injury Severity Score
- LOS:
-
Length of stay
- MT:
-
Massive transfusion
- OR:
-
Odds ratio
- PU:
-
Platelet units
- RBC:
-
Packed red blood cell units
- SAPS 2:
-
Simplified Acute Physiology Score
- SH:
-
Severe hemorrhage
- SM:
-
Supplementary material
- SOFA:
-
Sequential Organ Failure Assessment
- TIC:
-
Trauma-induced coagulopathy
References
Callcut RA, Kornblith LZ, Conroy AS, Robles AJ, Meizoso JP, Namias N, et al. The why and how our trauma patients die: a prospective Multicenter Western Trauma Association study. J Trauma Acute Care Surg. 2019;86:864–70.
Teixeira PGR, Inaba K, Hadjizacharia P, Brown C, Salim A, Rhee P, et al. Preventable or potentially preventable mortality at a mature trauma center. J Trauma. 2007;63:1338–46; discussion 1346–1347.
Kornblith LZ, Moore HB, Cohen MJ. Trauma-induced coagulopathy: the past, present, and future. J Thromb Haemost. 2019;17:852–62.
Floccard B, Rugeri L, Faure A, Saint Denis M, Boyle EM, Peguet O, et al. Early coagulopathy in trauma patients: an on-scene and hospital admission study. Injury. 2012;43:26–32.
Brown LM, Call MS, Knudson MM, Cohen MJ. A normal platelet count may not be enough: the impact of admission platelet count on mortality and transfusion in severely injured trauma patients. 2015;16.
Vulliamy P, Kornblith LZ, Kutcher ME, Cohen MJ, Brohi K, Neal MD. Alterations in platelet behavior after major trauma: adaptive or maladaptive? :11.
Vulliamy P, Gillespie S, Armstrong PC, Allan HE, Warner TD, Brohi K. Histone H4 induces platelet ballooning and microparticle release during trauma hemorrhage. Med Sci. 6.
Spahn DR, Bouillon B, Cerny V, Duranteau J, Filipescu D, Hunt BJ, et al. The European guideline on management of major bleeding and coagulopathy following trauma: fifth edition. Crit Care. 2019;23:98.
Holcomb JB, del Junco DJ, Fox EE, Wade CE, Cohen MJ, Schreiber MA, et al. The prospective, observational, multicenter, major trauma transfusion (PROMMTT) study: comparative effectiveness of a time-varying treatment with competing risks. JAMA Surg. 2013;148:127.
Balvers K, van Dieren S, Baksaas-Aasen K, Gaarder C, Brohi K, Eaglestone S, et al. Combined effect of therapeutic strategies for bleeding injury on early survival, transfusion needs and correction of coagulopathy. Br J Surg. 2017;104:222–9.
Cannon JW, Khan MA, Raja AS, Cohen MJ, Como JJ, Cotton BA, et al. Damage control resuscitation in patients with severe traumatic hemorrhage: a practice management guideline from the Eastern Association for the Surgery of Trauma. J Trauma Acute Care Surg. 2017;82:605–17.
Cardenas JC, Zhang X, Fox EE, Cotton BA, Hess JR, Schreiber MA, et al. Platelet transfusions improve hemostasis and survival in a substudy of the prospective, randomized PROPPR trial. 2018;2:9.
Vulliamy P, Gillespie S, Gall LS, Green L, Brohi K, Davenport RA. Platelet transfusions reduce fibrinolysis but do not restore platelet function during trauma hemorrhage. 2017;83:10.
Henriksen HH, Grand AG, Viggers S, Baer LA, Solbeck S, Cotton BA, et al. Impact of blood products on platelet function in patients with traumatic injuries: a translational study. J Surg Res. 2017;214:154–61.
Hamada SR, Delhaye N, Degoul S, Gauss T, Raux M, Devaud M-L, et al. Direct transport vs secondary transfer to level I trauma centers in a French exclusive trauma system: Impact on mortality and determinants of triage on road-traffic victims. 17.
Vandenbroucke JP, Von Elm E, Altman DG, Gøtzsche PC, Mulrow CD, Pocock SJ, et al. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): explanation and elaboration. PLoS Med. 2007;4:e297.
Hamada SR, Pirracchio R, Beauchesne J, Benlaldj MN, Meaudre E, Leone M, et al. Effect of fibrinogen concentrate administration on early mortality in traumatic hemorrhagic shock: a propensity score analysis. J Trauma Acute Care Surg. 2020;88:661–70.
Wannberg M, Miao X, Li N, Wikman A, Wahlgren C-M. Platelet consumption and hyperreactivity coexist in experimental traumatic hemorrhagic model. Platelets. 2020;31:777–83.
Hess JR, Lindell AL, Stansbury LG, Dutton RP, Scalea TM. The prevalence of abnormal results of conventional coagulation tests on admission to a trauma center. Transfusion. 2009;49:34–9.
Sambasivan CN, Kunio NR, Nair PV, Zink KA, Michalek JE, Holcomb JB, et al. High ratios of plasma and platelets to packed red blood cells do not affect mortality in nonmassively transfused patients. J Trauma. 2011;71:S329-336.
Kasotakis G, Starr N, Nelson E, Sarkar B, Burke PA, Remick DG, et al. Platelet transfusion increases risk for acute respiratory distress syndrome in non-massively transfused blunt trauma patients. Eur J Trauma Emerg Surg. 2019;45:671–9.
Hiippala ST, Myllylä GJ, Vahtera EM. Hemostatic factors and replacement of major blood loss with plasma-poor red cell concentrates. Anesth Analg. 1995;81:360–5.
Murray DJ, Olson J, Strauss R, Tinker JH. Coagulation changes during packed red cell replacement of major blood loss. Anesthesiology. 1988;69:839–45.
Bishop JF, Schiffer CA, Aisner J, Matthews JP, Wiernik PH. Surgery in acute leukemia: a review of 167 operations in thrombocytopenic patients. Am J Hematol. 1987;26:147–55.
McVay PA, Toy PT. Lack of increased bleeding after paracentesis and thoracentesis in patients with mild coagulation abnormalities. Transfusion. 1991;31:164–71.
Moyer J-D, Hamada SR, Josse J, Auliard O, Gauss T, Traumabase Group. Trauma reloaded: Trauma registry in the era of data science. Anaesth Crit Care Pain Med. 2021;40:100827.
Acknowledgements
Contributors from the Traumabase listed as investigators. Paer-Selim Abback, Département d'Anesthésie Réanimation, Hôpital Beaujon, HPUNVS, Clichy, France. Gérard Audibert, Département d'Anesthésie Réanimation, Hôpital de Nancy, Nancy, France. Tobias Gauss, Département d'Anesthésie Réanimation, Hôpital Beaujon, HPUNVS, Clichy, France. Thomas Geeraerts, Département d’Anesthésie Réanimation, Toulouse, France. Anatole Harrois, AP-HP, Département d'Anesthésie Réanimation, Hôpital du Kremlin Bicêtre, France. Olivier Langeron, Département d'Anesthésie Réanimation, Hôpitaux Universitaires Henri Mondor, Créteil, France. Marc Leone, Département d'Anesthésie Réanimation, Hôpital Nord, AP-HM, Marseille, France. Julien Pottecher, Département d'Anesthésie Réanimation, CHU de Strasbourg Hautepierre, Strasbourg, France. Laurent Stecken, Département d’Anesthésie-Réanimation, CHU de Bordeaux, Bordeaux, France. Jean-Luc Hanouz, Département d’Anesthésie-Réanimation, CHU de Caen, Caen, France.
Funding
The French Regional Health Agency of Ile De France has funded a part of the trauma registry. The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
Author information
Authors and Affiliations
Consortia
Contributions
SRH, AG and DG performed conception of the work, data collection, data analysis and interpretation, drafting the article, critical revision of the article, final approval of the version to be published. HN, AM, EM, AC, EG, GA, BV, JD and MB done data collection, critical revision of the article, final approval of the version to be published. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The Traumabase® is in accordance with all requirements from the Advisory Committee for the processing of research information in the field of health (CCTIRS), the French National Commission on Computing and Liberty (CNIL, authorization number 911461) and meets the requirements of the local and national ethics committee (Comité de Protection des Personnes, Paris VI). The national ethics committee waived the need for informed consent considering the retrospective nature of data collected.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1
. Table S1: Multivariate logistic regression analysis: predictors of 24-h all-cause mortality (on complete cases from the whole cohort, n = 12278). All p values < 10−6
Additional file 2
. Table S2: Multivariate analysis of 24-hour all-cause mortality in the MT subpopulation
Additional file 3
. Fig. S1: Cumulative transfusions of blood products (RBC, FFP, PU) during the trauma patient clinical pathway until ICU discharge
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Hamada, S.R., Garrigue, D., Nougue, H. et al. Impact of platelet transfusion on outcomes in trauma patients. Crit Care 26, 49 (2022). https://doi.org/10.1186/s13054-022-03928-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13054-022-03928-y