Abstract
Background
Recent multicenter studies identified COVID-19 as a risk factor for invasive pulmonary aspergillosis (IPA). However, no large multicenter study has compared the incidence of IPA between COVID-19 and influenza patients.
Objectives
To determine the incidence of putative IPA in critically ill SARS-CoV-2 patients, compared with influenza patients.
Methods
This study was a planned ancillary analysis of the coVAPid multicenter retrospective European cohort. Consecutive adult patients requiring invasive mechanical ventilation for > 48 h for SARS-CoV-2 pneumonia or influenza pneumonia were included. The 28-day cumulative incidence of putative IPA, based on Blot definition, was the primary outcome. IPA incidence was estimated using the Kalbfleisch and Prentice method, considering extubation (dead or alive) within 28 days as competing event.
Results
A total of 1047 patients were included (566 in the SARS-CoV-2 group and 481 in the influenza group). The incidence of putative IPA was lower in SARS-CoV-2 pneumonia group (14, 2.5%) than in influenza pneumonia group (29, 6%), adjusted cause-specific hazard ratio (cHR) 3.29 (95% CI 1.53–7.02, p = 0.0006). When putative IPA and Aspergillus respiratory tract colonization were combined, the incidence was also significantly lower in the SARS-CoV-2 group, as compared to influenza group (4.1% vs. 10.2%), adjusted cHR 3.21 (95% CI 1.88–5.46, p < 0.0001). In the whole study population, putative IPA was associated with significant increase in 28-day mortality rate, and length of ICU stay, compared with colonized patients, or those with no IPA or Aspergillus colonization.
Conclusions
Overall, the incidence of putative IPA was low. Its incidence was significantly lower in patients with SARS-CoV-2 pneumonia than in those with influenza pneumonia.
Clinical trial registration The study was registered at ClinicalTrials.gov, number NCT04359693.
Similar content being viewed by others
Background
Invasive pulmonary aspergillosis (IPA) was reported to be common in critically ill patients with chronic obstructive pulmonary disease (COPD) [1], acute respiratory distress syndrome (ARDS) [2], cirrhosis [3], acute hepatitis [4], or immunosuppression [5]. Previous studies also highlighted a relationship between IPA and outcomes, including mortality, duration of mechanical ventilation, and ICU length of stay [6]. Recently, critically ill patients receiving invasive mechanical ventilation for severe influenza were identified as a high-risk population for IPA [7]. Influenza-associated IPA (IAPA) was also reported to be associated with increased risk for mortality in this population.
Case series, rapidly followed by single-center and large multicenter studies, highlighted a link between COVID-19 pneumonia and IPA. The incidence of IPA ranges from 4.8 to 23% of patients with SARS-CoV-2 pneumonia receiving invasive mechanical ventilation [8,9,10,11,12,13,14,15,16,17]. Some of these studies also showed that COVID-19-associated IPA (CAPA) was associated with increased mortality and longer duration of mechanical ventilation, and ICU stay [16]. To the best of our knowledge, only one retrospective study compared the incidence of IPA between COVID-19 ARDS patients and other-viruses-related ARDS [18]. This study suggested that COVID-19 was associated with reduced incidence of IPA as compared to other ARDS patients. However, the number of included patients was limited (n = 172) and the study was performed in a single center.
Therefore, we conducted this planned ancillary study of the coVAPid European multicenter cohort to determine the incidence of putative IPA in SARS-CoV-2 pneumonia, compared to influenza pneumonia, in intubated critically ill patients. Secondary objectives were to determine the impact of putative IPA on morbidity and mortality, and the incidence of probable IPA, based on Verweij definition [19].
Methods
Study design and population
This study was a planned ancillary analysis of the coVAPid multicenter retrospective observational cohort, conducted in 36 ICUs in Europe. The methods used in the coVAPid study are described elsewhere [20]. Briefly, consecutive adult patients with SARS-CoV-2 pneumonia, influenza pneumonia, or no viral infection at ICU admission, who required invasive mechanical ventilation for more than 48 h, were included. Only patients with SARS-CoV-2 pneumonia, or influenza pneumonia, were eligible for the current ancillary study. Patients with missing data regarding the primary outcome were excluded from the current analysis.
The Ethics Committee and Institutional Review Board of the Lille University Hospital approved the study protocol (Comité de Protection des Personnes Ouest VI; approved by April 14, 2020; registration number RIPH:20.04.09.60039) as minimal-risk research using data collected for routine clinical practice and waived the requirement for informed consent. Patients (or their proxies) received written information about the study and could refuse to participate. The study was registered at ClinicalTrials.gov, number NCT04359693.
Definitions
Blot criteria were used for IPA diagnosis, as primary outcome [21]. When at least one criterion necessary for the diagnosis of putative IPA according to Blot definition was not met, the case was classified as Aspergillus colonization. Verweij criteria were used for probable IPA diagnosis, as a secondary outcome (Additional file 1: Table E1) [19]. Suspected IPA refers to clinical suspicion associated with any positive serum or respiratory sample for Aspergillus.
Outcomes
The primary outcome of our study was the incidence of putative IPA, according to Blot definition. The secondary outcomes included the incidence of probable IPA, according to Verweij definition; and outcomes of putative IPA, including mechanical ventilation duration, ICU length of stay, and 28-day mortality.
Statistical analysis
Quantitative variables were expressed as median (interquartile range) and categorical variables were expressed as numbers (percentage). Patient characteristics at ICU admission and during ICU stay were described, in each group, according to aspergillosis status (none, Aspergillus colonization, and putative IPA), without formal statistical comparisons. The 28-day cumulative incidence of putative or probable IPA, or combination of colonization and putative IPA were estimated using Kalbfleisch and Prentice method, considering extubation (dead or alive) within 28 days as competing event. For the incidence of putative IPA according to Blot definition, occurrence of Aspergillus colonization was treated as a competing event, in addition to extubation [22].
Regarding the causal relationship of interest, we assessed the association of study groups with IPA (according to both definitions, as well as combining together colonization and putative IPA) using cause-specific Cox’s proportional hazard models, with sandwich covariance estimation to account for center clustering effect. We considered previously cited competing events, before and after adjustment for pre-specified confounders (simplified acute physiology score (SAPS) II, COPD, immunosuppression, recent antibiotic treatment before ICU admission, ARDS on admission, corticosteroid treatment during ICU stay) [23]. Cause-specific hazard ratios (cHR) and their 95% confidence intervals (CIs) associated with SARS-CoV-2 pneumonia, against influenza pneumonia, were derived from Cox’s models as effect sizes.
We assessed the association of putative IPA with patient’s outcomes censored at day 28 (overall survival, mechanical ventilation duration, length of ICU stay) using a Cox’s regression model (with sandwich covariance estimation to account for center clustering effect) performed on the whole study population, combining the two groups), with cause-specific hazard for mechanical ventilation duration (considering extubation alive as event of interest and death under mechanical ventilation as competing event), and for length of ICU stay (considering ICU discharge alive as event of interest, and death during ICU as competing event), including study group, IPA, and interaction between IPA status and study group. IPA was treated as a time-dependent covariate, as 3-levels categorical variable: no putative IPA or Aspergillus colonization, versus Aspergillus colonization, and putative IPA. This model accounted for exposure time of IPA, by comparing at each follow-up time event point, the current IPA status of patients who have the event to patients who are at risk (without the event of interest and without the competing event for mechanical ventilation duration and length of ICU stay). The associations were further adjusted for the same previously mentioned confounders [24].
Statistical testing was performed at the two-tailed α level of 0.05. Data were analyzed using the SAS software package, release 9.4 (SAS Institute, Cary, NC).
Results
Patient characteristics at ICU admission
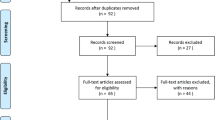
In total, 1047 patients were included (Fig. 1). Percentage of men, ARDS, and body mass index were higher in SARS-CoV-2 group than in influenza group. SAPS II, sequential organ failure assessment (SOFA) score, comorbidity scores, chronic diseases, rate of recent hospitalization, shock, cardiac arrest, neurological failure, or acute kidney injury were lower in SARS-CoV-2 pneumonia group, as compared to influenza pneumonia group (Table 1). The distribution of study patients in different centers is presented in Additional file 1: Table E3.
Patient characteristics during ICU stay
Percentage of prone positioning, as well as total duration of antimicrobial treatment were higher in SARS-CoV-2 pneumonia group than in influenza pneumonia group. Corticosteroid use, ECMO, and 28-day mortality rates were comparable in the two groups. The dose of corticosteroids was higher in SARS-CoV-2 pneumonia group, as compared to influenza group (Table 2).
Incidence of putative IPA according to Blot definition
Seventy-two patients, from 25 out of 36 participating centers, were suspected by clinicians as having IPA, including 23 in SARS-CoV-2 group, and 49 in influenza group. Of these 72 patients, 43 were classified as putative IPA, and 29 as Aspergillus colonization, according to Blot definition. No proven IPA was diagnosed in study patients.
The incidence of putative IPA was significantly lower in SARS-CoV-2 pneumonia group than in influenza pneumonia group (Fig. 2A, Table 3). This difference remained significant after adjustment for confounding factors. Similarly, when combining putative IPA and Aspergillus respiratory tract colonization, the incidence was still significantly lower in SARS-CoV-2 group than in influenza group (Fig. 3, Table 3). The classification of study patients, based on different definitions, is presented in Additional file 1: Table E2.
Cumulative incidence of putative or probable invasive pulmonary aspergillosis according to Blot (A) and Verweij (B) definitions. Cumulative incidence was estimated using Kalbfleisch and Prentice method, considering extubation (alive or due to death) within 28 days as competing event. Time axis starts at the day of intubation. IPA, invasive pulmonary aspergillosis, MV, mechanical ventilation
Cumulative incidence of putative invasive pulmonary aspergillosis or Aspergillus colonization according to Blot definition. Cumulative incidence was estimated using Kalbfleisch and Prentice method, considering extubation (alive or due to death) within 28 days as competing event. Time axis starts at the day of intubation. IPA, invasive pulmonary aspergillosis, MV, mechanical ventilation
Incidence of probable IPA according to Verweij definition
Among the 72 patients suspected by physicians as having IPA, 58 patients were classified as probable IPA according to Verweij definition. The incidence of probable IPA was also significantly lower in SARS-CoV-2 group, as compared to influenza group (Fig. 2B, Table 3). This difference remained significant after adjustment for confounding factors at ICU admission.
Outcomes of putative IPA
In the whole study population, putative IPA was associated with significant increase in 28-day mortality rate, and length of ICU stay, compared with colonized patients, or those with no IPA or Aspergillus colonization. These results were not confirmed in the subgroups of patients with SARS-CoV-2 or influenza pneumonia. Only in influenza group, duration of mechanical ventilation, and ICU stay were significantly longer in patients with putative IPA, as compared with those with no putative IPA or Aspergillus colonization (Fig. 4).
Association of putative invasive pulmonary aspergillosis, and Aspergillus colonization, according to Blot definition, with 28-day outcomes in overall population and according to study groups (SARS-CoV-2 pneumonia and influenza pneumonia). HRs were calculated using cause-specific proportional hazard models, considering death as competing event for mechanical ventilation and length of ICU stay. Adjusted HRs were calculated by including simplified acute physiology score II, chronic obstructive pulmonary disease, immunosuppression, recent antibiotic treatment before ICU admission, acute respiratory distress syndrome on admission, and corticosteroid treatment during ICU stay, as pre-specified covariates in Cox’s models (after handling missing values by multiple imputation). A HR > 1 indicates a decrease in survival (i.e., an increased risk for mortality), MV duration (i.e., an increased risk for extubation alive) and ICU length of stay (i.e., an increased risk for discharge alive) and a HR < 1 indicates an increase in survival (i.e., a decreased risk for mortality), MV duration (i.e., a decreased risk for extubation alive) and ICU length of stay (i.e., a decreased risk for discharge alive). P het indicates p value for heterogeneity in association of invasive pulmonary aspergillosis and 28-day outcomes across study groups (SARS-CoV-2 pneumonia vs. influenza pneumonia). * Not estimable, as no patient was discharged alive within 28 days. CI, confidence interval; HR, hazard ratio; ICU, intensive care unit; IPA, invasive pulmonary aspergillosis; MV, mechanical ventilation
Characteristics of patients with putative IPA
Median time from intubation to putative IPA diagnosis was longer in SARS-CoV-2 than in influenza group (11 vs. 6 days). Bronchoalveolar lavage was less frequently performed and antifungal treatment was less frequently prescribed in SARS-CoV-2 than in influenza group (Table 4).
Discussion
Overall, the incidence of putative IPA was low in patients with COVID-19 or influenza. Further, putative IPA incidence was significantly lower in SARS-CoV-2 pneumonia patients than in those with influenza pneumonia. Similar results were found regarding probable IPA, using Verweij definition. Putative IPA was associated with significantly higher 28-day mortality rate and length of ICU stay, compared with colonized patients, or those with no IPA or Aspergillus colonization. However, IPA was not significantly associated with increased duration of mechanical ventilation.
Incidence of invasive pulmonary aspergillosis
The incidence of IPA was low in our study, and some previous studies reported higher incidence of IAPA and CAPA [7, 12,13,14, 16, 17]. However, in most of these studies, screening for IPA was performed routinely. Further, patients with no routine screening were excluded. For example, in the recent multicenter Mycovid study [16], only patients with at least 3 screening samples performed within 2 weeks were analyzed, which resulted in overestimating the reported incidence of CAPA (15%). The population at risk are all patients receiving mechanical ventilation, and not only those receiving > 2 weeks of invasive mechanical ventilation. Another potential explanation for the high incidence of IPA reported in these studies is the false positive results of galactomannan in some patients, which is supported by the absence of positive impact of antifungal treatment on mortality, and the fact that some patients with CAPA survived in spite of absence of any antifungal treatment [13]. On the other hand, other well-performed single and multicenter studies reported lower incidence of IPA in influenza and COVID-19 patients [9, 10, 18, 25], which is in line with our findings. Geographical distribution and different case definitions might explain the variation in IPA incidence.
Comparison of invasive pulmonary aspergillosis incidence between COVID-19 and influenza patients
Our results suggest that IPA incidence might be lower in COVID-19 patients, compared with influenza patients. Several explanations could be provided for this result. First, the percentage of patients with immunosuppression at ICU admission was lower in COVID-19 than in influenza patients (8.8% vs. 22%). However, adjustment was performed for immunosuppression, as well as for other potential confounders. Second, BAL was performed less frequently in COVID-19 than in influenza patients, which might have underestimated the incidence of IPA in the first group. This could be explained by the fear of SARS-CoV-2 aerosolization and transmission to health workers at the beginning of the pandemic. Other factors, such as most severe ARDS, and more common prone position use in COVID-19 than in influenza patients could also explain the lower rate of BAL in COVID-19 patients. Third, the mechanism of entry of SARS-CoV-2, and influenza into the lower respiratory tract, and the pulmonary lesions associated with these viruses are different [26, 27]. This suggests that the lower incidence of IPA in COVID-19 patients might be specifically related to SARS-CoV-2 infection.
Impact of invasive pulmonary aspergillosis on outcomes
In the whole study population, combining COVID-19 and influenza patients, IPA was significantly associated with increased 28-day mortality and ICU length of stay. However, the relationship between IPA and duration of mechanical ventilation did not reach significance. In subgroup analyses, IPA was associated with increased duration of mechanical ventilation and ICU length of stay in influenza, but not in COVID-19 patients. Our study is probably underpowered to determine the relationship between IPA and outcomes, or the relationship between antifungal treatment and outcomes. However, previous studies have shown a negative impact on outcome in IAPA and CAPA patients [7, 12].
Strengths and limitations
To the best of our knowledge, our study is the first large multicenter cohort to compare the incidence of IPA between COVID-19 and influenza patients. Further, competing risk analysis, and cause-specific Cox models were used to adjust for potential confounders. However, several limitations should be acknowledged. First, the study was retrospective and there was no systematic screening for IPA, which might have underestimated the overall IPA incidence. Nevertheless, physicians prospectively identified IPA, based on clinical suspicion; and a recent taskforce recommended against routine screening for IPA in critically ill patients [23]. Second, no information was available on bronchoscopy macroscopic data, which may have also led to underestimating the incidence of IPA, because Aspergillus tracheobronchitis could not be diagnosed. Third, no information could be provided on galactomannan in some study patients, which might have also reduced the incidence of probable IPA. Fourth, the evaluation of the two diseases was not done simultaneously because of the absence of influenza during COVID-19 pandemic. Fifth, this study was conducted in Europe, mostly in France, and the results may not be generalizable to other parts of the world. Finally, we chose to use Blot definition for putative IPA, because this definition was validated using histological data in a large international study. However, galactomannan is not considered by this definition and some patients could have IPA with no Aspergillus identified in respiratory specimen. This might have also resulted in underestimating the overall incidence of IPA. However, Verweij definition was also used as a secondary outcome and although the overall IPA incidence was slightly higher in the two groups, IPA incidence was still significantly lower in COVID-19 than in influenza patients.
Conclusions
Overall, the incidence of IPA was low in study patients. Further, putative IPA incidence was lower in SARS-COV-2 pneumonia than in influenza pneumonia patients. Our study was performed at the beginning of COVID-19 pandemic, it would be interesting to determine how IPA incidence has evolved, especially with routine use of corticosteroids in COVID-19 patients. Screening for IPA should be performed, based on recent recommendations, in patients with clinical deterioration or absence of improvement.
Availability of data and materials
All data needed to evaluate the conclusions in this article are present and tabulated in the main text or the appendix. This article is the result of an original retrospective cohort. For individual de-identified raw data that underlie the results reported in this article, please contact the corresponding author.
Abbreviations
- ARDS:
-
Acute respiratory distress syndrome
- CAPA:
-
COVID-19-associated IPA
- CI:
-
Confidence interval
- COPD:
-
Chronic obstructive pulmonary disease
- COVID:
-
Coronavirus disease
- HR:
-
Hazard ratio
- ICU:
-
Intensive care unit
- IPA:
-
Invasive pulmonary aspergillosis
- IAPA:
-
Influenza-associated IPA
- MV:
-
Mechanical ventilation
- SAPS II:
-
Simplified acute physiology score II
- SARS-CoV-2:
-
Severe acute respiratory syndrome coronavirus 2
- SOFA:
-
Sequential organ failure assessment
References
Delsuc C, Cottereau A, Frealle E, Bienvenu A-L, Dessein R, Jarraud S, et al. Putative invasive pulmonary aspergillosis in critically ill patients with chronic obstructive pulmonary disease: a matched cohort study. Crit Care. 2015;19.
Contou D, Dorison M, Rosman J, Schlemmer F, Gibelin A, Foulet F, et al. Aspergillus-positive lower respiratory tract samples in patients with the acute respiratory distress syndrome: a 10-year retrospective study. Ann Intensive Care. 2016;6:52.
Levesque E, Ait-Ammar N, Dudau D, Clavieras N, Feray C, Foulet F, et al. Invasive pulmonary aspergillosis in cirrhotic patients: analysis of a 10-year clinical experience. Ann Intensive Care. 2019;9:31.
Gustot T, Maillart E, Bocci M, Surin R, Trépo E, Degré D, et al. Invasive aspergillosis in patients with severe alcoholic hepatitis. J Hepatol. 2014;60:267–74.
Pardo E, Lemiale V, Mokart D, Stoclin A, Moreau A-S, Kerhuel L, et al. Invasive pulmonary aspergillosis in critically ill patients with hematological malignancies. Intensive Care Med. 2019;45:1732–41.
Taccone FS, Van den Abeele A-M, Bulpa P, Misset B, Meersseman W, Cardoso T, et al. Epidemiology of invasive aspergillosis in critically ill patients: clinical presentation, underlying conditions, and outcomes. Crit Care. 2015;19:7.
Schauwvlieghe AFAD, Rijnders BJA, Philips N, Verwijs R, Vanderbeke L, Van Tienen C, et al. Invasive aspergillosis in patients admitted to the intensive care unit with severe influenza: a retrospective cohort study. Lancet Respir Med. 2018;6:782–92.
Koehler P, Bassetti M, Chakrabarti A, Chen SCA, Colombo AL, Hoenigl M, et al. Defining and managing COVID-19-associated pulmonary aspergillosis: the 2020 ECMM/ISHAM consensus criteria for research and clinical guidance. Lancet Infect Dis. 2021;21:e149–62.
Dellière S, Dudoignon E, Fodil S, Voicu S, Collet M, Oillic P-A, et al. Risk factors associated with COVID-19-associated pulmonary aspergillosis in ICU patients: a French multicentric retrospective cohort. Clin Microbiol Infect. 2020; 27. Available from: http://www.ncbi.nlm.nih.gov/pubmed/33316401
Fekkar A, Lampros A, Mayaux J, Poignon C, Demeret S, Constantin J-M, et al. Occurrence of invasive pulmonary fungal infections in patients with severe COVID-19 admitted to the ICU. Am J Respir Crit Care Med. 2021;203:307–17. https://doi.org/10.1164/rccm.202009-3400OC.
Prattes J, Wauters J, Giacobbe DR, Salmanton-García J, Maertens J, Bourgeois M, et al. Risk factors and outcome of pulmonary aspergillosis in critically ill coronavirus disease 2019 patients-a multinational observational study by the European Confederation of Medical Mycology. Clin Microbiol Infect. 2021. http://www.ncbi.nlm.nih.gov/pubmed/34454093
Prattes J, Wauters J, Giacobbe DR, Lagrou K, Hoenigl M, ECMM-CAPA Study Group. Diagnosis and treatment of COVID-19 associated pulmonary apergillosis in critically ill patients: results from a European confederation of medical mycology registry. Intensive Care Med. 2021. http://www.ncbi.nlm.nih.gov/pubmed/34269853
Bartoletti M, Pascale R, Cricca M, Rinaldi M, Maccaro A, Bussini L, et al. Epidemiology of invasive pulmonary aspergillosis among COVID-19 intubated patients: a prospective study. Clin Infect Dis. 2020. http://www.ncbi.nlm.nih.gov/pubmed/32719848
Permpalung N, Chiang TP-Y, Massie AB, Zhang SX, Avery RK, Nematollahi S, et al. COVID-19 associated pulmonary aspergillosis in mechanically ventilated patients. Clin Infect Dis. 2021. http://www.ncbi.nlm.nih.gov/pubmed/33693551
Salmanton-García J, Sprute R, Stemler J, Bartoletti M, Dupont D, Valerio M, et al. COVID-19-associated pulmonary aspergillosis, March-August 2020. Emerg Infect Dis. 2021;27:1077–86.
Gangneux jp, Dannaoui E FA. Fungal infections in mechanically ventilated COVID-19 patients in the ICU during the 1 first wave: the French multicenter MYCOVID study. Lancet Respir Med. 2021 (in press).
Janssen NAF, Nyga R, Vanderbeke L, Jacobs C, Ergün M, Buil JB, et al. Multinational observational cohort study of COVID-19-associated pulmonary aspergillosis1. Emerg Infect Dis.2021;27:2892–8.
Razazi K, Arrestier R, Haudebourg AF, Benelli B, Carteaux G, Decousser J, et al. Risks of ventilator-associated pneumonia and invasive pulmonary aspergillosis in patients with viral acute respiratory distress syndrome related or not to Coronavirus 19 disease. Crit Care [Internet]. BioMed Central; 2020 [cited 2021 Jan 22];24:699. https://ccforum.biomedcentral.com/articles/https://doi.org/10.1186/s13054-020-03417-0
Verweij PE, Rijnders BJA, Brüggemann RJM, Azoulay E, Bassetti M, Blot S, et al. Review of influenza-associated pulmonary aspergillosis in ICU patients and proposal for a case definition: an expert opinion. Intensive Care Med. 2020;46:1524–35.
Rouzé A, Martin-Loeches I, Povoa P, Makris D, Artigas A, Bouchereau M, et al. Relationship between SARS-CoV-2 infection and the incidence of ventilator-associated lower respiratory tract infections: a European multicenter cohort study. Intensive Care Med. 2021. http://www.ncbi.nlm.nih.gov/pubmed/33388794
Blot SI, Taccone FS, Van den Abeele A-M, Bulpa P, Meersseman W, Brusselaers N, et al. A clinical algorithm to diagnose invasive pulmonary aspergillosis in critically ill patients. Am J Respir Crit Care Med. 2012;186:56–64.
Prentice RL, Kalbfleisch JD, Peterson AV, Flournoy N, Farewell VT, Breslow NE. The analysis of failure times in the presence of competing risks. Biometrics. 1978;34:541–54.
Verweij PE, Brüggemann RJM, Azoulay E, Bassetti M, Blot S, Buil JB, et al. Taskforce report on the diagnosis and clinical management of COVID-19 associated pulmonary aspergillosis. Intensive Care Med. 2021;47:819–34.
Therneau T-M, Grambsch P-M. Modeling survival data: extending the cox model. New-York (2000).
Coste A, Frérou A, Raute A, Couturaud F, Morin J, Egreteau P-Y, et al. The extent of aspergillosis in critically ill patients with severe influenza pneumonia: a multicenter cohort study. Crit Care Med. 2021;49:934–42.
van de Veerdonk FL, Brüggemann RJM, Vos S, De Hertogh G, Wauters J, Reijers MHE, et al. COVID-19-associated Aspergillus tracheobronchitis: the interplay between viral tropism, host defence, and fungal invasion. Lancet Respir Med. 2021; 9:795–802.
Ackermann M, Verleden SE, Kuehnel M, Haverich A, Welte T, Laenger F, et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid-19. N Engl J Med. 2020; 383:120–8.
Acknowledgements
In addition to the authors, the coVAPid study group includes the following collaborators (information to include for individual PubMed record): Mathilde Bouchereau, CHU de Lille, Centre de Réanimation, F-59000 Lille, France. Boualem Sendid, INSERM U1285, CNRS UMR 8576, Glycobiology in Fungal Pathogenesis and Clinical Applications, Université de Lille ; and Pôle de Biologie-Pathologie-Génétique, Institut de Microbiologie, Service de Parasitologie Mycologie, CHU Lille, F-59000 Lille, France. Sean Boyd, Department of Intensive Care Medicine, Multidisciplinary Intensive Care Research Organization (MICRO), St. James's Hospital, Dublin, Ireland. Luis Coelho, Polyvalent Intensive Care Unit, Hospital de São Francisco Xavier, CHLO, Lisbon, Portugal. NOVA Medical School, CHRC, New University of Lisbon, Portugal. Julien Maizel, Service de médecine intensive réanimation, CHU Amiens Picardie, 80000 Amiens, France. Pierre Cuchet, Department of Medical Intensive Care, Caen University Hospital, F-14000 Caen, France. Wafa Zarrougui, Service de réanimation polyvalente, Centre hospitalier de Valenciennes, Valenciennes, France. Déborah Boyer, Medical Intensive Care Unit, Rouen University Hospital, 76000 Rouen, France. Jean-Pierre Quenot, Department of Intensive Care, François Mitterrand University Hospital, Dijon, France. Mehdi Imouloudene, Service de réanimation et de soins intensifs, Centre hospitalier de Douai, Douai, France. Marc Pineton de Chambrun, Service de Médecine Intensive Réanimation, Institut de Cardiologie, Groupe Hospitalier Pitié-Salpêtrière, Assistance Publique – Hôpitaux de Paris, Paris Cedex 13, France. Thierry Van Der Linden, Service de médecine intensive réanimation, Hôpital Saint Philibert GHICL, Université catholique, Lille, France. François Arrive, CHU de Poitiers, Médecine Intensive Réanimation, CIC 1402 ALIVE, Université de Poitiers, Poitiers, France. Sebastian Voicu, Department of Medical and Toxicological Critical Care, Lariboisière Hospital, INSERM UMRS-1144, Paris University, Paris, France. Elie Azoulay, Service de médecine intensive réanimation, Hôpital Saint-Louis, 75010 Paris, France. Edgard Moglia, Critical Care Department, Hospital Universitari Parc Taulí, Sabadell, Spain Frédéric Pene, Medical Intensive Care Unit, Cochin Hospital, Assistance Publique – Hôpitaux de Paris, Paris, France. Catia Cilloniz, Department of Pulmonology, Hospital Clinic of Barcelona, University of Barcelona, IDIBAPS, CIBERES, Barcelona, Spain. Didier Thevenin, Service de réanimation polyvalente, Centre Hospitalier de Lens, Lens, France. Charlotte Larrat, Service de Médecine Intensive Réanimation, CHU de Tours, Hôpital Bretonneau, 37044 Tours Cedex 9, France. Laurent Argaud, Service de Médecine Intensive—Réanimation, Hôpital Edouard Herriot, Hospices Civils de Lyon, 69437 Lyon Cedex 03, France. Bertrand Guidet, Service de Médecine Intensive Réanimation, Hôpital Saint-Antoine, Assistance Publique-Hôpitaux de Paris, 75012 Paris, France. Matthieu Turpin, Sorbonne Université, Assistance Publique-Hôpitaux de Paris, Service de Médecine Intensive Réanimation, Hôpital Tenon, Paris, France. Damien Contou, Service de réanimation polyvalente, CH Victor Dupouy, Argenteuil, France. Alexandra Beurton, Service de Médecine Intensive-Réanimation et Pneumologie, Assistance Publique-Hôpitaux de Paris, Hôpital Pitié Salpêtrière, France. Julien Demiselle, Département de Médecine Intensive Réanimation, CHU d'Angers, 49933 Angers Cedex 9, France. David Meguerditchian, Service de médecine intensive réanimation, CHU de Bordeaux, F-33000 Bordeaux, France. Keyvan Razazi, Assistance Publique-Hôpitaux de Paris, Hôpitaux Universitaires Henri-Mondor, Service de Médecine Intensive Réanimation ; Université Paris Est Créteil, CARMAS ; INSERM U955, Institut Mondor de recherche Biomédicale ; F-94010 Créteil, France. Romain Arrestier, Assistance Publique-Hôpitaux de Paris, Hôpitaux Universitaires Henri-Mondor, Service de Médecine Intensive Réanimation ; Université Paris Est Créteil, CARMAS ; INSERM U955, Institut Mondor de recherche Biomédicale ; F-94010 Créteil, France. Vassiliki Tsolaki, Intensive Care Unit, University Hospital of Larissa, University of Thessaly, Biopolis Larissa, 41110 Greece. Mehdi Marzouk, Intensive Care Unit, Hôpital de Béthune, 62408 Béthune, France. Guillaume Brunin, Service de réanimation, Hôpital Duchenne, 62200 Boulogne-sur-Mer, France. Nicolas Weiss, Sorbonne Université, Assistance Publique-Hôpitaux de Paris, Sorbonne Université, Hôpital de la Pitié-Salpêtrière, Département de Neurologie, Unité de Médecine Intensive Réanimation Neurologique, Paris, France. Luis Morales, Intensive Care Unit, Hospital Universitari Sagrat Cor, Barcelona, Spain.
Funding
This study was supported in part by a grant from the French government through the « Programme Investissement d’Avenir» (I-SITE ULNE) managed by the Agence Nationale de la Recherche (coVAPid project). Prof. Ignacio Martin-Loeches has been supported by SFI (Science Foundation Ireland), Grant Number 20/COV/0038. The funders of the study had no role in the study design, data collection, analysis, or interpretation, writing of the report, or decision to submit for publication.
Author information
Authors and Affiliations
Consortia
Contributions
AR, EL, IML, PP, AD, JL, and SN conceptualized and designed the study. All authors acquired the data, drafted or critically revised the manuscript for important intellectual content, and gave final approval of the submitted version. AR, EL, IML, PP, RN, AT, AD, JL, and SN analyzed and interpreted the data. SN was guarantor of the paper. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The Ethics Committee and Institutional Review Boards approved the study protocol (Comité de Protection des Personnes Ouest VI; approved by April 14, 2020; registration number RIPH:20.04.09.60039) as minimal-risk research using data collected for routine clinical practice and waived the requirement for informed consent.
Consent for publication
Not applicable.
Competing interests
AR received personal fees from Maat Pharma, IML received personal fees from MSD, and Gilead. AA received personal fees from Lilly Foundation, and grants from Grifols and Fisher & Paykel. CEL received personal fees from Bayer, Merck, Aerogen, Biomérieux, ThermoFisher Brahms, and Carmat. SN received personal fees from MSD, Bio-Rad, BioMérieux, Gilead, Fisher and Paykel, and Pfizer. All other authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1
. Further details on methods and results.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Rouzé, A., Lemaitre, E., Martin-Loeches, I. et al. Invasive pulmonary aspergillosis among intubated patients with SARS-CoV-2 or influenza pneumonia: a European multicenter comparative cohort study. Crit Care 26, 11 (2022). https://doi.org/10.1186/s13054-021-03874-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13054-021-03874-1