Abstract
There is ongoing demographic ageing and increasing longevity of the population, with previously devastating and often-fatal diseases now transformed into chronic conditions. This is turning multi-morbidity into a major challenge in the world of critical care. After many years of research and innovation, mainly in geriatric care, the concept of multi-morbidity now requires fine-tuning to support decision-making for patients along their whole trajectory in healthcare, including in the intensive care unit (ICU). This article will discuss current challenges and present approaches to adapt critical care services to the needs of these patients.
Similar content being viewed by others
Introduction
Multi-morbidity is defined as the co-occurrence of multiple, usually two or more, chronic conditions in an individual [1]. There have been several attempts to establish criteria for the conditions which qualify for this count. These initiatives range from refining the set of eligible conditions and including the number of affected body systems to considering patterns of recurrences and deterioration [2, 5]. Pearson-Stuttard et al. [6] have suggested multi-morbidity metrics based on the onset and sequence of diseases and the clustering of conditions. However, none of these new approaches have been universally accepted so far.
The combination of certain diseases can trigger super-additive interactions [7] resulting in an enhanced effect on functional abilities, quality of life as well as life expectancy and, eventually, may create complex health needs [8, 9]. This especially affects old individuals with an age-related decline in organ function and increase of vulnerability to stress even in the absence of multi-morbidity [10]. Since advanced age is the most important risk factor for multi-morbidity, the prevalence of multi-morbidity is close to 90% in patients aged 85 years or older [11]. Previously devastating and often-fatal diseases have been transformed by modern medicine into chronic conditions. Since the longevity of the population is also increasing, these developments are turning multi-morbidity into a major challenge in the world of critical care [12]. Even in intensive care units (ICUs) designed to manage single-organ conditions, such as cardiac/coronary ICU's, multi-morbidity has become highly prevalent and an important contributor to outcome prediction [13].
After many years of research and innovation, mainly in primary and geriatric care, the concept of multi-morbidity now requires fine-tuning to support decision-making for patients along their whole trajectory in healthcare. Critical care medicine has a particular need for rapid improvement and development since it is mostly organ/system-centred, with survival being the main outcome measure. The holistic view needed for multi-morbid elderly patients, and their individual requirements, still remains a work in progress [14]. During the COVID-19 pandemic, the National Institute for Health and Care Excellence in the UK had to recommend a more holistic approach beyond single scores for organ failure or frailty for deciding about admission to critical care. In particular, it advised that comorbidities and underlying health conditions should be considered when assessing the potential benefit of critical care for the individual patient [15]. This article will discuss challenges and present approaches to integrate multi-morbidity into the decision-making processes in critical care.
What does multi-morbidity mean in the critically ill patient?
Multi-morbidity is heterogeneous in phenotype and outcome. There still is no universal concept that describes the burden and impact on individual patients and which would thereby provide useful and actionable information for critical care patients [1]. Merely counting the number of chronic conditions appears too simplistic in this regard. For example, an individual with well-controlled hypertension and osteoporosis is currently considered to be multi-morbid (i.e. by having two chronic conditions), as is someone with end stage chronic kidney disease and chronic obstructive lung disease requiring home oxygen. Clearly, these two situations are not equivalent in terms of prognosis or level of care required. Moreover, some conditions prevalent in older patients, such as sarcopenia or chronic pain, do not influence the early treatment of critical illnesses, although their consequences, e.g. difficult weaning, may necessitate later consideration in their care pathway. Recent findings from the second Very Old Intensive Care Patients study (VIP 2) [16] illustrated the problem of distinguishing chronic conditions, which affect the outcome of critical diseases, from those that do not (Fig. 1A) [16]. However, a more detailed analysis of patient trajectories in ICU suggests that the number of comorbidities still has a role for predicting the clinical course in subgroups of patients (Fig. 1B) [17].
Multimorbidity and ICU outcome in 2103 patients aged 80 years or older from the VIP2 study cohort [16] who were admitted to ICU for more than 24 h and did not have limitations of life-sustaining treatment. Among those, 1455 patients received invasive ventilation, vasopressors or renal replacement therapy. (a) Box plot of the number of chronic comorbidities for ICU survivors (n= 1805) and nonsurvivors (n = 298). Logistic regression did not show a significant association with ICU outcome. (b) Multi-state modeling with multimorbidity, frailty and baseline SOFA score as covariates [17]. The panel depicts transitions and significant hazard ratios (95% confidence intervals) determined for each point of increase of the number of comorbidities (nCM), clinical frailty scale (CFS) and SOFA score. The number of comorbidities is associated with transition from low-intensity care to death in ICU indicating a role of chronic organ impairments for outcome at that stage.
To reflect the quantitative role of individual conditions on the overall impact of multi-morbidity, Min et al. weighed the contribution of each condition with its specific survival rate [18]. This new index outperformed biomarkers of acute physiology for predicting mortality in 440 000 older ICU patients. Moreover, a registry study of 230 000 ICU patients in Denmark suggested that detailed data from the patient's medical history can improve mortality prediction which, however, performs best when combined with characteristics of the acute physiology of organ failure [19]. In a systematic review, Stirland et al. investigated 35 different multi-morbidity indices [20]. Only a minority of these indices passed the authors' threshold for usefulness with respect to the prediction of hospital admissions or mortality. This leaves the essential topic of conceptualizing multi-morbidity for clinical practice open for further research.
Phenotyping multi-morbidity by clustering diseases
Although multi-morbidity is heterogeneous, the co-occurrence of diseases is mostly non-random and organised in clusters (Table 1). This is obvious for conditions that share the same pathophysiology, such as cardiovascular disorders. For other clusters, the knowledge about the joint pathogenesis is incomplete, such as in cardio-renal syndromes [21]. Almagro et al. recently described gender-specific clusters with neurological and osteoarticular conditions being more frequent in women, while respiratory disorders dominated in men [22]. New clusters can emerge due to exposure to new treatments, e.g. immune checkpoint inhibitors.
Data-driven research demonstrated that different multi-morbidity clusters are associated with different outcomes in critical care [23]. In addition to predictive modelling, stratifying patients by multi-morbidity patterns would enable targeted interventions in a similar way as single conditions benefit from a more precise understanding of disease phenotypes [24]. In individuals associated with an arteriosclerosis cluster, for example, early adjustment of haemodynamic management might be necessary to protect organs that are at an increased risk for malperfusion, but without detectable dysfunction on presentation. Also important are safety issues which arise when organ-specific therapies can cause collateral damage in other organs with chronic impairments [12]. Eventually, this new approach may give rise to "cluster medicine" which considers multi-morbidity as a set of mostly predictable clusters due to common genetic or environmental pathways [25]. This way of thinking could also change the diagnostic process—a condition that has not yet been diagnosed, but is known to be part of a cluster identified in an individual, is considered present until proven otherwise. For example, critically ill patients with diabetes and long-standing hypertension may need urgent investigation for cardiac conditions to guide fluid resuscitation.
Critical care is embedded in a data-rich environment providing a continuous flow of clinical data. This necessitates the rapid detection of distinct phenotypes of acute diseases, notably in those with substantial heterogeneity. This heterogeneity is partly caused by the presence of co-morbidities [26]. In this context, "cluster medicine", instead of being a nebulous concept, may be seen as an implementation of precision medicine for multi-morbid individuals in ICU. If sufficiently informative data about pre-existing conditions and their association with specific clusters become available, this paradigm could enable prognostication and management of these patients with a high degree of precision.
Managing multi-morbidity
Management of multi-morbidity is difficult. A recent meta-analysis of various intervention strategies in primary care found only small differences in clinical outcome. Critical illness adds another layer of complexity. Multi-morbidity and the often-associated polypharmacy alter the clinical presentation of many critical conditions. These problems may delay their detection, e.g. sepsis in patients receiving beta-blockers and paracetamol, and interfere with interventions, e.g. fluid resuscitation in congestive heart failure and chronic kidney disease. Of note, the dynamics of some multi-morbidity patterns are contrary to logical expectations, which are based on assuming independence of the underlying conditions. This is shown by the lower mortality in obese individuals with sepsis compared to non-obese patients [27].
Since multi-morbid patients have been frequently excluded from clinical trials, there is a paucity of evidence and guidelines to manage organ dysfunction in these patients [28]. Importantly, applying recommendations devised for the treatment of single conditions could be confusing or even detrimental in this setting [29]. In the absence of a robust framework for evidence-based medicine, being vigilant and implementing a comprehensive, e.g. geriatric, model of care [30] are currently the most pragmatic ways of dealing with the uncertainties of managing multi-morbidity in ICU patients. The concept of comprehensive geriatric assessment can provide standardized screening and assessment tools for chronic conditions and disabilities [31]. Thereafter, a contribution by geriatricians to decisions about objectives and suitable levels of critical care can further support a holistic view and prevent inappropriate interventions. However, the specific approach to these challenges and, eventually, the outcome quality depend on the structure and workflows of the healthcare organisation [32].
The decision-making about tailoring critical care for multi-morbid individuals, especially in the very old, require consideration and weighting of patient-centred outcome measures such as quality of life vs burden of treatment [33]. Short- and long-term goals should be determined by the expectations of the individual patient, which may differ from recommendations for managing single conditions in younger people. However, a recent review was unable to identify methodologically robust studies about understanding personal preferences of multi-morbid patients presenting with acute diseases [34]. Thus, a precise adjustment of critical care to the personal needs of these individuals still remains elusive.
Multi-morbidity and prognostication
Pre-admission characteristics, notably past trajectories of overall health, are known to be at least as important for predicting long-term outcome of critical care as the severity of the acute illness. Frailty and functional disabilities [35] are regarded as both long-term consequences of multi-morbidity [36, 37] and predictors for post-ICU outcome including functional status [16, 38]. In fact, frailty, as a measure of reduced resilience to physical stress, was discussed as the link between advanced multi-morbidity and increased mortality [39]. During the COVID-19 pandemic, multi-morbidity, frailty as well as the severity of the acute condition were all strong predictors of in-hospital death [40]. A recent study showed the additive role of functional disabilities for mortality in very old multi-morbid individuals [41]. Although multi-morbidity, frailty and functional disabilities overlap in many older patients [42, 43], there are individuals with multi-morbidity who cannot be classified as frail or disabled. This indicates the existence of distinct patterns of vulnerability among multi-morbid patients, which may benefit from new and different treatment approaches in critical care [8]. Importantly, multi-morbidity patterns and outcome are influenced by socioeconomic and ethnic factors which was highlighted by the COVID-19 pandemic [44].
Conclusions
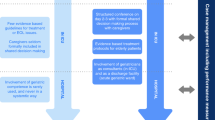
What needs to be done to provide tailored and patient-centred critical care to multi-morbid patients? (Fig. 2). Firstly, multi-morbidity requires universal recognition in healthcare institutions historically structured to treat single conditions. Including multi-morbidity, frailty and functional status into the pre-operative assessment for post-operative prognostication and care planning was already suggested a decade ago [45]. Secondly, the (cluster) analysis of data obtained in realistic scenarios will help to develop quantitative, individually precise and, thus, clinically useful concepts of multi-morbidity. In combination with biomarkers for organ failure, that approach can give rise to composite risk prediction scores. The 'where' and 'when' of interventions should be defined by a more granular analysis of patients' trajectories in critical care. However, we have to pay attention to geographic and cultural characteristics of medical care [46]. Thirdly, we should deal with medical uncertainties with time-limited trials, where multi-morbid patients are admitted to ICU with patient-centred goals and clearly defined limitations concerning treatment escalation to enable the initiation of end of life care if necessary [47]. This framework also provides the opportunity to obtain longitudinal data, i.e. time series of observations, for a more precise predictive modelling [48]. Fourthly, long-term and patient-centred outcome crucially depends on post-ICU care, which should be planned and managed in a multi-disciplinary way, involving geriatricians as well as caregivers in the community [30]. Although much work still needs to be done, critical care can be better prepared for the coming wave of multi-morbid very old intensive care patients by tackling these issues [49].
Availability of data and materials
This is a viewpoint paper with no study data.
References
The Academy of Medical Sciences. Multi-morbidity: a priority for global health research. April 2018. https://acmedsci.ac.uk/filedownload/82222577
Aubert CE, Schnipper JL, Roumet M, Marques-Vidal P, Stirnemann J, Auerbach AD, Zimlichman E, Kripalani S, Vasilevskis EE, Robinson E, Fletcher GS, Aujesky D, Limacher A, Donzé J. Best Definitions of multi-morbidity to identify patients with high health care resource utilization. Mayo Clin Proc Innov Qual Outcomes. 2020;4:40–9.
Hafezparast N, Turner EB, Dunbar-Rees R, Vodden A, Dodhia H, Reynolds B, Reichwein B, Ashworth M. Adapting the defnition of multi-morbidity. BMC Fam Pract. 2021;22:124.
St Sauver JL, Chamberlain AM, Bobo WV, Boyd CM, Finney Rutten LJ, Jacobson DJ, McGree ME, Grossardt BR, Rocca WA. Implementing the US Department of Health and Human Services definition of multi-morbidity. BMJ Open. 2021;11:e042870.
Lee YAJ, Xie Y, Lee PSS, Lee ES. Comparing the prevalence of multi-morbidity using different operational definitions in primary care in Singapore based on a cross-sectional study using retrospective, large administrative data. BMJ Open. 2020;10:e039440.
Pearson-Stuttard J, Ezzati M, Gregg EW. Multi-morbidity-a defining challenge for health systems. Lancet Public Health. 2019;4:e599–600.
Mujica-Mota RE, Roberts M, Abel G, Elliott M, Lyratzopoulos G, Roland M, Campbell J. Common patterns of morbidity and multi-morbidity and their impact on health-related quality of life: evidence from a national survey. Qual Life Res. 2015;24(4):909–18. https://doi.org/10.1007/s11136-014-0820-7.
Yarnall AJ, Sayer AA, Clegg A, Rockwood K, Parker S, Hindle JV. New horizons in multi-morbidity in older adults. Age Ageing. 2017;46(6):882–8. https://doi.org/10.1093/ageing/afx150.
Borrie M, Cooper T, Basu M, Kay K, Prorok JC, Seitz D. Ontario geriatric specialist physician resources 2018. Can Geriatr J. 2020;23(3):219–27. https://doi.org/10.5770/cgj.23.448.
Calderón-Larrañaga A, Vetrano DL, Ferrucci L, Mercer SW, Marengoni A, Onder G, Eriksdotter M, Fratiglioni L. Multi-morbidity and functional impairment-bidirectional interplay, synergistic effects and common pathways. J Intern Med. 2019;285:255–71.
Nguyen H, Manolova G, Daskalopoulou C, Vitoratou S, Prince M, Prina AM. Prevalence of multi-morbidity in community settings: a systematic review and meta-analysis of observational studies. J Comorb. 2019. https://doi.org/10.1177/2235042X19870934.
Damluji AA, Forman DE, van Diepen S, Alexander KP, Page RL, Hummel SL, Menon V, Katz JN, Albert NM, Afilalo J, Cohen MG. American Heart Association Council on Clinical Cardiology and Council on Cardiovascular and Stroke Nursing. Older Adults in the Cardiac Intensive Care Unit: Factoring Geriatric Syndromes in the Management, Prognosis, and Process of Care: A Scientific Statement From the American Heart Association. Circulation. 2020;141(2):e6–32. https://doi.org/10.1161/CIR.0000000000000741.
Miller PE, Thomas A, Breen TJ, Chouairi F, Kunitomo Y, Aslam F, Damluji AA, Anavekar NS, Murphy JG, van Diepen S, Barsness GW, Brennan J, Jentzer J. Prevalence of noncardiac multi-morbidity in patients admitted to two cardiac intensive care units and their association with mortality. Am J Med. 2021;134(5):653–6615. https://doi.org/10.1016/j.amjmed.2020.09.035.
Haines KJ, Hibbert E, McPeake J, Anderson BJ, Bienvenu OJ, Andrews A, Brummel NE, Ferrante LE, Hopkins RO, Hough CL, Jackson J, Mikkelsen ME, Leggett N, Montgomery-Yates A, Needham DM, Sevin CM, Skidmore B, Still M, van Smeden M, Collins GS, Harhay MO. Prediction models for physical, cognitive, and mental health impairments after critical illness: a systematic review and critical appraisal. Crit Care Med. 2020;48(12):1871–80.
https://www.nice.org.uk/news/article/nice-updates-rapid-covid-19-guideline-on-critical-care
Guidet B, de Lange DW, Boumendil A, Leaver S, Watson X, Boulanger C, Szczeklik W, Artigas A, Morandi A, Andersen F, Zafeiridis T, Jung C, Moreno R, Walther S, Oeyen S, Schefold JC, Cecconi M, Marsh B, Joannidis M, Nalapko Y, Elhadi M, Fjølner J, Flaatten H. VIP2 study group The contribution of frailty, cognition, activity of daily life and comorbidities on outcome in acutely admitted patients over 80 years in European ICUs: the VIP2 study. Intensive Care Med. 2020;46(1):57–69.
Jackson CH. Multi-state models for panel data: the msm package for R. J Statist Software. 2011;38:1–28.
Min H, Avramovic S, Wojtusiak J, Khosla R, Fletcher RD, Alemi F, Kheirbek R. A Comprehensive multi-morbidity index for predicting mortality in intensive care unit patients. J Palliat Med. 2017;20(1):35–41. https://doi.org/10.1089/jpm.2015.0392.
Nielsen AB, Thorsen-Meyer H-C, Belling K, Nielsen AP, Thomas CE, Chmura PJ, Lademann M, Moseley PL, Heimann M, Dybdahl L, Spangsege L, Hulsen P, Perner A, Brunak S. Survival prediction in intensive-care units based on aggregation of long-term disease history and acute physiology: a retrospective study of the Danish National Patient Registry and electronic patient records. Lancet Digit Health. 2019;1(2):e78–89. https://doi.org/10.1016/S2589-7500(19)30024-X.
Stirland LE, González-Saavedra L, Mullin DS, Ritchie CW, Muniz-Terrera G, Russ TC. Measuring multi-morbidity beyond counting diseases: systematic review of community and population studies and guide to index choice. BMJ. 2020;368:m160. https://doi.org/10.1136/bmj.m160.
Canetta PA, Troost JP, Mahoney S, Kogon AJ, Carlozzi N, Bartosh SM, Cai Y, Davis TK, Fernandez H, Fornoni A, Gbadegesin RA, Herreshoff E, Mahan JD, Nachman PH, Selewski DT, Sethna CB, Srivastava T, Tuttle KR, Wang CS, Falk RJ, Gharavi AG, Gillespie BW, Greenbaum LA, Holzman LB, Kretzler M, Robinson BM, Smoyer WE, Guay-Woodford LM, Reeve B, Gipson DS, CureGN Consortium. Health-related quality of life in glomerular disease. Kidney Int. 2019;95(5):1209–24. https://doi.org/10.1016/j.kint.2018.12.018.
Almagro P, Ponce A, Komal S, de la Asunción Villaverde M, Castrillo C, Grau G, Simon L, de la Sierra A. Multi-morbidity gender patterns in hospitalized elderly patients. PLoS ONE. 2020;15(1):e0227252. https://doi.org/10.1371/journal.pone.0227252.
Zador Z, Landry A, Cusimano MD, Geifman N. Multi-morbidity states associated with higher mortality rates in organ dysfunction and sepsis: a data-driven analysis in critical care. Crit Care. 2019;23(1):247. https://doi.org/10.1186/s13054-019-2486-6.
Seymour CW, Kennedy JN, Wang S, Chang C-CH, Elliott CF, Xu Z, Berry S, Clermont G, Cooper G, Gomez H, Huang DT, Kellum JA, Mi Q, Opal SM, Talisa V, van der Poll T, Visweswaran S, Vodovotz Y, Weiss JC, Yealy DM, Yende S, Angus DC. Derivation, validation, and potential treatment implications of novel clinical phenotypes for sepsis. JAMA. 2019;321(20):2003–17. https://doi.org/10.1001/jama.2019.5791.
Whitty CJM, MacEwen C, Goddard A, Alderson D, Marshall M, Calderwood C, Atherton F, McBride M, Atherton J, Stokes-Lampard H, Reid W, Powis S, Marx C. Rising to the challenge of multi-morbidity. BMJ. 2020;368:l6964. https://doi.org/10.1136/bmj.l6964.
Vincent JL. The coming era of precision medicine for intensive care. Crit Care. 2017;21(Suppl 3):314.
Pepper DJ, Demirkale CY, Sun J, Rhee C, Fram D, Eichacker P, Klompas M, Suffredini AF, Kadri SS. Does obesity protect against death in sepsis? A retrospective cohort study of 55,038 adult patients. Crit Care Med. 2019;47(5):643–50. https://doi.org/10.1097/CCM.0000000000003692.
Forman DE, Maurer MS, Boyd C, Brindis R, Salive ME, Horne FM, Bell SP, Fulmer T, Reuben DB, Zieman S, Rich MW. Multi-morbidity in older adults with cardiovascular disease. J Am Coll Cardiol. 2018;71(19):2149–61. https://doi.org/10.1016/j.jacc.2018.03.022.
Damluji AA, Forman DE, van Diepen S, Alexander KP, Page RL, Hummel SL, Menon V, Katz JN, Albert NM, Afilalo J, Cohen MG. American Heart Association Council on Clinical Cardiology and Council on Cardiovascular and Stroke Nursing.Older Adults in the Cardiac Intensive Care Unit: Factoring Geriatric Syndromes in the Management, Prognosis, and Process of Care: A Scientific Statement From the American Heart Association. Circulation. 2020;141(2):e6–32. https://doi.org/10.1161/CIR.0000000000000741.
Ungar A, Rivasi G, Petrovic M, Schönenberger A, Martínez-Sellés M, Gasowski J, Bahat-Ozturk G, Bo M, Dallmaier D, Fumagalli S, Grodzicki T, Kotovskaya Y, Maggi S, Mattace-Raso F, Polidori MC, Rajkumar R, Strandberg T, Werner N, Benetos A, From the EuGMS Special Interest Group on Cardiovascular Medicine. Toward a geriatric approach to patients with advanced age and cardiovascular diseases: position statement of the EuGMS Special Interest Group on Cardiovascular Medicine. Eur Geriatr Med. 2020;11(1):179–84. https://doi.org/10.1007/s41999-019-00267-0.
Mooijaart SP, Nickel CH, Conroy SP, Lucke JA, van Tol LS, Olthof M, Blomaard LC, Buurman BM, Dundar ZD, de Groot B, Gasperini B, Heeren P, Karamercan MA, McNamara R, Mitchell A, van Oppen JD, Martin Sanchez FJ, Schoon Y, Singler K, Spode R, Skúldóttir S, Thorrsteindottir T, van der Velde M, Wallace J. A European research agenda for geriatric emergency medicine. Eur Geriatr Med. 2021;12:413–22.
Santana Baskar P, Cordato D, Wardman D, Bhaskar S. In-hospital acute stroke workflow in acute stroke—systems-based approaches. Acta Neurol Scand. 2021;143:111–20.
Kivelitz L, Schäfer J, Kanat M, Mohr J, Glattacker M, Voigt-Radloff S, Dirmaier J. Patient-centeredness in older adults with multi-morbidity: results of an online expert Delphi study. Gerontologist. 2021. https://doi.org/10.1093/geront/gnaa223.
McNelly AS, et al. Attitudes of multimorbid patients to surviving future acute illness and subsequent functional disability: a systematic review. MedRxiv. 2020. https://doi.org/10.1101/2020.06.03.20121293.
Beard JR, Jotheeswaran AT, Cesari M, de Carvalho IA. The structure and predictive value of intrinsic capacity in a longitudinal study of ageing. BMJ Open. 2019;9(11):e026119. https://doi.org/10.1136/bmjopen-2018-026119.
Boeckxstaens P, De Sutter An, Vaes B, Degryse J-M. Should we keep on measuring multi-morbidity? J Clin Epidemiol. 2016;71:113–4. https://doi.org/10.1016/j.jclinepi.2015.05.015.
Storeng SH, Vinjerui KH, Sund ER, Krokstad S. Associations between complex multi-morbidity, activities of daily living and mortality among older Norwegians. A prospective cohort study: the HUNT Study, Norway. BMC Geriatr. 2020;20(1):21. https://doi.org/10.1186/s12877-020-1425-3.
Flaatten H, De Lange DW, Morandi A, Andersen FH, Artigas A, Bertolini G, Boumendil A, Cecconi M, Christensen S, Faraldi L, Fjølner J, Jung C, Marsh B, Moreno R, Oeyen S, Öhman CA, Pinto BB, Soliman IW, Szczeklik W, Valentin A, Watson X, Zaferidis T, Guidet B, VIP1 Study Group. The impact of frailty on ICU and 30-day mortality and the level of care in very elderly patients (≥ 80 years). Intensive Care Med. 2017;43(12):1820–8.
Ferrante LE, Pisani MA, Murphy TE, Gahbauer EA, Leo-Summers LS, Gill TM. Functional trajectories among older persons before and after critical illness. JAMA Intern Med. 2015;175(4):523–9.
Covino M, Russo A, Salini S, De Matteis G, Simeoni B, Della Polla D, Sandroni C, Landi F, Gasbarrini A, Franceschi F. Frailty assessment in the emergency department for risk stratification of COVID-19 patients aged ≥80 years. J Am Med Dir Assoc. 2021;S1525–8610(21):00643–5.
Oude Voshaar RC, Jeuring HW, Borges MK, van den Brink RHS, Marijnissen RM, Hoogendijk EO, van Munster B, Aprahamian I. Course of frailty stratified by physical and mental multi-morbidity patterns: a 5-year follow-up of 92,640 participants of the LifeLines cohort study. BMC Med. 2021;19(1):29. https://doi.org/10.1186/s12916-021-01904-x.
Landi F, Liperoti R, Russo A, Capoluongo E, Barillaro C, Pahor M, Bernabei R, Onder G. Disability, more than multi-morbidity, was predictive of mortality among older persons aged 80 years and older. J Clin Epidemiol. 2010;63(7):752–9. https://doi.org/10.1016/j.jclinepi.2009.09.007.
Boeckxstaens P, Vaes B, Legrand D, Dalleur O, De Sutter An, Degryse J-M. The relationship of multi-morbidity with disability and frailty in the oldest patients: a cross-sectional analysis of three measures of multi-morbidity in the BELFRAIL cohort. Eur J Gen Pract. 2015;21(1):39–44. https://doi.org/10.3109/13814788.2014.914167.
St Sauver JL, Boyd CM, Grossardt BR, Bobo WV, Finney Rutten LJ, Roger VL, Ebbert JO, Therneau TM, Yawn BP, Rocca WA. Risk of developing multi-morbidity across all ages in an historical cohort study: differences by sex and ethnicity. BMJ Open. 2015;5:e006413.
Church S, Rogers E, Rockwood K, Theou O. A scoping review of the clinical frailty scale. BMC Geriatr. 2020;20(1):393. https://doi.org/10.1186/s12877-020-01801-7.
Robinson TN, Eiseman B, Wallace JI, Church SD, McFann KK, Pfister SM, Sharp TJ, Moss M. Redefining geriatric preoperative assessment using frailty, disability and co-morbidity. Ann Surg. 2009;250(3):449–55. https://doi.org/10.1097/SLA.0b013e3181b45598.
Shrime MG, Ferket BS, Scott DJ, Lee J, Barragan-Bradford D, Pollard T, Arabi YM, Al-Dorzi HM, Baron RM, Hunink MG, Celi LA, Lai PS. Time-limited trials of intensive care for critically Ill patients with cancer: how long is long enough? JAMA Oncol. 2016;2:76–83.
Futoma J, Simons M, Panch T, Doshi-Velez F, Celi LA. The myth of generalisability in clinical research and machine learning in health care. Lancet Digit Health. 2020;2(9):e489–92. https://doi.org/10.1016/S2589-7500(20)30186-2.
Beil M, Sviri S, Flaatten H, De Lange DW, Jung C, Szczeklik W, Leaver S, Rhodes A, Guidet B, van Heerden PV. On predictions in critical care: the individual prognostication fallacy in elderly patients. J Crit Care. 2021;61:34–8. https://doi.org/10.1016/j.jcrc.2020.10.006.
Acknowledgements
None.
Funding
None.
Author information
Authors and Affiliations
Contributions
All authors contributed to the conceptualization of the paper, drafted and reviewed the manuscript and take responsibility for the content. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
Not required as there are no study subjects.
Consent for publication
Not required as there are no study subjects.
Competing interests
None.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Beil, M., Flaatten, H., Guidet, B. et al. The management of multi-morbidity in elderly patients: Ready yet for precision medicine in intensive care?. Crit Care 25, 330 (2021). https://doi.org/10.1186/s13054-021-03750-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13054-021-03750-y