Abstract
Background
Uncertainty about the optimal respiratory support strategies in critically ill COVID-19 patients is widespread. While the risks and benefits of noninvasive techniques versus early invasive mechanical ventilation (IMV) are intensely debated, actual evidence is lacking. We sought to assess the risks and benefits of different respiratory support strategies, employed in intensive care units during the first months of the COVID-19 pandemic on intubation and intensive care unit (ICU) mortality rates.
Methods
Subanalysis of a prospective, multinational registry of critically ill COVID-19 patients. Patients were subclassified into standard oxygen therapy ≥10 L/min (SOT), high-flow oxygen therapy (HFNC), noninvasive positive-pressure ventilation (NIV), and early IMV, according to the respiratory support strategy employed at the day of admission to ICU. Propensity score matching was performed to ensure comparability between groups.
Results
Initially, 1421 patients were assessed for possible study inclusion. Of these, 351 patients (85 SOT, 87 HFNC, 87 NIV, and 92 IMV) remained eligible for full analysis after propensity score matching. 55% of patients initially receiving noninvasive respiratory support required IMV. The intubation rate was lower in patients initially ventilated with HFNC and NIV compared to those who received SOT (SOT: 64%, HFNC: 52%, NIV: 49%, p = 0.025). Compared to the other respiratory support strategies, NIV was associated with a higher overall ICU mortality (SOT: 18%, HFNC: 20%, NIV: 37%, IMV: 25%, p = 0.016).
Conclusion
In this cohort of critically ill patients with COVID-19, a trial of HFNC appeared to be the most balanced initial respiratory support strategy, given the reduced intubation rate and comparable ICU mortality rate. Nonetheless, considering the uncertainty and stress associated with the COVID-19 pandemic, SOT and early IMV represented safe initial respiratory support strategies. The presented findings, in agreement with classic ARDS literature, suggest that NIV should be avoided whenever possible due to the elevated ICU mortality risk.
Similar content being viewed by others
Background
Coronavirus disease 2019 (COVID-19) has generated a surge of critically ill patients who require invasive mechanical ventilation (IMV) overburdening intensive care units (ICU) worldwide.
Traditionally, the treatment of acute respiratory distress syndrome (ARDS) has focused mainly on IMV and its optimization [1]; nonetheless, in the last decade new approaches have been increasingly explored, primarily high-flow oxygen therapy by nasal cannula (HFNC) and noninvasive positive-pressure ventilation (NIV) [2, 3]. At the onset of the COVID-19 pandemic, most clinicians supported by the recommendations of international guidelines employed either standard oxygen therapy (SOT) or early IMV for the treatment of COVID-19-induced ARDS (CARDS) [4]. This choice was probably influenced by the numerous uncertainties regarding the new pathology, but also to avoid endangering hospital personnel by generating aerosols with HFNC and NIV. Nonetheless, in certain areas and centers, a lack of mechanical ventilators and adequately trained ICU staff forced clinicians to use noninvasive techniques to treat CARDS [5].
The high mortality rate associated with CARDS observed at the start to the pandemic has decreased over time [6, 7]. While many factors may explain this improvement, the decision to use invasive or noninvasive respiratory support remains one of the most controversial ones [8]. Expert opinions range widely. While some eminent authors urge for early intubation at the first signs of respiratory fatigue, to prevent patient self-inflicted lung injury (P-SILI) [9,10,11,12], others argue that all noninvasive options should be exhausted before proceeding to IMV [13,14,15,16,17,18]. Nevertheless, there is a surprising lack of evidence regarding the optimal respiratory support strategy.
The present study was designed in the context of the ubiquitous uncertainty surrounding respiratory support strategies in critically ill COVID-19 patients. This study consists of a subanalysis of the data collected prospectively in the RISC-19-ICU registry [19]. The main objective was to determine which respiratory support strategy employed during the first months of the COVID-19 pandemic was associated with a better overall prognosis. To reflect the early intubation trend followed during the first months of the pandemic, patients directly intubated on ICU admission but with matched severity characteristics to the noninvasively supported patients were also included in the analysis, constituting an independent respiratory support strategy.
Methods
This was a retrospective subanalysis of data from the prospective RISC-19-ICU registry, which contains a standardized dataset of all critically ill COVID-19 patients admitted to the collaborating centers during the ongoing pandemic.
The RISC-19-ICU registry was deemed exempt from the need for additional ethics approval and patient informed consent by the ethics committee of the canton of Zurich (KEK 2020-00322, ClinicalTrials.gov Identifier: NCT04357275). The present study complies with the tenets of the Declaration of Helsinki, the Guidelines on Good Clinical Practice (GCP-Directive) issued by the European Medicines Agency, as well as Swiss law and Swiss regulatory authority requirements. All collaborating centers have complied with all local legal and ethical requirements. As of October 1, 2020, 63 collaborating centers in 10 countries, were actively contributing to the RISC-19-ICU registry. For further specifications on the RISC-19-ICU registry structure and data collection, see Additional file 1: e-Appendix 1.
Inclusion and exclusion criteria
Patients were included in the present substudy if they required SOT (≥10 L/min [20]), HFNC, NIV, or IMV at the time point of admission to the ICU defined as day 0. Patients without a full ICU outcome data set, with SOT <10 L/min, or with a do-not-intubate order at day 0 were excluded. For the days ensuing ICU admission, the daily respiratory support therapy was defined as the main strategy used during the chart day.
Initial ventilation support group definitions
For study purposes, patients were categorized into four groups according to their maximal respiratory support at ICU admission (day 0), as follows: (1) SOT group: patients receiving SOT with an oxygen flow of ≥10 L/min (FiO2 was approximated based on the delivered oxygen flow as described by Farias et al. [21]); (2) HFNC group: patients receiving HFNC, defined as a device delivering humidified and heated oxygen at a flow rate above 30 L/min; (3) NIV group: patients receiving NIV, irrespective of interface, mode and ventilator type employed; and (4) IMV group: intubated patients receiving IMV.
Statistical analysis
Missing data handling is described in Additional file 1: e-Appendix 2. Comparisons of population characteristics were performed using the analysis of variance or Kruskal–Wallis test, as appropriate, and the Chi-squared test for categorical variables. Nearest neighbor matching with a propensity score caliper distance of 0.1 was employed to select IMV patients with ICU admission characteristics comparable to those of the patients in the SOT, HFNC and NIV groups. Patients having received IMV in another institutions ICU before admission to the RISC-19-ICU center were excluded from the matching process. To enable comparability between IMV and the noninvasive respiratory support strategies, Sequential Organ Failure Assessment (SOFA) and Simplified Acute Physiology II (SAPS II) scores were used without the mechanical ventilation and neurologic sub-scores for the matching process. An optimal quality match was defined as a standardized mean difference (SMD) ≤0.1 per matching variable between patients in the IMV group and the other groups (SOT, HFNC and NIV) [22].
Univariable Cox proportional hazard models coupled to the Kaplan–Meier estimator were employed to analyze the effects, represented by hazard ratios (HR), of the different respiratory support strategies on the incidence of intubation, ICU mortality and discharge from ICU. Multivariable adjusted HRs were calculated for every model independently by means of an iterative, step-wise, maximum likelihood optimizing algorithm, controlling for collinearity, interactions, and effect size variation in every iteration. The maximum number of covariates per model was chosen to ensure 1 to 10 events per covariate. Comparison of survival distributions among the various respiratory support strategies was approached by means of the log-rank test. Proportional hazard assumptions were assessed through inspection of Schoenfeld residuals.
Generalized linear regression model (GLM) analysis, considering all recorded baseline characteristics at ICU admission, was employed to determine the best predictive model for mortality in patients initially receiving HFNC and NIV and requiring delayed IMV. Multivariable GLM analysis was performed by means of an iterative, step-wise, maximum likelihood optimizing algorithm initially considering all variables with p<0.1 on the univariable analysis. First-order interaction terms between the predictor variables were tested for all models, and excluded if not improving the final model fit. For the final GLM model, a prognostic score and nomogram were generated, and receiver operating characteristics (ROC) analysis was employed alongside minimal Euclidean distance fitting to the (0, 1) point to determine the optimal cut-off value for the generated score. 95% confidence intervals (CI) and p values comparing the prognostic score to classic severity scores were generated by means of the bootstrap percentile method.
Statistical analysis was performed through a fully scripted data management pathway using the R environment for statistical computing version 3.6 .1. Due to the observational, prospective nature of this cohort study no power calculations were performed. A two-sided p <0.05 was considered statistically significant. Values are given as medians with interquartile ranges (IQR) or counts and percentages as appropriate.
Results
Baseline and matching
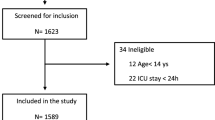
Between March 13 and September 6, 2020, 1421 patients were included into the RISC-19-ICU registry. Of these 877 met the inclusion criteria at ICU admission (Fig. 1). During the first 24 hours of ICU stay, 618 (70%) patients had been intubated and were receiving mechanical ventilation; of the remaining 259 patients, 85 (10%) were being treated with SOT, 87 (10%) with HFNC and 87 (10%) with NIV. Compared to the other three groups, patients under IMV presented higher severity scores, including increased need for vasoactive medication (Additional file 1: e-Table 1).
To allow for an unbiased assessment of respiratory strategies, a comparable population of IMV patients was extracted by propensity score matching against the other three groups based on 22 clinical, severity and laboratory parameters at admission (Additional file 1: e-Figure 1). After the matching process, 351 patients (85 SOT, 87 HFNC, 87 NIV and 92 IMV) were included in the final analysis. Matching quality was considered excellent, as reflected by an SMD ≤ 0.1 for all matching variables, excepting SAPS II (SMD = 0.13), bilirubin (SMD = 0.12), and mean arterial pressure (SMD = 0.11), in which the mean distributional difference between groups was nonetheless negligibly small (Additional file 1: e-Figure 1).
Characteristics of the overall population
After the matching process, the baseline characteristics across all four groups at ICU admission were similar (Table 1, Additional file 1: e-Table 2). Patients were treated at 49 different ICUs, all of which followed different ventilation approaches. Until IMV was required or the patient could be weaned, no obvious crossovers between ventilation therapies seem to have been present (Additional file 1: e-Table 3). Further, there was no obvious temporal relationship between the period of the pandemic during which patients were admitted to the ICU and the use of a specific respiratory support strategy or mortality rate (Additional file 1: e-Figure 2 and e-Figure 3).
Kaplan–Meier curves for the a incidence of intubation and b intensive care unit mortality stratified by respiratory support strategy at intensive care unit admission. Forest plots reporting crude and multivariable adjusted (*italic) hazard ratios with 95% confidence intervals are displayed below the Kaplan–Meier curves for each respiratory support strategy. †p values for between groups survival curve difference were calculated by means of the log-rank test
Kaplan–Meier curves for a intensive care unit mortality and b intensive care unit length of stay stratified by respiratory support strategy at intensive care unit admission (only intubated patients). Forest plots reporting crude and multivariable adjusted (*italic) hazard ratios with 95% confidence intervals are displayed below the Kaplan–Meier curves for each respiratory support strategy. †p values for between groups survival curve difference were calculated by means of the log-rank test
Of the patients who were not intubated and invasively ventilated on ICU admission, 55% required intubation and IMV between the first and second day of their ICU stay. A smaller proportion of patients (p = 0.025) in the HFNC (52%) and NIV (49%) groups progressed toward delayed IMV, compared to those in the SOT group (64%) (Fig. 2a).
Overall, the ICU mortality rate was higher (p = 0.016) in patients initially ventilated with NIV than in the other groups (SOT: 18%, HFNC: 20%, NIV: 37%, IMV: 25%) (Fig. 2b). In patients who did not progress toward intubation, the ICU mortality rates were as follows: 10% in the SOT, 7% in the HFNC, and 36% in the NIV group (Additional file 1: e-Table 4). The amount of therapy withdrawals was similar between groups (p = 0.408).
Characteristics of patients progressing toward intubation and invasive mechanical ventilation
The median duration of the in-hospital stay until intubation was longer (p<0.001) in the NIV group (4 [IQR, 3–7] days) compared to the other three groups (SOT: 3 [1–5] days, HFNC: 3 [2–6] days, IMV: 1 [0–3] days) (Table 2). At the day of intubation, patients progressing toward mechanical ventilation had an overall median partial pressure of arterial oxygen to inspired fraction of oxygen (P/F) ratio of 137 [95–179] mmHg, with no variations between groups (p = 0.256) (Table 2). In all groups the initial ventilator settings and static compliance were similar. The use of corticosteroids and prone positioning were also comparable between groups. Patients under early IMV experienced less pronounced C-reactive protein (CRP) dynamics, with a lower proportional peak increase and a larger proportional decrease over the initial 7 days of ICU stay compared to patients in the noninvasive respiratory support groups (p = 0.02) (Additional file 1: e-Figure 4; Additional file 1: e-Table 5). Patients who received initial NIV therapy had a greater need for vasopressors during the ICU stay (p = 0.029).
ICU mortality in patients requiring IMV was 28% (65) with a median length of stay of 16 [9–26] days. Patients initially treated with NIV who progressed toward IMV presented a trend (p = 0.073) toward higher ICU mortality (37%) as opposed to patients in the other groups (SOT: 21%, HFNC: 31%) when compared to the early IMV group (25%) (Fig. 3a). Patients who were initially treated with HFNC and NIV, and later required IMV, had longer (p = 0.018) ICU lengths of stay than patients under initial SOT when compared to early IMV (Fig. 3b).
After multivariable adjustment for covariates, NIV was independently associated with a higher overall ICU mortality (adjusted HR 2.67, 95% CI [1.14–6.25]) as well as with an increased ICU mortality rate (adjusted HR 2.96, 95% CI [1.07–8.23]) and a prolonged length of ICU stay (adjusted HR 0.57, 95% CI [0.33–0.97]) in patients failing NIV and requiring delayed IMV, as opposed to the other respiratory support strategies (Figs. 2b, 3a, b; Additional file 1: e-Figures 5–8).
Predictors of mortality in patients initially treated with HFNC or NIV patients with delayed intubation and invasive mechanical ventilation
To identify the HFNC and NIV patients with the worst ICU outcomes after progression to intubation and IMV, an iterative, multivariable GLM analysis was performed. The model identified age, respiratory rate and diagnosis of diabetes mellitus as independent prognostic factors of mortality (Additional file 1: e-Table 6; Additional file 1: e-Figure 9A). A prognostic score, based on the previously described model, presented a moderate prognostic ability (area under the receiver operating curve: 0.75, 95% CI [0.63–0.85]) for ICU mortality in these patients. This prognostic score was superior to all other tested prognostic scores at ICU admission (Additional file 1: e-Figure 9B; Additional file 1: e-Table 9). The Kaplan–Meier estimator presented in Additional file 1: e-Figure 9C shows the excellent (p<0.0001) dichotomizing capacity of a prognostic score of 134 points (Positive Likelihood Ratio for Mortality: 2.4) to identify patients with a higher risk of ICU mortality.
Discussion
In this subpopulation of a prospective, critically ill COVID-19 cohort during the first peak of the pandemic, 70% of patients were intubated and mechanically ventilated on the day of admission to the ICU. Use of SOT, HFNC and NIV was limited to 10% of the patients, respectively. The incidence of intubation and IMV in patients initially supported with HFNC and NIV was 12–15% lower than in patients with SOT. Compared to the other respiratory support strategies, NIV was associated with higher ICU mortality rates. A prognostic score considering age, respiratory rate and diabetes mellitus at ICU admission performed moderately in identifying HFNC and NIV patients with increased mortalities after delayed intubation and may help to discern patients who are at lower risk for increased ICU mortality during a HFNC or NIV trial.
International guidelines in place at the onset of the pandemic recommended early IMV for critically ill COVID-19 patients; HFNC and NIV were not recommended, mainly due to safety concerns related to the production of aerosols, which could jeopardize the health of hospital staff [23]. Notwithstanding those recommendations, the proportion of patients ventilated with noninvasive respiratory support strategies in this study was comparable to that described in the setting of the LUNGSAFE study, in which 15% of patients received noninvasive respiratory support [24]. Numerous COVID-19 cohort studies conducted in Europe and the United States have described similar proportions of noninvasive support measures [7, 18, 25,26,27].
Although contradictory results have been reported regarding the value of HFNC to avoid intubation [20, 28], this technique has been shown to reduce mortality rates in cases of acute hypoxemic failure [20], thus finding its place in international respiratory support recommendations [29]. In critically ill COVID-19 patients, other studies have shown—consistent with the data presented in our study—lower intubation and IMV rates, but without any reduction in ICU mortality [30]. The initially postulated risk of virus aerosolisation can probably be minimized by using conventional type I surgical masks over the nasal cannula [31]. By contrast, NIV remains controversial in the treatment of ARDS, a debate that is evident in the absence of unambiguous recommendations in clinical guidelines [32]. Although the use of NIV has been correlated with a reduced need for IMV and lower mortality rates in mild ARDS [33], the available evidence in severer expressions of ARDS indicates higher mortality rates [20, 24, 34]. In ARDS of viral etiology especially, the use of NIV is associated with high failure rates (up to 85%) [35].
Patients may—in an attempt to maintain homeostasis—initiate a vicious cycle through vigorous breathing efforts, exacerbating their lungs pathology by means of extremely elevated transpulmonary forces, leading to excessive stress and increased pulmonary inflammation [36, 37]. In our study, this patient-induced biotrauma might be one of the factors explaining the pronounced CRP dynamics in the noninvasively supported groups as opposed to those receiving early IMV [38]. Consequently, the prolonged use of noninvasive ventilation, delaying intubation in patients who ultimate fail and thus require IMV, has been associated with higher mortality rates in ARDS [39,40,41,42,43], as well as in critically ill COVID-19 patients [44,45,46]. The excess mortality observed in patients treated with NIV in this study might thus be explained by the longer period of harmful spontaneous breathing in patients failing NIV therapy, exacerbated by an increased respiratory rate and disproportionate tidal volumes induced by NIV therapy [24, 47].
If faced with a choice, physicians will intuitively prioritize avoidance of intubation and IMV, provided that this strategy does not imply any increase in mortality risk. Thus, the data presented in this study suggests that the best strategy appears to be an initial closely monitored HFNC trial with thorough assessment of clinical improvement, followed by proactive intubation and IMV in patients with a high risk of failure and mortality. The use of prognostic scores, such as the one exemplified in this study, may support clinical decision making to differentiate between patients who are treatable with noninvasive respiratory support strategies from those likely to have a worse outcome if intubation is delayed [48, 49]. To which degree static scores or dynamic scores taking advantage of the temporal assessment of patients, such as the ROX score, may improve ICU outcome nevertheless remains to be assessed [18, 49].
The present study has several limitations. First, the lack of randomization between respiratory support groups and it being a retrospective analysis, lead to many possible outcome modifying biases, such as the inability to assess the influence of human and material resources on treatment outcomes. Nonetheless, the lack of randomization was minimized through the application of propensity score matching to numerous variables at ICU admission, thus ensuring the comparability of the study groups in terms of the most objectively assessable patient characteristics. Second, the lack of a universal respiratory support protocol implies a high level of center- and clinician-related variability and prevents a mechanistic reasoning behind the described effects. On the other hand, the observational nature of this study potentially reflects the clinicians’ expertise more than a protocolized, randomized four-arm study, thereby reducing bias caused by variations in clinical experience or disfavour of a specific type of respiratory support strategy. Consequently the present study offers a representative view of the respiratory support strategies employed during the first peak of the pandemic. Third, some of the crude mortality trends observed in this study lacked statistical significance. However, given the moderate numbers of patients in each ventilation strategy, the large number of centers, the lack of a centralized protocol, and the statistical significance in the adjusted analyses, the observed signals provide a certain robustness for clinical decision-making and the development of hypotheses for future confirmatory, controlled studies. Fourth, the available registry data did not allow to determine the time on IMV for all patients, thus preventing an analysis of ventilator-free time. Fifth, the data underlying the prognostic score analysis were assessed on a daily basis, thus diminishing the prognostic capacity for scores, which require higher temporal resolution. Finally, the here proposed prognostic score has not been validated in other NIV and HFNC populations, thus caution is advised when employing it in a clinical framework; external validation is warranted.
Conclusion
Given that patients who received HFNC in this cohort had lower intubation rates but comparable ICU mortality, the most reasonable initial ventilation strategy in critically ill COVID-19 patients appears to be a closely monitored trial of HFNC, prioritizing rapid intubation and IMV in patients with a high risk of failure. Nonetheless, considering the highly uncertain and stressful clinical setting experienced during the first wave of the COVID-19 pandemic, SOT and early IMV both represent safe and “cautious” initial respiratory support strategies. The presented findings, in agreement with classic ARDS literature, suggest that NIV should be avoided whenever possible due to an associated elevated ICU mortality risk.
Availability of data and materials
Any intensive care unit or other center treating critically ill COVID-19 patients is invited to join the RISC-19-ICU registry at https://www.risc-19-icu.net. While the registry protocol prevents the deposition of the full registry dataset in a third-party repository, analyses on the full dataset may be requested by any collaborating center after approval of the study protocol by the registry board. Reproducibility of the results in the present study was ensured by providing code for registry-specific data transformation and statistical analysis for collaborative development on the GitHub and Zenodo repositories. The registry protocol and data dictionary is publicly accessible at https://www.risc-19-icu.net.
Abbreviations
- ARDS:
-
Acute respiratory distress syndrome
- CI:
-
Confidence interval
- CARDS:
-
COVID-19 ARDS
- COVID-19:
-
Coronavirus disease 2019
- CRP:
-
C-reactive protein
- GLM:
-
Generalized linear regression model
- HR:
-
Hazard ratio
- HFNC:
-
High-flow oxygen therapy by nasal cannula
- ICU:
-
Intensive care unit
- IMV:
-
Invasive mechanical ventilation
- IQR:
-
Interquartile range
- NIV:
-
Noninvasive positive-pressure ventilation
- P/F:
-
Partial pressure of arterial oxygen to inspired fraction of oxygen
- P-SILI:
-
Patient self-inflicted lung injury
- ROC:
-
Receiver operating characteristics
- SOT:
-
Standard oxygen therapy
- SMD:
-
Standardized mean difference
- SOFA:
-
Sequential Organ Failure Assessment
- SAPS II:
-
Simplified Acute Physiology Score II
- STROBE:
-
Strengthening the Reporting of Observational Studies in Epidemiology
References
Curley GF, Laffey JG, Zhang H, Slutsky AS. Biotrauma and ventilator-induced lung injury: clinical implications. Chest. 2016;150(5):1109–17.
Frat J-P, Coudroy R, Marjanovic N, Thille AW. High-flow nasal oxygen therapy and noninvasive ventilation in the management of acute hypoxemic respiratory failure. Ann Transl Med. 2017;5(14):16.
Ricard J-D, Roca O, Lemiale V, Corley A, Braunlich J, Jones P, Kang BJ, Lellouche F, Nava S, Rittayamai N, et al. Use of nasal high flow oxygen during acute respiratory failure. Intensive Care Med. 2020;46(12):2238–47.
World Health Organization. Clinical management of severe acute respiratory infection when novel coronavirus (2019-nCoV) infection is suspected: interim guidance, 28 January 2020. In. Geneva: World Health Organization; 2020.
Raoof S, Nava S, Carpati C, Hill NS. High-flow, noninvasive ventilation and awake (nonintubation) proning in patients with Coronavirus disease 2019 with respiratory failure. Chest. 2020;158(5):1992–2002.
Dennis JM, McGovern AP, Vollmer SJ, Mateen BA. Improving survival of critical care patients with coronavirus disease 2019 in England: a national cohort study, March to June 2020. Crit Care Med. 2021;49(2):209–214.
COVID-ICU Group on behalf of the REVA Network and the COVID-ICU Investigators. Clinical characteristics and day-90 outcomes of 4244 critically ill adults with COVID-19: a prospective cohort study. Intensive Care Med. 2021;47(1):60–73.
Rahmanzade R, Rahmanzadeh R, Tabarsi P, Hashemian SM. Noninvasive versus invasive ventilation in COVID-19: one size does not fit all! Anesth Analgesia. 2020;131(2):66.
Marini JJ, Gattinoni L. Management of COVID-19 respiratory distress. JAMA. 2020;323(22):2329–30.
Gattinoni L, Marini JJ, Busana M, Chiumello D, Camporota L. Spontaneous breathing, transpulmonary pressure and mathematical trickery. Ann Intensive Care. 2020;10(1):88.
Gattinoni L, Marini JJ, Chiumello D, Busana M, Camporota L. COVID-19: scientific reasoning, pragmatism and emotional bias. Ann Intensive Care. 2020;10(1):134.
Gershengorn HB, Hu Y, Chen J-T, Hsieh SJ, Dong J, Gong MN, Chan CW. Reply: optimal respiratory assistance strategy for patients with COVID-19. Ann Am Thorac Soc. 2021;18(5):917–18.
Tobin MJ, Laghi F, Jubran A. Caution about early intubation and mechanical ventilation in COVID-19. Ann Intensive Care. 2020;10(1):78.
Tobin MJ, Laghi F, Jubran A. P-SILI is not justification for intubation of COVID-19 patients. Ann Intensive Care. 2020;10(1):105.
Tobin MJ, Jubran A, Laghi F. P-SILI as justification for intubation in COVID-19: readers as arbiters. Ann Intensive Care. 2020;10(1):156.
Tobin MJ. Does making a diagnosis of ARDS in patients with coronavirus disease 2019 matter? Chest. 2020;158(6):2275–7.
Hatipoğlu U, Chatburn R, Duggal A. Optimal respiratory assistance strategy for patients with COVID-19. Ann Am Thorac Soc. 2020. https://doi.org/10.1513/AnnalsATS.202010-1339LE.
Zucman N, Mullaert J, Roux D, Roca O, Ricard J-D, Longrois D, Dreyfuss D. Contributors: prediction of outcome of nasal high flow use during COVID-19-related acute hypoxemic respiratory failure. Intensive Care Med. 2020;46(10):1924–6.
Wendel Garcia PD, Fumeaux T, Guerci P, Heuberger DM, Montomoli J, Roche-Campo F, Schuepbach RA, Hilty MP. Prognostic factors associated with mortality risk and disease progression in 639 critically ill patients with COVID-19 in Europe: initial report of the international RISC-19-ICU prospective observational cohort. EClinicalMedicine. 2020;25:100449.
Frat J-P, Thille AW, Mercat A, Girault C, Ragot S, Perbet S, Prat G, Boulain T, Morawiec E, Cottereau A, et al. High-flow oxygen through nasal cannula in acute hypoxemic respiratory failure. N Engl J Med. 2015;372(23):2185–96.
Farias E, Rudski L, Zidulka A. Delivery of high inspired oxygen by face mask. J Crit Care. 1991;6(3):119–24.
Normand S-LT, Landrum MB, Guadagnoli E, Ayanian JZ, Ryan TJ, Cleary PD, McNeil BJ. Validating recommendations for coronary angiography following acute myocardial infarction in the elderly: a matched analysis using propensity scores. J Clin Epidemiol. 2001;54(4):387–98.
Alhazzani W, Møller MH, Arabi YM, Loeb M, Gong MN, Fan E, Oczkowski S, Levy MM, Derde L, Dzierba A, et al. Surviving sepsis campaign: guidelines on the management of critically ill adults with coronavirus disease 2019 (COVID-19). Crit Care Med. 2020;48(6):66.
Bellani G, Laffey JG, Pham T, Madotto F, Fan E, Brochard L, Esteban A, Gattinoni L, Bumbasirevic V, Piquilloud L, et al. Noninvasive ventilation of patients with acute respiratory distress syndrome insights from the LUNG SAFE study. Am J Respir Crit Care Med. 2016;195(1):67–77.
Cummings MJ, Baldwin MR, Abrams D, Jacobson SD, Meyer BJ, Balough EM, Aaron JG, Claassen J, Rabbani LE, Hastie J, et al. Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York City: a prospective cohort study. The Lancet. 2020;395(10239):1763–70.
Karagiannidis C, Mostert C, Hentschker C, Voshaar T, Malzahn J, Schillinger G, Klauber J, Janssens U, Marx G, Weber-Carstens S, et al. Case characteristics, resource use, and outcomes of 10 021 patients with COVID-19 admitted to 920 German hospitals: an observational study. Lancet Respir Med. 2020;8(9):853–62.
Botta M, Tsonas AM, Pillay J, Boers LS, Algera AG, Bos LDJ, Dongelmans DA, Hollmann MW, Horn J, Vlaar APJ, Schultz MJ, Neto AS, Paulus F. PRoVENT-COVID Collaborative Group. Ventilation management and clinical outcomes in invasively ventilated patients with COVID-19 (PRoVENTCOVID): a national, multicentre, observational cohort study. Lancet Respir Med. 2021;9(2):139–148.
Ferreyro BL, Angriman F, Munshi L, Del Sorbo L, Ferguson ND, Rochwerg B, Ryu MJ, Saskin R, Wunsch H, da Costa BR, et al. Association of noninvasive oxygenation strategies with all-cause mortality in adults with acute hypoxemic respiratory failure: a systematic review and meta-analysis. JAMA. 2020;324(1):57–67.
Rochwerg B, Einav S, Chaudhuri D, Mancebo J, Mauri T, Helviz Y, Goligher EC, Jaber S, Ricard J-D, Rittayamai N, et al. The role for high flow nasal cannula as a respiratory support strategy in adults: a clinical practice guideline. Intensive Care Med. 2020;46(12):2226–37.
Demoule A, Vieillard Baron A, Darmon M, Beurton A, Géri G, Voiriot G, Dupont T, Zafrani L, Girodias L, Labbé V, et al. High-flow nasal cannula in critically iii patients with severe COVID-19. Am J Respir Crit Care Med. 2020;202(7):1039–42.
Leonard S, Atwood CW, Walsh BK, DeBellis RJ, Dungan GC, Strasser W, Whittle JS. Preliminary findings on control of dispersion of aerosols and droplets during high-velocity nasal insufflation therapy using a simple surgical mask: implications for the high-flow nasal cannula. Chest. 2020;158(3):1046–9.
Rochwerg B, Brochard L, Elliott MW, Hess D, Hill NS, Nava S, Navalesi P, Antonelli M, Brozek J, Conti G, et al. Official ERS/ATS clinical practice guidelines: noninvasive ventilation for acute respiratory failure. Eur Respir J. 2017;50(2):1602426.
Xu X-P, Zhang X-C, Hu S-L, Xu J-Y, Xie J-F, Liu S-Q, Liu L, Huang Y-Z, Guo F-M, Yang Y, et al. Noninvasive ventilation in acute hypoxemic nonhypercapnic respiratory failure: a systematic review and meta-analysis. Crit Care Med. 2017;45(7):66.
Schettino G, Altobelli N, Kacmarek RM. Noninvasive positive-pressure ventilation in acute respiratory failure outside clinical trials: experience at the Massachusetts General Hospital*. Crit Care Med. 2008;36(2):66.
Kumar A, Zarychanski R, Pinto R, Cook DJ, Marshall J, Lacroix J, Stelfox T, Bagshaw S, Choong K, Lamontagne F, et al. Critically ill patients with 2009 influenza A(H1N1) infection in Canada. JAMA. 2009;302(17):1872–9.
Yoshida T, Uchiyama A, Matsuura N, Mashimo T, Fujino Y. Spontaneous breathing during lung-protective ventilation in an experimental acute lung injury model: high transpulmonary pressure associated with strong spontaneous breathing effort may worsen lung injury*. Crit Care Med. 2012;40(5):66.
Brochard L, Slutsky A, Pesenti A. Mechanical ventilation to minimize progression of lung injury in acute respiratory failure. Am J Respir Crit Care Med. 2016;195(4):438–42.
Forel J-M, Roch A, Marin V, Michelet P, Demory D, Blache J-L, Perrin G, Gainnier M, Bongrand P, Papazian L. Neuromuscular blocking agents decrease inflammatory response in patients presenting with acute respiratory distress syndrome*. Crit Care Med. 2006;34(11):66.
Ozyilmaz E, Ugurlu AO, Nava S. Timing of noninvasive ventilation failure: causes, risk factors, and potential remedies. BMC Pulmonary Med. 2014;14(1):19.
Mosier JM, Sakles JC, Whitmore SP, Hypes CD, Hallett DK, Hawbaker KE, Snyder LS, Bloom JW. Failed noninvasive positive-pressure ventilation is associated with an increased risk of intubation-related complications. Ann Intensive Care. 2015;5(1):4.
Kang BJ, Koh Y, Lim C-M, Huh JW, Baek S, Han M, Seo H-S, Suh HJ, Seo GJ, Kim EY, et al. Failure of high-flow nasal cannula therapy may delay intubation and increase mortality. Intensive Care Med. 2015;41(4):623–32.
Kangelaris KN, Ware LB, Wang CY, Janz DR, Zhuo H, Matthay MA, Calfee CS. Timing of intubation and clinical outcomes in adults with acute respiratory distress syndrome*. Crit Care Med. 2016;44(1):66.
Miller DC, Pu J, Kukafka D, Bime C. Failure of high flow nasal cannula and subsequent intubation is associated with increased mortality as compared to failure of non-invasive ventilation and mechanical ventilation alone: a real-world retrospective analysis. J Intensive Care Med. 2020;0885066620968041.
Pandya A, Kaur NA, Sacher D, O'Corragain O, Salerno D, Desai P, Sehgal S, Gordon M, Gupta R, Marchetti N, Zhao H, Patlakh N, Criner GJ. University T; COVID-19 Research Group. Ventilatory Mechanics in Early vs Late Intubation in a Cohort of Coronavirus Disease 2019 Patients With ARDS: A Single Center’s Experience. Chest. 2021;159(2):653–56.
Hyman JB, Leibner ES, Tandon P, Egorova NN, Bassily-Marcus A, Kohli-Seth R, Arvind V, Chang HL, Lin H-M, Levin MA. Timing of intubation and in-hospital mortality in patients with coronavirus disease 2019. Crit Care Explor. 2020;2(10):66.
Calligaro GL, Lalla U, Audley G, Gina P, Miller MG, Mendelson M, Dlamini S, Wasserman S, Meintjes G, Peter J, et al. The utility of high-flow nasal oxygen for severe COVID-19 pneumonia in a resource-constrained setting: a multi-centre prospective observational study. EClinicalMedicine. 2020;28:56.
Carteaux G, Millán-Guilarte T, De Prost N, Razazi K, Abid S, Thille AW, Schortgen F, Brochard L, Brun-Buisson C, Mekontso Dessap A. Failure of noninvasive ventilation for de novo acute hypoxemic respiratory failure: role of tidal volume*. Crit Care Med. 2016;44(2):66.
Duan J, Han X, Bai L, Zhou L, Huang S. Assessment of heart rate, acidosis, consciousness, oxygenation, and respiratory rate to predict noninvasive ventilation failure in hypoxemic patients. Intensive Care Med. 2017;43(2):192–9.
Roca O, Caralt B, Messika J, Samper M, Sztrymf B, Hernández G, García-de-Acilu M, Frat J-P, Masclans JR, Ricard J-D. An index combining respiratory rate and oxygenation to predict outcome of nasal high-flow therapy. Am J Respir Crit Care Med. 2018;199(11):1368–76.
Acknowledgements
We thank Prof. Dr. Jordi Mancebo for critical revision and Bradley Londres for professional language editing of this manuscript. We also want to thank all of the physicians and nurses in our collaborating centers for their tireless and brave efforts in patient treatment and care, as without them it would have been impossible to contain this ongoing health care emergency. The authors wish to acknowledge all of our collaborators from the RISC-19-ICU registry as collaborating authors, listed in alphabetical order: Angela Algaba-Calderon, Janina Apolo, Theodoros Aslanidis, Barna Babik, Filippo Boroli, Jan Brem, Mirko Brenni, Silvio D. Brugger, Giovanni Camen, Emanuele Catena, Roberto Ceriani, Ivan Chau, Andreas Christ, Chiara Cogliati, Pablo Concha, Gauthier Delahaye, Iris Drvaric, Jesús Escós-Orta, Silvia Fabbri, Francesca Facondini, Miodrag Filipovic, Jorge Gámez-Zapata, Peter Gerecke, Diederik Gommers, Thomas Hillermann, Can Ince, Beatrice Jenni-Moser, Marija Jovic, Geoffrey Jurkolow, Alexander Klarer, Adriana Lambert, Jean-Christophe Laurent, Jerome Lavanchy, Barbara Lienhardt-Nobbe, Pascal Locher, Marie-Reine Losser, Roger F. Lussman, Aurora Magliocca, Antoni Margarit, Alberto Martínez, Romano Mauri, Eric Mayor-Vázquez, Jens Meier, Mallory Moret-Bochatay, Martina Murrone, Didier Naon, Thomas Neff, Emmanuel Novy, Lina Petersen, Jerome Pugin, Anne-Sylvie Ramelet, Jonathan Rilinger, Peter C. Rimensberger, Michael Sepulcri, Karim Shaikh, Marianne Sieber, Maria Sole Simonini, Savino Spadaro, Govind Oliver Sridharan, Klaus Stahl, Dawid L. Staudacher, Xiana Taboada-Fraga, Adrian Tellez, Severin Urech, Giovanni Vitale, Gerardo Vizmanos-Lamotte, Tobias Welte, Begoña Zalba-Etayo, Nuria Zellweger.
Funding
The RISC-19-ICU registry is supported by the Swiss Society of Intensive Care Medicine and funded by internal resources of the Institute of Intensive Care Medicine, of the University Hospital Zurich and by unrestricted grants from CytoSorbents Europe GmbH (Berlin, Germany) and Union Bancaire Privée (Zurich, Switzerland). The sponsors had no role in the design of the study, the collection and analysis of the data, or the preparation of the manuscript.
Author information
Authors and Affiliations
Consortia
Contributions
PDWG and FRC conceived and designed this study. PDWG, HAB, PKB, MAF, BY, SD, TT, TW, AK, AF, GRK, MAW, RC, FT, AP, ER, RRG, PC, ALA, MCMD, HLG, RE, MPM, NG, PS, MS, LM, JW, MMJ, ML, PS, FH, AD, HK, SC, SG, CB, JM, IF, MHP, ABW, SC, KM, TH, HR, MS, MS, DS, UP, AR, AH, FMB, MFL, PF, TG, CH, EC, DMH, TF, JM, PG, RAS, MPH and FRC acquired, analyzed, and/or interpreted the data. PDWG performed statistical analysis. PDWG and FRC drafted the manuscript. PDWG, HAB, PKB, MAF, BY, SD, TT, TW, AK, AF, GRK, MAW, RC, FT, AP, ER, RRG, PC, ALA, MCMD, HLG, RE, MPM, NG, PS, MS, LM, JW, MMJ, ML, PS, FH, AD, HK, SC, SG, CB, JM, IF, MHP, ABW, SC, KM, TH, HR, MS, MS, DS, UP, AR, AH, FMB, MFL, PF, TG, CH, EC, DMH, TF, JM, PG, RAS, MPH, and FRC critically revised the manuscript for important intellectual content. PDWG, TF, JM, PG, RAS, and MPH supervised the registry. PDWG and FRC had full access to the entirety of the study data and take full responsibility for the integrity and accuracy of the data analysis. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was approved by the cantonal ethics committee of Zurich (KEK 2020-00322, ClinicalTrials.gov Identifier: NCT04357275). Informed consent to participate was either waived or obtained from the patients or from their next of kin, in accordance with the stipulations issued by the center-specific local ethics committee. All collaborating centers have complied with all local legal and ethical requirements.
Consent for publication
Informed consent for publication was either waived or obtained from the patients or from their next of kin, in accordance with the stipulations issued by the center-specific local ethics committee. All collaborating centers have complied with all local legal and ethical requirements.
Competing interests
The authors declare that they have no competing interests regarding the present study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
RISC-19-ICU Investigators are listed in the acknowledgements of the manuscript and should be added as Collaborators to the Articles Indexing.
Supplementary Information
Additional file 1.
e-Appendix 1: Specifications on the RISC-19-ICU registry structure and data collection. e-Appendix 2: Missing data handling. e-Table 1: Overall “unmatched” baseline characteristics on Day 0. e-Figure 1: “Love Plot” presenting Standardized Mean Differences between the unmatched and matchedcohort. e-Table 2: Demographics, characteristics at ICU admission, progression of respiratory support and outcome; stratified by survivor status. e-Table 3: Progression of respiratory support stratified by respiratory support strategy at ICU admission. e-Figure 2: Temporal relationship between the period of admission to the intensive care unit and the use of respiratory support strategies or mortality rate. e-Figure 3: Kaplan Meier curves for intensive care unit mortality stratified by the period of admission to the intensive care unit. e-Table 4: Demographics, characteristics at ICU admission, progression of respiratory support and outcome for patients never requiring intubation and invasive mechanical ventilation. e-Figure 4: C-Reactive Protein stratified by respiratory support strategy on Day 0 over the first week of ICU stay. e-Table 5: Mixed Effect Model of C-Reactive Protein stratified by respiratory support strategy on Day 0 over the first week of ICU stay. e-Figure 5: Multivariable adjusted COX regression model for the incidence of intubation. e-Figure 6: Multivariable adjusted COX regression model for overall ICU mortality. e-Figure 7: Multivariable adjusted COX regression model for ICU mortality (intubated patients only). e-Figure 8: Multivariable adjusted COX regression model for ICU discharge (intubated patients only). e-Table 6: Prognostic Model for the identification of patients with lower ICU mortality risk after a failed HFNC or NIV trial. e-Figure 9: Nomogram, Receiver Operating Curves and stratified Kaplan Meier curve for a prognostic model identifying patients with lower ICU mortality risk after a failed HFNC or NIV trial. e-Table 7: Area Under the Receiver Operating Curves (AUROCs) for the Prognostic Score versus classic severity scores.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Wendel Garcia, P.D., Aguirre-Bermeo, H., Buehler, P.K. et al. Implications of early respiratory support strategies on disease progression in critical COVID-19: a matched subanalysis of the prospective RISC-19-ICU cohort. Crit Care 25, 175 (2021). https://doi.org/10.1186/s13054-021-03580-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13054-021-03580-y