Abstract
Background
While obesity confers an increased risk of death in the general population, numerous studies have reported an association between obesity and improved survival among critically ill patients. This contrary finding has been referred to as the obesity paradox. In this retrospective study, two causal inference approaches were used to address whether the survival of non-obese critically ill patients would have been improved if they had been obese.
Methods
The study cohort comprised 6557 adult critically ill patients hospitalized at the Intensive Care Unit of the Ghent University Hospital between 2015 and 2017. Obesity was defined as a body mass index of ≥ 30 kg/m2. Two causal inference approaches were used to estimate the average effect of obesity in the non-obese (AON): a traditional approach that used regression adjustment for confounding and that assumed missingness completely at random and a robust approach that used machine learning within the targeted maximum likelihood estimation framework along with multiple imputation of missing values under the assumption of missingness at random. 1754 (26.8%) patients were discarded in the traditional approach because of at least one missing value for obesity status or confounders.
Results
Obesity was present in 18.9% of patients. The in-hospital mortality was 14.6% in non-obese patients and 13.5% in obese patients. The raw marginal risk difference for in-hospital mortality between obese and non-obese patients was − 1.06% (95% confidence interval (CI) − 3.23 to 1.11%, P = 0.337). The traditional approach resulted in an AON of − 2.48% (95% CI − 4.80 to − 0.15%, P = 0.037), whereas the robust approach yielded an AON of − 0.59% (95% CI − 2.77 to 1.60%, P = 0.599).
Conclusions
A causal inference approach that is robust to residual confounding bias due to model misspecification and selection bias due to missing (at random) data mitigates the obesity paradox observed in critically ill patients, whereas a traditional approach results in even more paradoxical findings. The robust approach does not provide evidence that the survival of non-obese critically ill patients would have been improved if they had been obese.
Similar content being viewed by others
Background
Obesity is a chronic disease associated with cardiovascular disease, chronic kidney disease, diabetes mellitus, some cancers, and musculoskeletal disorders. It has become one of the most important public health problems in many high- and middle-income countries, entailing a heavy economic burden [1,2,3]. The prevalence of obesity is steadily increasing worldwide and, on average, approximately one in five patients admitted to the intensive care unit (ICU) is obese [4, 5]. Although obesity confers an increased risk of morbidity and mortality in the general population and poses additional challenges that may compromise prognosis in critically ill patients, a growing body of literature has found an association between obesity and improved ICU outcomes, including lower mortality. This contrary finding has been referred to as the obesity paradox [4,5,6,7,8,9,10,11].
While some authors postulate underlying pathophysiologic mechanisms to support its biological plausibility [4, 9, 10, 12, 13], others remain skeptical and provide methodological explanations. The first potential type of bias is confounding bias. Confounding factors of the obesity-mortality relationship include age, sex, ethnicity, smoking status, alcohol consumption, income, education, physical activity, and dietary pattern, among others [14]. Failure to adequately control for confounding will result in bias [15]. Bias may in particular arise as a consequence of reverse causation, whereby pre-existing disease leads to unintended weight loss and higher mortality, making obesity appear protective [16, 17].
Secondly, studies restricted to patients with an obesity-related disease may suffer from so-called collider stratification bias. The causal diagram presented in Fig. 1 depicts the causal relations between obesity, ICU admission, and mortality. Obesity may lead to earlier ICU admission, as clinicians tend to consider obese patients at higher risk of worse outcome [4, 12]. The node U represents common causes of ICU admission and mortality that are usually unmeasured or difficult to quantify. Examples include perceived reversibility of the acute illness, anticipated quality of life, patient wishes, ceiling of care, peer standards, and bed occupancy status [18, 19]. Because ICU admission may be affected by both obesity as well as these unmeasured prognostic factors of mortality, restriction of the study population to patients admitted to the ICU may induce collider stratification bias, a form of selection bias. In particular, non-obese patients will generally have more potent risk factors U for mortality. Indeed, the presence of more potent risk factors (and not the absence of obesity) may explain why these patients got admitted to the ICU [17, 20].
Causal diagram representing causal relations between obesity, ICU admission, and mortality. The circle around variable U indicates that it has not been measured. The box around variable ICU indicates that the analysis conditions on it by design, since the study cohort only comprised patients admitted to the intensive care unit. This causal diagram has been simplified for the purpose of illustrating collider stratification bias. ICU, intensive care unit; U, unmeasured factors
Other potential types of bias include body mass index (BMI) group misallocation by estimating rather than actually measuring height and weight in critically ill patients [21], treatment bias due to closer monitoring and different treatment of obese patients [4, 22, 23], and publication bias due to selective reporting of unusual findings such as the obesity paradox.
An important clinical question that arises from the obesity paradox is whether the survival of non-obese critically ill patients would have been improved if they had been obese. This demands a causal inference approach. While the necessity of adequate confounding control for valid causal inference has been recently emphasized by the editors of many critical care journals, no recommendations have been made on which method to apply [24]. This study purported to address the aforementioned clinical question using two different approaches: a traditional approach that used regression adjustment for confounding and a more robust approach that used state-of-the-art machine learning techniques within a causal inference framework along with missing data imputation.
Methods
Study design and data collection
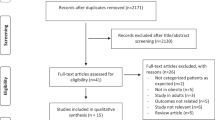
This retrospective study focused on the relationship between obesity and in-hospital mortality in adult (≥ 16 years) critically ill patients consecutively hospitalized at the ICU of the Ghent University Hospital between January 1, 2015, and December 31, 2017. During the study period 11,244 adult patients were admitted to the ICU. Fifty-five patients were excluded from the analysis because their reason for ICU admission was directly related to underweight or obesity. In case of multiple ICU admissions during the study period (n = 1762), only the first was considered. Patients with a Simplified Acute Physiology Score II (SAPS II) of < 32 points (first quartile, n = 2063) or missing SAPS II (n = 807) at ICU admission were discarded in order to exclude patients who were admitted to the ICU for the sole purpose of monitoring and in whom critical illness was less likely to be present. The final study cohort comprised 6557 patients. A flow diagram is presented in Fig. 2.
Obesity was the exposure of interest and was dichotomously defined as a BMI of ≥ 30 kg/m2 according to the classification of the National Institutes of Health and the World Health Organization [1, 25]. A sensitivity analysis was also performed using a BMI cut-off value of ≥ 25 kg/m2 and ≥ 35 kg/m2. The patient’s height and weight were usually estimated at ICU admission by critical care nurses. A BMI of < 10 or > 80 kg/m2 was considered as biologically implausible, thus consequently attributed to input error and handled as missing value. In-hospital mortality was used as outcome variable.
The following potential confounders of the obesity-mortality relationship were extracted or derived from the prospectively maintained Intensive Care Information System database (GE Healthcare Centricity® Critical Care): age, sex, ethnicity, smoking status, alcohol consumption, physical activity, hypothyroidism, chronic glucocorticoid therapy, solid malignancy, hematologic malignancy, dementia, human immunodeficiency virus infection or acquired immunodeficiency syndrome, and calendar time (in years since start of study). As annual income was not available at the patient level, the median annual income (net taxable income in 2015 as provided by Statbel [26]) per statistical sector to which the patient belongs based on home address was used as proxy variable. Variables related to obesity or ICU stay were also obtained. Obesity-related conditions included Charlson comorbidity index, cardiovascular disease, hypertension, diabetes mellitus, chronic kidney disease, chronic liver disease, and chronic pulmonary disease. Variables related to ICU stay included SAPS II within 36 h after ICU admission, type of admission, and ICU mortality. The data on these sociodemographic characteristics and comorbidities contained in the Intensive Care Information System database were inputted manually at ICU admission by critical care physicians. Data discrepancies were resolved by expert opinion or by reviewing the electronic health record.
The estimand of interest was the difference in in-hospital mortality in non-obese patients if all had been obese versus as observed (i.e., if they stayed non-obese). This corresponds with the average treatment effect in the untreated (ATU), which is the marginal causal risk difference in the subpopulation that was not exposed (to obesity at ICU admission). We considered this effect to be of particular interest because it is less susceptible to selection bias. The reason is that non-obese patients would likely also have been admitted to the ICU and have had a SAPS II of ≥ 32 points at ICU admission if they had been obese (so that their selection is not related to their obesity status). By contrast, this seems less plausible to hold for obese patients if they had been non-obese, making the average treatment effect in the treated (and also the overall average treatment effect) more prone to selection bias. Identifying the effect of obesity on in-hospital mortality in non-obese patients requires adequate confounding control [15]. In the following two sections, we describe the two approaches that we considered for this.
Traditional approach
In line with common approaches [7, 8], a logistic regression model was fitted with in-hospital mortality as the outcome, obesity as a dichotomous covariate, and the main effects of the 14 confounders as other covariates. Model selection procedures were not performed to reduce the risk of residual confounding, leaving all variables in the model. An estimate of the ATU was obtained by direct standardization, also known as G-computation [15, 27]. First, the logistic regression model was used to predict the outcome for each patient as if the patient had been obese and the outcome as if the patient had been non-obese. These predictions were then averaged only across patients who were non-obese. The difference between both averages gave an estimate of the ATU.
This approach has several limitations. First, logistic regression assumes that the log odds of in-hospital mortality are linear in the covariates. Incorrect modeling of the true relationship between confounders and outcome leaves residual confounding bias. This is a major concern, especially when there is little overlap in the confounder values between obese and non-obese patients, for then regression-based estimators can severely rely on extrapolation, without this being visible from the results. For instance, that extrapolations are being made is only subtly hinted at via slightly increased standard errors, thereby possibly leaving an optimistically precise yet biased estimator of the causal effect. Additionally, regression models require complete cases of the data, discarding cases with at least one missing value. Complete case analysis is, however, inefficient and can be biased when missingness is informative [15].
Robust approach
In view of the foregoing concern about parametric model misspecification, the one-step targeted maximum likelihood estimation (TMLE) framework was used to enable the use of machine learning methods with the aim to reduce bias against model misspecification, while ensuring that valid confidence intervals for the treatment effect can be constructed [27,28,29,30,31]. Here, machine learning was based on a so-called super learning procedure. This is an ensemble method that selects the optimally weighted combination of multiple candidate algorithms by applying a metalearning algorithm that minimizes the cross-validated risk associated with chosen prediction error loss functions. The resulting super learner is designed to perform as well as or better than the best-fitting candidate algorithm (in large sample sizes) [27, 29, 32, 33]. In this study, super learning with stratified 10-fold cross-validation was performed using following library of 15 candidate algorithms: null estimator (unconditional mean); main effects logistic regression model; stepwise logistic regression model; 5 penalized regression models using elastic net with mixing parameter of 0 (ridge penalty), 0.25, 0.50, 0.75, or 1.0 (lasso penalty); random forests; extreme gradient boosting; support vector machines; Bayesian additive regression trees model; and 3 general additive models with polynomial terms to the second, third, or fourth degree. Three super learners for the outcome mechanism and for the exposure mechanism were created by applying the squared error (L2) loss, log loss, and the rank loss functions as metalearning algorithm. Details of the super learning procedure, including the hyperparameter settings of each candidate algorithm, the optimal super learner weights that minimized the corresponding loss function, and the performance measures obtained by nested cross-validation, are presented in Additional file 1. The best performance for the data at hand was shown for the log loss super learners, which were therefore used in the subsequent TMLE procedure. Truncation levels of 2.5% and 97.5% were used for extreme inverse probability weights. Standard errors for the TMLE were calculated based on the influence curve.
TMLE combined with super learning reduces model misspecification bias [27, 29], but it does not tackle the potential problem of selection bias due to missing data. Therefore, multiple imputation by chained equations (MICE) was used to impute missing values. MICE is an iterative algorithm based on fully conditional specification, where the imputation model is specified separately for each incomplete variable in function of all other (possibly incompletely) measured variables. It relies on the missing at random assumption, which states that the probability of a value being missing does not depend on the unobserved data conditional on the observed data [34]. Specification of the imputation model is given in Additional file 2. The number of imputations was set to 50. Each imputed dataset was analyzed separately using the one-step TMLE and the log loss super learner for the outcome and exposure mechanism. The separate estimates and variances for each of the imputed datasets were pooled into an overall estimate and variance using Rubin’s rule [35].
Data analysis was performed in R version 3.3.2 using the stdReg version 3.0.0, SuperLearner version 2.0-24, rBayesianOptimization version 1.1.0, tmle version 1.3.0-2, and mice version 3.6.0 packages [36].
Results
Baseline characteristics
The final study cohort comprised 6557 patients. Obesity was present in 18.9% of patients. The respective ICU and in-hospital mortality was 9.7% and 14.4% in the overall cohort, 9.8% and 14.6% in non-obese patients, and 9.1% and 13.5% in obese patients. The raw marginal risk difference for in-hospital mortality between obese and non-obese patients was − 1.06% (95% confidence interval (CI) of − 3.23 to 1.11%, P = 0.337). These findings are consistent with the obesity paradox. Baseline characteristics are summarized in Tables 1 and 2.
Traditional approach
The results of the multivariate logistic regression model are presented in Table 3. This model indicated that the odds for in-hospital mortality were 20% lower in obese patients compared with non-obese patients with the same level of the confounders that were controlled for (adjusted odds ratio of 0.80, 95% CI 0.64 to 0.99, P = 0.048). Using standardization, this translated into a marginal causal risk difference—in particular an ATU—of − 2.48% (95% CI − 4.80 to − 0.15%, P = 0.037). This suggested that the in-hospital mortality would be significantly smaller if all non-obese patients had been obese. This regression-based approach thus resulted in even more paradoxical findings, despite presumed adjustment for confounding.
Although only 2.7% of values for exposure and confounders were missing, 1754 (26.8%) patients had at least one missing value and were thereby discarded in the traditional approach. However, the complete cases formed a selective, non-random sample in which the association between obesity and in-hospital mortality became more pronounced. Indeed, the raw marginal risk difference for in-hospital mortality between obese and non-obese patients went from − 1.06% (95% CI − 3.23 to 1.11%, P = 0.337) in patients with complete cases for exposure to − 2.25% (95% CI − 4.65 to 0.15%, P = 0.067) in patients with complete cases for exposure and confounders. Missingness was equally common in obese and non-obese patients (24.2% versus 23.9%, P = 0.828).
Additionally, and somewhat counterintuitively, the above regression adjustment seemed to indicate that the bias arising from measured confounders was towards the null. Indeed, the marginal causal risk difference of − 2.48% was further away from zero than the raw marginal risk difference of − 2.25% in patients with complete cases for exposure and confounders. This suggested that a seemingly protective effect of obesity on in-hospital mortality had been partially masked by worse baseline confounders in obese patients. However, this may also be the result of model misspecification and, hence, of inadequate removal of confounding bias.
Robust approach
We therefore provided a re-analysis based on TMLE and missing data imputation which, interestingly, mitigated the obesity paradox by yielding an ATU of − 0.59% (95% CI − 2.77 to 1.60%, P = 0.599). A summary table of the traditional versus robust approach is given in Table 4. This table also provides the separate impact of performing TMLE or missing data imputation on the marginal causal risk difference. Traditional regression adjustment on the imputed data resulted in a pooled ATU of − 1.46% (95% CI − 3.58 to 0.66%, P = 0.178), while performing TMLE on the complete cases (thus without missing data imputation) gave a similar ATU of − 1.52% (95% CI − 3.85 to 0.81%, P = 0.201). Only the combination of both techniques attenuated evidence for an obesity paradox. Truncation of the exposure weights was necessary in 0.1% and 0.6% of patients in the TMLE analysis without and with missing data imputation, respectively.
A sensitivity analysis was performed by repeating the above analysis for different BMI cut-off values. Details are given in Additional file 3. The raw marginal risk difference for in-hospital mortality between patients with a BMI of ≥ 25 kg/m2 (54.8% of patients) and patients with a BMI of < 25 kg/m2 was − 1.40% (95% CI − 3.14 to 0.35%, P = 0.117). The traditional approach resulted in an ATU of − 2.11% (95% CI − 4.10 to − 0.12%, P = 0.038), whereas the robust approach yielded an ATU of − 0.01% (95% CI − 1.81 to 1.79%, P = 0.992), thereby debunking this overweight/obesity paradox. Since only a small proportion of patients had a BMI ≥ 35 kg/m2 (5.2% of patients), the confidence intervals of the effect estimates were too wide to make a meaningful comparison between the traditional and robust approach in patients with a BMI ≥ 35 kg/m2 versus patients with a BMI < 35 kg/m2.
Discussion
The obesity paradox refers to the counterintuitive association between obesity and improved survival rates in critically ill patients and may arise from several methodological pitfalls, including confounding bias and collider stratification bias. The underlying question that clinicians may ask is whether the survival of non-obese critically ill patients would have been improved if they had been obese. This has, to our knowledge, not yet been addressed by prior clinical research and demands a causal inference approach.
In this study, the raw marginal risk difference for in-hospital mortality between obese and non-obese patients admitted to the ICU who have a SAPS II of ≥ 32 points at ICU admission was − 1.06% (95% CI − 3.23 to 1.11%, P = 0.337). Two causal inference approaches were used to estimate the ATU: an approach that used traditional regression adjustment for confounding and that assumed missingness completely at random, and a robust approach that used super learning within the TMLE framework along with multiple imputation of missing values under the assumption of missingness at random. The traditional approach resulted in an ATU of − 2.48% (95% CI − 4.80 to − 0.15%, P = 0.037), which was an even more paradoxical finding. This may, however, be subject, among others, to selection bias due to missing data and residual confounding bias due to model misspecification. By contrast, the robust approach that combined targeted learning with multiple imputation to deal with both types of biases yielded an ATU of − 0.59% (95% CI − 2.77 to 1.60%, P = 0.599) and thereby mitigated the obesity paradox. Thus, this study did not provide evidence that the survival of non-obese critically ill patients would have been improved if they had been obese, nor did it prove that their survival would have been worse.
Nevertheless, caution is warranted in the interpretation of the study findings. First, despite the inclusion of a large number of confounders, residual confounding due to inaccurate proxy variables or unmeasured variables cannot be ruled out. In particular, data on the patient’s dietary pattern and educational level were lacking and may have confounded the obesity-mortality relationship. Additionally, the inclusion of some confounders can be disputed. Indeed, the obesity status measured upon admission to the ICU is the result of a potentially long time-varying process, which makes, for example, control for malignancy potentially not entirely appropriate for eliminating confounding bias, as it may eliminate indirect effects of obesity via malignancy, while also introducing collider stratification bias [16]. Furthermore, it should be noted that 1754 (26.8%) patients were discarded in the traditional approach because of at least one missing value for exposure or confounders.
Secondly, while our analysis focused on the subgroup of non-obese patients to dampen the possible effect of collider stratification bias due to the selection of patients with a SAPS II of at least 32 points who have been admitted to the ICU, such bias in the ATU cannot be entirely ruled out. It may be biased when obesity affects the probability of either ICU admission or SAPS II being ≥ 32 points, as is likely the case. Overcoming the above two problems would necessitate a population cohort study, where individuals’ body weight and the evolution of confounders such as malignancy can be monitored over time [37]. Unfortunately, such data are currently lacking.
A third limitation of the current analysis is that the obtained estimate and its standard error may be subject to residual bias when the dependence of the exposure or outcome on the confounders is too complex to approximate well by super learning at the considered sample sizes [29]. Fourthly, while the MICE algorithm relaxes the implicit missing completely at random assumption of a complete case analysis (as in the first approach), it still relies on the untestable missing at random assumption and on correct specification of the parametric imputation model, which may further bias the TMLE estimator [34]. Finally, although a sensitivity analysis using different BMI cut-off values did not change the interpretation of the results, a possibly non-linear dose-response relationship between BMI and in-hospital mortality among critically ill patients may be inadequately captured by the binary nature of the definition used for obesity.
Conclusions
A causal inference approach that is robust to residual confounding bias due to model misspecification and selection bias due to missing (at random) data mitigates the obesity paradox observed in critically ill patients, whereas a traditional approach results in even more paradoxical findings. The robust approach does not provide evidence that the survival of non-obese critically ill patients would have been improved if they had been obese.
Availability of data and materials
The dataset used and analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- ATU:
-
Average treatment effect in the untreated
- BMI:
-
Body mass index
- CI:
-
Confidence interval
- ICU:
-
Intensive care unit
- MICE:
-
Multiple imputation by chained equations
- SAPS II:
-
Simplified Acute Physiology Score II
- TMLE:
-
Targeted maximum likelihood estimation
References
World Health Organization. Obesity: preventing and managing the global epidemic. Report of a WHO consultation. World Health Organ Tech Rep Ser. 2000;894(i-xii):1–253.
NCD Risk Factor Collaboration (NCD-RisC). Trends in adult body-mass index in 200 countries from 1975 to 2014: a pooled analysis of 1698 population-based measurement studies with 19.2 million participants. Lancet. 2016;387(10026):1377–96.
The GBD 2015 Obesity Collaborators. Health effects of overweight and obesity in 195 countries over 25 years. N Engl J Med. 2017;377(1):13–27.
Schetz M, De Jong A, Deane AM, Druml W, Hemelaar P, Pelosi P, et al. Obesity in the critically ill: a narrative review. Intensive Care Med. 2019;45(6):757–69.
Sakr Y, Alhussami I, Nanchal R, Wunderink RG, Pellis T, Wittebole X, et al. Being overweight is associated with greater survival in ICU patients: results from the intensive care over nations audit. Crit Care Med. 2015;43(12):2623–32.
Hutagalung R, Marques J, Kobylka K, Zeidan M, Kabisch B, Brunkhorst F, et al. The obesity paradox in surgical intensive care unit patients. Intensive Care Med. 2011;37(11):1793–9.
Pepper DJ, Demirkale CY, Sun J, Rhee C, Fram D, Eichacker P, et al. Does obesity protect against death in sepsis? A retrospective cohort study of 55,038 adult patients. Crit Care Med. 2019;47(5):643–50.
Pepper DJ, Sun J, Welsh J, Cui X, Suffredini AF, Eichacker PQ. Increased body mass index and adjusted mortality in ICU patients with sepsis or septic shock: a systematic review and meta-analysis. Crit Care. 2016;20(1):181.
Pickkers P, de Keizer N, Dusseljee J, Weerheijm D, van der Hoeven JG, Peek N. Body mass index is associated with hospital mortality in critically ill patients: an observational cohort study. Crit Care Med. 2013;41(8):1878–83.
Zhao Y, Li Z, Yang T, Wang M, Xi X. Is body mass index associated with outcomes of mechanically ventilated adult patients in intensive critical units? A systematic review and meta-analysis. PLoS One. 2018;13(6):e0198669.
Zhi G, Xin W, Ying W, Guohong X, Shuying L. "Obesity paradox" in acute respiratory distress syndrome: asystematic review and meta-analysis. PLoS One. 2016;11(9):e0163677.
Ball L, Serpa Neto A, Pelosi P. Obesity and survival in critically ill patients with acute respiratory distress syndrome: a paradox within the paradox. Crit Care. 2017;21(1):114.
Patel JJ, Rosenthal MD, Miller KR, Codner P, Kiraly L, Martindale RG. The critical care obesity paradox and implications for nutrition support. Curr Gastroenterol Rep. 2016;18(9):45.
Aune D, Sen A, Prasad M, Norat T, Janszky I, Tonstad S, et al. BMI and all cause mortality: systematic review and non-linear dose-response meta-analysis of 230 cohort studies with 3.74 million deaths among 30.3 million participants. BMJ. 2016;353:i2156.
Hernan MA, Robins JM. Causal inference: what if. Boca Raton: Chapman & Hall/CRC; 2020.
Banack HR, Kaufman JS. Estimating the time-varying joint effects of obesity and smoking on all-cause mortality using marginal structural models. Am J Epidemiol. 2016;183(2):122–9.
Banack HR, Stokes A. The 'obesity paradox' may not be a paradox at all. Int J Obes. 2017;41(8):1162–3.
Emerson P, Brooks D, Quasim T, Puxty A, Kinsella J, Lowe DJ. Factors influencing intensive care admission: a mixed methods study of EM and ICU. Eur J Emerg Med. 2017;24(1):29–35.
Smith G, Nielsen M. ABC of intensive care. Criteria for admission. BMJ (Clinical research ed). 1999;318(7197):1544–7.
Banack HR, Kaufman JS. Does selection bias explain the obesity paradox among individuals with cardiovascular disease? Ann Epidemiol. 2015;25(5):342–9.
Toft-Petersen AP, Wulff J, Harrison DA, Ostermann M, Margarson M, Rowan KM, et al. Exploring the impact of using measured or estimated values for height and weight on the relationship between BMI and acute hospital mortality. J Crit Care. 2018;44:196–202.
Wacharasint P, Boyd JH, Russell JA, Walley KR. One size does not fit all in severe infection: obesity alters outcome, susceptibility, treatment, and inflammatory response. Crit Care. 2013;17(3):R122.
Selim BJ, Ramar K, Surani S. Obesity in the intensive care unit: risks and complications. Hosp Pract (1995). 2016;44(3):146–56.
Lederer DJ, Bell SC, Branson RD, Chalmers JD, Marshall R, Maslove DM, et al. Control of confounding and reporting of results in causal inference studies. Guidance for authors from editors of respiratory, sleep, and critical care journals. Ann Am Thorac Soc. 2019;16(1):22–8.
National Institutes of Health. Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults - the evidence report. Obes Res. 1998;6(Suppl 2):51S–209S.
The Belgian statistical office (Statbel). Fiscal statistics on income by statistical sector [accessed on 24 Apr 2018]. Available from: https://statbel.fgov.be/nl/themas/huishoudens/fiscale-inkomens/plus.
Schuler MS, Rose S. Targeted maximum likelihood estimation for causal inference in observational studies. Am J Epidemiol. 2017;185(1):65–73.
van der Laan MJ, Rubin D. Targeted maximum likelihood learning. Int J Biostat. 2006;2(1):Art 11.
van der Laan M, Rose S. Targeted learning in data science: causal inference for complex longitudinal studies. Cham: Springer International Publishing; 2018.
van der Laan M, Gruber S. One-step targeted minimum loss-based estimation based on universal least favorable one-dimensional submodels. Int J Biostat. 2016;12(1):351–78.
Lendle SD, Subbaraman MS, van der Laan MJ. Identification and efficient estimation of the natural direct effect among the untreated. Biometrics. 2013;69(2):310–7.
LeDell E, van der Laan MJ, Petersen M. AUC-maximizing ensembles through metalearning. Int J Biostat. 2016;12(1):203–18.
van der Laan MJ, Polley EC, Hubbard AE, et al. Stat Appl Genet Mol Biol. 2007;6:Article25.
van Buuren S, Groothuis-Oudshoorn K. mice: multivariate imputation by chained equations in R. J Statistical Software. 2011;45(3):67.
Rubin D. Multiple imputation for nonresponse in surveys. Hoboken: Wiley; 1987.
R Core Team. R: a language and environment for dtatistical computing. Vienna; 2016. Available from: https://www.R-project.org/.
Vansteelandt S. Asking too much of epidemiologic studies: the problem of collider bias and the obesity paradox. Epidemiology (Cambridge, Mass). 2017;28(5):e47–e9.
Acknowledgements
We would like to thank Bram Gadeyne, Data Engineer for the Intensive Care Unit of the Ghent University Hospital, for the data extraction from the Intensive Care Information System database, as well as Cloë Ost, Statistical Product Manager for Statbel, for providing the list of the statistical sectors to which each patient belongs.
Funding
None.
Author information
Authors and Affiliations
Contributions
AD, JS, JD, and SV conceived the study. AD and DB applied for the ethics approval. AD, KC, DB, and JD contributed to the acquisition of data. AD, JS, and SV conceived, performed, and interpreted the statistical analysis. AD drafted the manuscript. All authors were involved in the data interpretation and critically revised the draft versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was approved by the local ethics committee of the Ghent University Hospital (project number 2018/0536) and was conducted in accordance with the Declaration of Helsinki. The need for informed consent was waived due to its retrospective nature.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Additional file 1.
Details of the super learning procedure.
Additional file 2.
Specification of the imputation model.
Additional file 3.
Sensitivity analysis using different BMI cut-off values.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Decruyenaere, A., Steen, J., Colpaert, K. et al. The obesity paradox in critically ill patients: a causal learning approach to a casual finding. Crit Care 24, 485 (2020). https://doi.org/10.1186/s13054-020-03199-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13054-020-03199-5