Abstract
This article is one of ten reviews selected from the Annual Update in Intensive Care and Emergency Medicine 2019. Other selected articles can be found online at https://www.biomedcentral.com/collections/annualupdate2019. Further information about the Annual Update in Intensive Care and Emergency Medicine is available from http://www.springer.com/series/8901.
Similar content being viewed by others
Introduction
The acute respiratory distress syndrome (ARDS) is a hypoxemic syndrome primarily treated using supportive mechanical ventilation. Although mechanical ventilation is life-saving, it can cause ventilator-induced lung injury (VILI). Therefore, the goal of mechanical ventilation is to achieve adequate gas exchange while minimizing lung injury. Multiple mechanical ventilation strategies have been developed to limit VILI. These strategies are based on the pathophysiological concept that alveolar overdistention, shear-stress, and atelectrauma (i.e., the cyclical opening and closing of unstable alveoli) are possible mechanisms that result in VILI. Targets that might aggravate or attenuate VILI, notably tidal volume and positive end-expiratory pressure (PEEP), have become the subject of extensive research.
The ARDS Network (ARDSNet) trial aimed to reduce overdistention, if necessary at the cost of suboptimal gas exchange [1]. This trial demonstrated that a ventilation strategy with low tidal volume and limited plateau pressure (Pplat ≤30 cmH2O) reduced mortality rate. Lachmann proposed to reduce atelectrauma and shear-stress by using recruitment maneuvers and subsequent use of higher PEEP: the “open lung concept” [2].
The open lung concept, combined with low tidal volume seems appealing from a pathophysiological perspective, and has been very promising in experimental ARDS models [3, 4]. However, clinical evidence is inconsistent. A meta-analysis comparing higher PEEP (13–15 cmH2O) and low PEEP ventilation strategies reported a reduction in mortality rate, but only in a subgroup analysis of patients with moderate to severe ARDS [5]. Another meta-analysis reported a reduced mortality rate in patients with ARDS treated according to the open lung concept [6]. Amato and colleagues demonstrated in a multilevel mediation analysis that an increase in PEEP reduced mortality rate in patients with ARDS, but only if this resulted in a decreased driving pressure [7].
The recent Alveolar Recruitment for ARDS Trial (ART) renewed the controversies about the efficacy of recruitment maneuvers and application of higher PEEP levels [8]. This trial reported that a recruitment maneuver combined with higher PEEP increased mortality rate in patients with moderate to severe ARDS. It was proposed that the overdistention caused by recruitment maneuvers and higher PEEP might be more harmful than the shear-stress and atelectrauma it prevents [9]. This raises the following question: should we abandon the open lung concept in our patients with ARDS?
In this chapter, we will briefly discuss the pathophysiology of ARDS and VILI, limitations and indications of the open lung concept, bedside monitoring to guide the open lung concept, and airway pressure release ventilation (APRV) as an alternative.
The pathophysiology of ARDS and VILI
The pathophysiology of ARDS is based on the triad of alveolar-capillary membrane injury, high-permeability (alveolar) edema and inflammation [10]. Histologically this is characterized by diffuse alveolar damage [11]. The “baby lung” model describes the pathophysiological effects of ARDS, mainly edema, on lung mechanics [12]. It is based on observations that atelectasis and edema are preferentially distributed to dependent lung regions, whereas independent lung regions are relatively well-aerated. The amount of collapse and edema formation correlates with ARDS severity. Although intrinsic elasticity of the independent lung region is nearly normal, lung function is restricted by the collapsed dependent lung region. Because the ARDS lung is small and not stiff, the term “baby lung” was proposed [12].
The pathophysiological triad cannot be routinely measured in clinical practice. Therefore, arterial hypoxemia and bilateral opacities on chest imaging are used as clinical surrogates in the Berlin definition of ARDS [13]. Because the Berlin definition is not based on pathophysiological criteria, it poses several limitations in clinical research. Only half of clinically diagnosed patients with ARDS have diffuse alveolar damage at autopsy [14]. In addition, pulmonary and extrapulmonary insults may induce ARDS, both with a different response to PEEP [15]. As a consequence, ARDS is a heterogeneous syndrome.
The Berlin definition of ARDS specified disease severity according to the PaO2/FiO2 ratio at a PEEP level of at least 5 cmH2O. This classification is important, as recruitability is dependent on disease severity. However, PEEP has a major effect on the PaO2/FiO2 ratio and application of high PEEP could mask ARDS severity. Caironi and colleagues [16] reported that 54% of patients with mild ARDS at clinical PEEP (i.e., > 5 cmH2O) were reclassified as either moderate or severe ARDS at 5 cmH2O PEEP. In addition, the correlation between ARDS severity and lung recruitability improved significantly at 5 cmH2O [16]. Therefore, a fixed PEEP level should be used to assess disease severity and recruitability.
Injurious mechanical ventilation in experimental models results in diffuse alveolar damage, including interstitial and alveolar edema, hyaline membrane formation, and cell infiltration [17]. Therefore, VILI cannot be distinguished from ARDS and is potentially the most important insult that sustains or aggravates ARDS. As ARDS is characterized by baby lungs, alveolar overdistention of the independent lung is considered to be a major contributor to VILI. Initially it was unclear whether high tidal volume, high airway pressure, or both resulted in VILI. Dreyfuss and colleagues distinguished tidal volume from airway pressures in a rat model [18]. Pulmonary edema formation was assessed after 20 min of mechanical ventilation according to the following protocols: (1) high pressure (45 cmH2O) and high tidal volume (40 mL/kg); (2) high pressure (45 cmH2O) and lower tidal volume (19 mL/kg)—lower tidal volume was achieved by a thoracoabdominal strap avoiding chest wall distention; and (3) negative inspiratory pressure (iron lung) and high tidal volume (44 mL/kg). They observed that edema increased significantly in groups 1 and 3 compared to group 2, indicating that high volume and not high pressure caused lung injury. In addition, in a fourth group they reported that 10 cmH2O PEEP reduced edema formation.
Protti and colleagues demonstrated the beneficial effect of PEEP in combination with a reduced tidal volume [3]. In a pig model, they divided the end-inspiratory lung volume (i.e., strain) into a component generated by PEEP (static strain = PEEP volume/functional residual capacity) and a component generated by tidal volume (dynamic strain = tidal volume/functional residual capacity). Four groups were ventilated with a total strain of 2.5 (close to total lung capacity): (1) VPEEP 0% and tidal volume 100%; (2) VPEEP 25% and tidal volume 75%; (3) VPEEP 50% and tidal volume 50%; and (4) VPEEP 75% and tidal volume 25%. After 54 h, all pigs in the tidal volume 100% group had died due to massive lung edema, whereas none of the pigs in the VPEEP 75% and tidal volume 25% group had died or developed pulmonary edema. At the end of the experiment, sudden removal of PEEP in the last group did not result in pulmonary edema formation, indicating that the integrity of the alveolar-capillary barrier was preserved and PEEP did not only counteract the extravasation of plasma. PEEP has a protective effect, but tidal volume should be reduced during application of high PEEP levels.
The use of higher PEEP levels is accompanied by an increase in Pplat > 30 cmH2O. Since the ARDSNet trial reported that a combination of low tidal volume and a Pplat ≤30 cmH2O reduced mortality rates, physicians are cautious with the use of high airway pressures. However, Pplat is exerted over the entire respiratory system, including the lungs and chest wall. Chest wall elastance varies widely in patients with ARDS and contributes between 20 and 50% to total respiratory system elastance (ERS) [19]. A Pplat of 30 cmH2O exerted at a stiff chest wall (50% of ERS) results in a transpulmonary pressure of 15 cmH2O, whereas a similar Pplat exerted at a normal chest wall (20% of ERS) results in a transpulmonary pressure of 24 cmH2O. Therefore, Pplat provides little information about the transpulmonary pressure, i.e., the distending force on the lung.
In conclusion, there is sufficient experimental evidence that high tidal volume and not high airway pressure is important in the development of VILI. In addition, higher PEEP levels are beneficial if tidal volume is reduced in order to limit the total strain (overdistention). Thus, a combination of higher PEEP and low tidal volume should be applied to reduce the development of VILI.
The open lung concept
In 1970, Mead and colleagues developed a mathematical model to estimate intrapulmonary pressures in a heterogeneously ventilated lung [20]. They stated that at the interfaces of open and collapsed lung, a transpulmonary pressure of 30 cmH2O could result in local pressures of 140 cmH2O. Based on these estimates, Lachmann hypothesized that shear-stress might be the major cause of structural damage and VILI [2]. In order to minimize shear-stress and atelectrauma in heterogeneously ventilated lungs, he proposed to “open up the lung and keep the lung open”.
Traditionally the open lung concept consists of a recruitment maneuver to open up the collapsed lung and high PEEP to maintain alveolar stability. According to the LaPlace law (P = 2γ/r, where P is the pressure within an alveolus, γ is the surface tension of the alveolar wall, and r is the radius of the alveolus), more pressure is required to open a collapsed or deflated alveolus in comparison to an open alveolus. Surfactant impairment in severe ARDS further increases opening pressure as a result of increased surface tension. In addition, the opening pressure of collapsed alveoli has to overcome the alveolar retractive force and the compressing force on the alveolus by surrounding lung tissue. The sum of these pressures is estimated to be 45–60 cmH2O in patients with ARDS [9].
An elegant example of opening the dependent lung, although not by using high airway pressures, is the application of prone positioning. In the supine position, the weight of the ventral lungs, heart and abdominal viscera increases pleural pressure in the dorsal lung regions. The decrease in transpulmonary pressure (airway pressure minus pleural pressure) results in a reduced distending force on the dependent lung. In addition, pulmonary edema in ARDS gradually increases lung mass. Eventually the dependent lung collapses under its own weight and ventilation is redistributed to the baby lung. Application of the prone position changes gravitational forces; the dorsal lung becomes the independent lung region and is re-aerated. Due to conformational shape matching (the anatomic tendency to overdistend ventral lung regions despite gravitational forces) and a greater lung mass on the dorsal side, aeration in the prone position is more homogeneously distributed [21]. Perfusion is also distributed more homogeneously in the prone position. As a result, ventilation-perfusion matching and oxygenation improves [22]. Early large randomized controlled trials did not confirm the theoretical advantages of prone positioning. However, a meta-analysis suggested a reduction in mortality rate in patients with severe ARDS [23]. The beneficial effects of prone positioning were confirmed by the PROSEVA trial [24]. Patients with severe ARDS (PaO2/FiO2 ratio < 150 mmHg) assigned to the prone group had a significantly lower 28-day mortality rate (16.0%) compared to the supine group (32.8%). Therefore, opening up the lung by prone positioning is recommended in severe ARDS.
The open lung concept in mild to moderate ARDS
The American Thoracic Society Clinical Practice Guideline for mechanical ventilation in adult patients with ARDS recommends limiting Pplat to 30 cmH2O, in line with the ARDSNet trial [25]. This raises the following question: can a lung be fully open at a Pplat ≤30 cmH2O? Cressoni and colleagues investigated whether mechanical ventilation with a Pplat of 30 cmH2O actually recruited the lung [26]. They included 33 patients with mild to severe ARDS. Four computed tomography (CT) scans were done: one at 5 cmH2O PEEP, and three at Pplat of 19 ± 0, 28 ± 0, and 40 ± 2 cmH2O during a < 5 s breath holding episode. Lung recruitment was defined as the amount of lung tissue (grams) that regained inflation as a result of the applied airway pressures (Fig. 1). They found that the amount of lung recruitment achieved with a Pplat increase from 30 to 45 cmH2O was negligible in patients with mild to moderate ARDS. In contrast, a similar increase in Pplat in patients with severe ARDS resulted in a significant amount of lung recruitment. These results confirm that the amount of recruitable tissue increases with ARDS severity.
Lung recruitment as a function of airway pressure. This figure represents the amount of lung tissue (grams) recruited as a function of applied airway pressure. Estimates were based on computed tomography (CT) images of patients with acute respiratory distress syndrome (ARDS). Green: mild ARDS, blue: moderate ARDS, red: severe ARDS, dark red: severe ARDS with venovenous extracorporeal membrand oxygenation (VV-ECMO). From [26] with permission
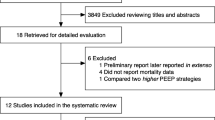
Multiple clinical studies have assessed the effects of recruitment maneuvers in patients with ARDS. A recent meta-analysis included 15 randomized controlled trials (a total of 3134 patients) that compared the open lung concept with other mechanical ventilation strategies in patients with ARDS [6]. The authors reported a reduced mortality rate in the patients treated according to the open lung concept. However, this meta-analysis was performed prior to the ART trial. The multicenter ART included 1010 patients with moderate to severe ARDS [8]. The objective of the study was to compare recruitment maneuvers with PEEP titrated according to best respiratory system compliance (“High PEEP”) to the ARDSNet protocol (“Low PEEP”). The initial recruitment maneuver consisted of PEEP increments up to a maximum Pplat of 60 cmH2O. Subsequently, a decremental PEEP trial was performed and the PEEP associated with the best compliance plus 2 cmH2O was applied. After three cases of resuscitated cardiac arrests, the recruitment maneuver was modified to a maximum Pplat of 50 cmH2O. The high PEEP strategy resulted in an increased 28-day mortality rate (55.3% vs. 49.3%). There are two major explanations for the increased mortality rate after a recruitment maneuver. A first explanation is the included study population, as 599 of 1010 patients (59.3%) had moderate ARDS. According to Fig. 1, an increase of Pplat to 60 cmH2O in moderate ARDS results in a negligible amount of recruited lung tissue at the cost of overdistention. A subgroup analysis supports this hypothesis, as the increase in mortality rate was more pronounced in patients with moderate ARDS, whereas mortality was similar in the two groups in patients with severe ARDS. Gattinoni and colleagues estimated the power delivered to the lung during the ART trial. They found that the power delivered to mild ARDS lungs was three times greater than to severe ARDS lungs (1169 J vs. 390 J) [9]. Second, the ART trial did not distinguish between responders and non-responders. A mean reduction in driving pressure of only 2 cmH2O was found, indicating that the recruitment maneuver was inadequate to open up the lung and increase functional residual capacity in most patients. In conclusion, this study found an increased mortality rate after the application of a mild recruitment maneuver and subsequent PEEP titration based on best compliance in patients with moderate ARDS.
In addition, a trial comparing high-frequency oscillatory ventilation (HFOV) with the ARDSNet protocol in patients with moderate to severe ARDS was terminated prematurely, as a trend towards increased mortality was observed in the HFOV group [27]. In this trial, HFOV was applied in accordance with the open lung concept strategy: first a recruitment maneuver was performed by increasing the distending pressure to 40 cmH2O. Subsequently, mean airway pressure was set at 30 cmH2O and reduced based on target oxygenation in combination with very low tidal volume (1–2 mL/kg) and high respiratory frequency. However, as in the ART trial, a subgroup analysis demonstrated that mortality rate was not increased if HFOV was applied in patients with severe ARDS. An individual patient data meta-analysis of four HFOV trials (1552 patients with ARDS) found that HFOV might even reduce mortality rate in patients with severe ARDS, whereas mortality was increased in patients with mild ARDS [28]. This suggests that a strategy of higher mean airway pressure results in an increased mortality rate in patients with moderate ARDS due to PEEP or distending pressure, whereas in patients with severe ARDS higher mean airway pressure might be beneficial.
The open lung concept in severe ARDS
In patients with severe refractory hypoxemia under the ARDSNet protocol there are three possible treatment strategies: (1) maintain ARDSNet protocol and accept hypoxemia; (2) convert to venovenous extracorporeal membrane oxygenation (VV-ECMO); or (3) initiate mechanical ventilation according to the open lung concept, thus accepting airway pressures > 30 cmH2O (Fig. 2). The EOLIA trial compared early application of VV-ECMO with the ARDSNet protocol in patients with very severe ARDS [29]. The authors reported that VV-ECMO did not reduce 60-day mortality rate. In addition, VV-ECMO is associated with a high complication rate (up to 40%), including intracranial hemorrhage resulting in death [30].
In a retrospective analysis of patients treated according to the open lung concept who met the EOLIA inclusion criteria, we observed a 30-day mortality rate of 25% as compared to 35–46% in the EOLIA trial [29]. This supports our hypothesis that there is an indication for the open lung concept in patients with severe ARDS. However, it is essential that recruitment maneuvers and high Pplat are guided by strict monitoring.
Inspiratory pressure is limited by transpulmonary pressure instead of Pplat. Transpulmonary pressure is estimated with an esophageal balloon catheter. An inspiratory transpulmonary pressure of < 25 cmH2O is considered to be lung protective ventilation regardless of Pplat [19]. Grasso and colleagues measured transpulmonary pressure in 14 patients with severe ARDS who were referred to their ICU for VV-ECMO [19]. In half of the patients, transpulmonary pressure was > 25 cmH2O and in these patients VV-ECMO was initiated. In the other patients, transpulmonary pressure was < 25 cmH2O and therefore PEEP was increased from 17 to 22 cmH2O until transpulmonary pressure was equal to 25 cmH2O. The authors accepted airway pressures up to 38 cmH2O. In these patients, oxygenation improved and they did not require VV-ECMO.
In order to prevent overdistention, it is important to distinguish responders to a recruitment maneuver from non-responders. Responders can be identified by an increase in oxygenation, compliance and/or a significant reduction in driving pressure. The reduction in driving pressure is a direct result of opening up the lung, thereby increasing functional residual capacity. In our experience, driving pressure is reduced rapidly after a recruitment maneuver in responders. The extent to which the driving pressure has to decrease in order to be a responder is unclear. The multilevel mediation analysis by Amato and colleagues suggests that a driving pressure of ≤15 cmH2O reduces mortality rate in patients with ARDS [7]. However, in the ART trial, driving pressure was reduced from 13.5 to 11.5 cmH2O after a recruitment maneuver and still resulted in an increased mortality rate. Although driving pressure decreased initially, an increase was observed afterwards, whereas driving pressure in the control group remained stable. This suggests that maintaining a low stable driving pressure might be more important than the absolute value of the driving pressure. In non-responders, functional residual capacity does not increase after a recruitment maneuver. Thus, PEEP should not be increased as this results in increased overdistention of the baby lung (Fig. 3).
Responders and non-responders to the open lung concept (OLC). Lung aeration at expiration is schematically depicted in the ARDSNet protocol (left) and in responders (middle) and non-responders (right) to the OLC, i.e., a recruitment maneuver and higher positive end-expiratory pressure (PEEP) levels. In responders, functional residual capacity increases in response to recruitment, resulting in reduced strain and driving pressure. In non-responders, functional residual capacity does not increase following a recruitment maneuver. Subsequent application of higher PEEP levels results in alveolar overdistention. Very light blue: overdistention; light blue: normally aerated lung tissue; dark blue: collapsed alveoli
Slow recruitment with airway pressure release ventilation
Time is an important variable in both alveolar recruitment and stabilization, yet often overlooked. The application of 30 cmH2O to a lung inflated at 5 cmH2O for 2 s opens up approximately 75% of alveoli [31]. Continuation of 30 cmH2O for 40 s gradually increases the proportion of open alveoli to 85%. In the expiratory phase, there is a delay of approximately 0.17 s before alveolar collapse commences and at 0.25 s an alveolus is collapsed [32]. Inspiration time in the ARDSNet protocol is too short to recruit the majority of alveoli and too long to prevent the alveoli from collapsing. APRV might address both problems. APRV consists of a continuous positive airway pressure (Phigh) with a brief intermittent release phase (Plow) for expiration and CO2 removal. Patients are allowed to breath spontaneously independent of ventilator cycles. Phigh slowly recruits the lung and a short Plow prevents alveolar collapse. Eventually, the lung is open and stable. However, in experimental models, heterogeneity is increased if Plow is set too long, giving the alveoli sufficient time to collapse [33]. Zhou and colleagues compared APRV 50% with the ARDSNet protocol in patients with moderate to severe ARDS [34]. They reported a trend towards a reduced ICU mortality in the APRV group: 19.7% vs. 34.3%. The number of ventilator-free days, oxygenation and respiratory system compliance were in favor of the APRV group. In this study, the investigators aimed for a spontaneous minute ventilation of at least 30% of total minute ventilation. The contraction of the diaphragm during spontaneous breathing is more pronounced in the dorsal lung region and assists in opening up even the most dependent lung regions. In conclusion, APRV results in an open lung by slow recruitment, alveolar stabilization and contraction of the diaphragm.
Conclusion
The objective of the open lung concept is to achieve an open and homogeneously ventilated lung. From a pathophysiological perspective the open lung concept seems beneficial, because shear-stress and atelectrauma are reduced. An open and more homogeneously ventilated lung can be achieved by the application of prone position or high airway pressures. In patients with severe ARDS, prone position has been shown to reduce mortality rates.
Multiple studies using recruitment maneuvers with airway pressures up to 50–60 cmH2O showed improved oxygenation and not reduced mortality rates. The ART trial found an increased mortality rate when a recruitment maneuver was combined with decremental PEEP titration based on best compliance in patients with moderate to severe ARDS [8]. The application of HFOV and high mean airway pressures in patients with ARDS increased mortality rate as well [27]. However, subgroup analyses of both trials showed that mortality rate increased in patients with moderate ARDS, but was similar or even reduced in patients with severe ARDS [28]. Apparently, the application of higher PEEP or distending pressures increases mortality in patients with moderate ARDS due to overdistention, despite best PEEP titration. This observation indicates that high airway pressures should not be used in patients with moderate ARDS.
We propose that the open lung concept should be applied in patients with severe ARDS with refractory hypoxemia under the ARDSNet protocol, but only if a patient is a responder to recruitment. In patients who do not respond to recruitment, PEEP should be reduced and VV-ECMO may be considered. As both the open lung concept and VV-ECMO require clinical expertise, we recommend that this strategy be applied in tertiary referral centers. The exact definition of a responder remains to be elucidated. After a recruitment maneuver, driving pressure, oxygenation, and compliance should improve, but to what extent remains unclear.
References
Brower R, Matthay M, Morris A, et al. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. The acute respiratory distress syndrome network. N Engl J Med. 2000;342:1301–8.
Lachmann B. Open up the lung and keep the lung open. Intensive Care Med. 1992;18:319–21.
Protti A, Andreis DT, Monti M, et al. Lung stress and strain during mechanical ventilation: any difference between statics and dynamics? Crit Care Med. 2013;41:1046–55.
Jain SV, Kollisch-Singule M, Satalin J, et al. The role of high airway pressure and dynamic strain on ventilator-induced lung injury in a heterogeneous acute lung injury model. Intensive Care Med Exp. 2017;5:25.
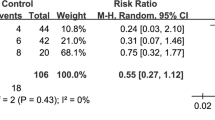
Briel M, Meade M, Mercat A, et al. Higher vs lower positive end-expiratory pressure in patients with acute lung injury and acute respiratory distress syndrome: systematic review and meta-analysis. JAMA. 2010;303:865–73.
Lu J, Wang X, Chen M, et al. An open lung strategy in the management of acute respiratory distress syndrome: a systematic review and meta-analysis. Shock. 2017;48:43–53.
Amato MB, Meade MO, Slutsky AS, et al. Driving pressure and survival in the acute respiratory distress syndrome. N Engl J Med. 2015;372:747–55.
Cavalcanti AB, Suzumura EA, Laranjeira LN, et al. Effect of lung recruitment and titrated positive end-expiratory pressure (PEEP) vs low PEEP on mortality in patients with acute respiratory distress syndrome: a randomized clinical trial. JAMA. 2017;318:1335–45.
Cipulli F, Vasques F, Duscio E, Romitti F, Quintel M, Gattinoni L. Atelectrauma or volutrauma: the dilemma. J Thorac Dis. 2018;10:1258–64.
Thompson BT, Chambers RC, Liu KD. Acute respiratory distress syndrome. N Engl J Med. 2017;377:1904–5.
Tomashefski JF Jr. Pulmonary pathology of acute respiratory distress syndrome. Clin Chest Med. 2000;21:435–66.
Gattinoni L, Marini JJ, Pesenti A, Quintel M, Mancebo J, Brochard L. The “baby lung” became an adult. Intensive Care Med. 2016;42:663–73.
Ranieri VM, Rubenfeld GD, Thompson BT, et al. Acute respiratory distress syndrome: the Berlin definition. JAMA. 2012;307:2526–33.
Kao KC, Hu HC, Chang CH, et al. Diffuse alveolar damage associated mortality in selected acute respiratory distress syndrome patients with open lung biopsy. Crit Care. 2015;19:228.
Gattinoni L, Pelosi P, Suter PM, Pedoto A, Vercesi P, Lissoni A. Acute respiratory distress syndrome caused by pulmonary and extrapulmonary disease. Different syndromes? Am J Respir Crit Care Med. 1998;158:3–11.
Caironi P, Carlesso E, Cressoni M, et al. Lung recruitability is better estimated according to the Berlin definition of acute respiratory distress syndrome at standard 5 cmH2O rather than higher positive end-expiratory pressure: a retrospective cohort study. Crit Care Med. 2015;43:781–90.
Slutsky AS, Ranieri VM. Ventilator-induced lung injury. N Engl J Med. 2013;369:2126–36.
Dreyfuss D, Soler P, Basset G, Saumon G. High inflation pressure pulmonary edema. Respective effects of high airway pressure, high tidal volume, and positive end-expiratory pressure. Am Rev Respir Dis. 1988;137:1159–64.
Grasso S, Terragni P, Birocco A, et al. ECMO criteria for influenza a (H1N1)-associated ARDS: role of transpulmonary pressure. Intensive Care Med. 2012;38:395–403.
Mead J, Takishima T, Leith D. Stress distribution in lungs: a model of pulmonary elasticity. J Appl Physiol. 1970;28:596–608.
Gattinoni L, Taccone P, Carlesso E, Marini JJ. Prone position in acute respiratory distress syndrome. Rationale, indications, and limits. Am J Respir Crit Care Med. 2013;188:1286–93.
Henderson AC, Sa RC, Theilmann RJ, Buxton RB, Prisk GK, Hopkins SR. The gravitational distribution of ventilation-perfusion ratio is more uniform in prone than supine posture in the normal human lung. J Appl Physiol (1985). 2013;115:313–24.
Sud S, Friedrich JO, Taccone P, et al. Prone ventilation reduces mortality in patients with acute respiratory failure and severe hypoxemia: systematic review and meta-analysis. Intensive Care Med. 2010;36:585–99.
Guerin C, Reignier J, Richard JC, et al. Prone positioning in severe acute respiratory distress syndrome. N Engl J Med. 2013;368:2159–68.
Fan E, Del Sorbo L, Goligher EC, et al. An official American Thoracic Society/European Society of Intensive Care Medicine/Society of Critical Care Medicine clinical practice guideline: mechanical ventilation in adult patients with acute respiratory distress syndrome. Am J Respir Crit Care Med. 2017;195:1253–63.
Cressoni M, Chiumello D, Algieri I, et al. Opening pressures and atelectrauma in acute respiratory distress syndrome. Intensive Care Med. 2017;43:603–11.
Ferguson ND, Cook DJ, Guyatt GH, et al. High-frequency oscillation in early acute respiratory distress syndrome. N Engl J Med. 2013;368:795–805.
Meade MO, Young D, Hanna S, et al. Severity of hypoxemia and effect of high-frequency oscillatory ventilation in acute respiratory distress syndrome. Am J Respir Crit Care Med. 2017;196:727–33.
Combes A, Hajage D, Capellier G, et al. Extracorporeal membrane oxygenation for severe acute respiratory distress syndrome. N Engl J Med. 2018;378:1965–75.
Vaquer S, de Haro C, Peruga P, Oliva JC, Artigas A. Systematic review and meta-analysis of complications and mortality of veno-venous extracorporeal membrane oxygenation for refractory acute respiratory distress syndrome. Ann Intensive Care. 2017;7:51.
Albert SP, Dirocco J, Allen GB, et al. The role of time and pressure on alveolar recruitment. J Appl Physiol (1985). 2009;106:757–65.
Hasan D, Satalin J, van der Zee P, et al. Excessive extracellular ATP desensitizes P2Y2 and P2X4 ATP receptors provoking surfactant impairment ending in ventilation-induced lung injury. Int J Mol Sci. 2018;19:1185.
Kollisch-Singule M, Emr B, Smith B, et al. Airway pressure release ventilation reduces conducting airway micro-strain in lung injury. J Am Coll Surg. 2014;219:968–76.
Zhou Y, Jin X, Lv Y, et al. Early application of airway pressure release ventilation may reduce the duration of mechanical ventilation in acute respiratory distress syndrome. Intensive Care Med. 2017;43:1648–59.
Acknowledgments
We thank Nathalie Timmerman for her support in the preparation of figures.
Funding
Publications costs were funded by the Department of Adult Intensive Care Medicine, Erasmus MC Rotterdam, The Netherlands.
Availability of data and materials
Not applicable.
Author information
Authors and Affiliations
Contributions
All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
Diederik Gommers received speakers fee and travel expenses from Dräger, GE healthcare (medical advisory board 2009–2012), Maquet, and Novalung (medical advisory board).
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
van der Zee, P., Gommers, D. Recruitment Maneuvers and Higher PEEP, the So-Called Open Lung Concept, in Patients with ARDS. Crit Care 23, 73 (2019). https://doi.org/10.1186/s13054-019-2365-1
Published:
DOI: https://doi.org/10.1186/s13054-019-2365-1