Abstract
Background
Post-resuscitation care after out-of-hospital cardiac arrest (OHCA) is challenging due to the threat of organ failure and difficult prognostication. Our aim was to examine whether urine biomarkers could give an early prediction of acute kidney injury (AKI) and outcome.
Methods
This was a prospective observational study of comatose OHCA patients at Oslo University Hospital Ullevål, Norway. Risk factors were clinical parameters and biomarkers measured in spot urine (cystatin C, neutrophil gelatinase-associated lipocalin (NGAL) and the product of tissue inhibitor of metalloproteinase 2 (TIMP-2) and insulin-like growth factor-binding protein 7 (IGFBP7)) at admission and day 3. Outcome variables were AKI within 3 days using the Kidney Disease Improving Global Outcomes definition, 6-month mortality, and poor neurological outcome (PNO) defined as cerebral performance category 3–5.
Results
Among 195 included patients (85 % males, mean age 60 years), 88 (45 %) died, 96 (49 %) had PNO, and 88 (45 %) developed AKI. In univariate analysis, increased urine cystatin C and NGAL concentration sampled at admission and day 3 were independent risk factors for AKI, mortality and PNO. Increased urine TIMP-2 × IGFBP7 levels was associated with AKI only at admission. In multivariate analyses combining clinical parameters and biomarker concentrations, the area under the receiver operating characteristics curve (AuROC) with 95 % confidence interval (CI) were 0.774 (0.700–0.848), 0.812 (0.751–0.873), and 0.819 (0.759–0.878) for AKI, mortality and PNO, respectively.
Conclusions
In comatose OHCA patients, urine levels of cystatin C and NGAL at admission and day 3 were independent risk factors for AKI, 6-month mortality and PNO.
Trial registration
Clinicaltrials.gov NCT01239420. Registered 10 November 2010.
Similar content being viewed by others
Background
Out-of-hospital cardiac arrest (OHCA) is a major health problem, with an incidence rate in Europe of 84 per 100,000 inhabitants per year [1]. Among those admitted to hospital with return of spontaneous circulation (ROSC), mortality at 30 days, or to hospital discharge is on average 58 % [1], but with large variations across sites. Due to the reperfusion injury seen in the post cardiac arrest (CA) syndrome [2, 3], these patients are disposed to develop multiple organ failure [4], with acute kidney injury (AKI) affecting about half of the survivors [5, 6]. Even though most organ functions recover, some patients suffer long-time disability with poor neurological outcome (PNO) [7]. A huge challenge for clinicians is the lack of reliable predictors of AKI, mortality, and neurological outcome after OHCA. An early diagnostic and/or prognostic biomarker could potentially optimize targeted post-resuscitation care and reduce the burden of futile treatment to patients, relatives and the healthcare system [8].
There are many candidate biomarkers that aim to predict AKI and prognosis after CA, but none of these have discriminating power high enough to be recommended for routine use [9, 10]. Promising biomarkers of AKI are cystatin C [11], neutrophil gelatinase-associated lipocalin (NGAL) [12], tissue inhibitor of metalloproteinase 2 (TIMP-2), and insulin-like growth factor-binding protein 7 (IGFBP7) [13]. Recent studies of CA patients revealed that NGAL measured in blood was a predictor of AKI [14], mortality [14, 15], and neurological outcome [14]. In one of the studies, enrolment serum NGAL actually performed better than neuron-specific enolase and S100B in predicting survival to hospital discharge [14]. However, we lack data on the diagnostic and prognostic utility of the AKI biomarkers cystatin C, NGAL, TIMP-2, and IGFBP7 measured in urine early after OHCA.
The primary aim of this study was to examine the ability of urine biomarkers to predict AKI, mortality and PNO after OHCA. The secondary aim was to find the optimal biomarker and sampling time, and to investigate if the discriminating power was improved in models combining clinical parameters and biomarker concentrations.
Methods
Study design and setting
Patients were consecutively enrolled in this prospective study of OHCA patients as an a priori planned substudy of the yet not published Norwegian Cardiorespiratory Arrest Study (NORCAST) (NCT01239420). The primary aim of the NORCAST study was to assess early predictors of patient outcome after OHCA. Oslo University Hospital Ullevål is a community hospital for approximately 200,000 people, and a regional hospital for 1.4 million people in Norway, with around 45,000 admissions per year. The Regional Committee for Medical Research Ethics of Eastern and Southern Norway approved the study.
Study population
Adult (≥18 years) comatose (Glasgow Coma Scale ≤8 at admission) OHCA patients with ROSC admitted between 8 September 2010 and 13 January 2014 were included. Patients with known chronic kidney disease (CKD), or who died within 24 h of intensive care unit (ICU) stay, or for some reason did not receive active treatment, were excluded. Patients were treated according to our own standard operating procedure (SOP) for OHCA, including the use of targeted temperature management (TTM), with target set at 33 °C for 24 h. Patients were followed in detail during their hospital stay until an extensive 6-month post-arrest consultation.
Study definitions
OHCA was defined as the absence of spontaneous respiration in a comatose patient receiving cardiopulmonary resuscitation (CPR). ROSC was identified as sustained electrical activity on the electrocardiogram generating a palpable pulse. AKI and CKD were classified according to Kidney Disease Improving Global Outcomes (KDIGO) guidelines [16, 17], but only data from the first 3 days of ICU stay were assessed. In the definition of AKI the patients’ steady-state creatinine concentrations prior to CA remain unknown, and we used relative changes from creatinine levels at admission. The worst of the serum creatinine and urine output criteria was considered, and all patients undergoing renal replacement therapy (RRT) were classified as stage 3. PNO was defined as a cerebral performance category 3–5 [7]. Severity of illness was assessed using the Simplified Acute Physiology Score (SAPS) II [18], and the extent of organ failure was considered utilizing the Sequential Organ Failure Assessment (SOFA) score [19].
Data collection
Baseline characteristics such as age, weight, sex, and prior medical history were prospectively collected. Traditional prehospital data following the Utstein criteria [20] were obtained from the paramedic records and CA registry. Patient data from the first days was collected from the ICU charts, including blood sample results from routine laboratory investigations, fluid balance (perspiration not included), and severity of illness scores (SOFA and SAPS II). Additional data on mortality and neurological outcome were obtained during an extensive consultation 6 months post-arrest.
Biochemical sampling and analyses
Spot urine samples were collected from urine catheters at admission (0 to 6 h post-arrest) and on day 3 after the OHCA. Samples were stored in a refrigerator for up to 72 h before being frozen at –70 °C. After thawing, samples were centrifuged for 5 min at 20 °C and 500 RCF, aliquoted, and refrozen. Thereafter, the urine samples were re-thawed and identically re-centrifuged before they were diluted 1:200 and run in duplicate according to the manufacturers’ instructions. Cystatin C and NGAL were quantified using Bio-Plex Pro RBM Human Kidney Toxicity Assays panel 2 on the Bio-Plex 200 system (Bio-Rad Laboratories, Hercules, CA, USA). The concentrations of TIMP-2 and IGFBP7 were measured using the NephroCheck™ Test (Astute Medical, San Diego, CA, USA), calculating the product of both biomarker concentrations (TIMP-2 × IGFBP7). For all biomarkers, results below the lower range were set as 0, and results above the upper range were set as 100,000. A pilot study revealed that the studied biomarkers in urine were stable when they were stored in a refrigerator for up to 72 h prior to freezing, and when centrifuged after thawing [21].
Statistical methods
Data are presented as number (percentage), median (interquartile range (IQR)) or mean (standard deviation (SD)). Univariate analyses were performed using the Pearson’s Chi square test and Fisher’s exact test when appropriate. The association between potential risk factors and the outcomes AKI, mortality and PNO were quantified by odds ratio (OR) with 95 % confidence interval (CI). Variables with p < 0.25 in the univariate analyses were considered candidates for the multivariate model if they had less than 15 % missing data. Independent risk factors were identified using a multivariate logistic regression model and a manual backward stepwise elimination procedure. Multivariate analyses were preceded by estimation of correlation between risk factors. The predictive accuracy of the models was assessed by calibration and discrimination. Calibration was evaluated by the Hosmer and Lemeshow goodness-of-fit test. A statistically non-significant Hosmer and Lemeshow result (p > 0.05) suggests that the model predicts accurately on average. Discrimination was evaluated by analysis of the area under the receiver operating characteristics curve (ROC) curve, and acceptable discriminatory capability was defined as an area under the ROC curve (AuROC) above 0.7. Chi-square tests for equality of AuROCs were performed using Stata 14 (StataCorp, College Station, TX, USA); all other statistical analyses were performed using SPSS 21 for Windows (IBM SPSS, Chicago, IL, USA). Two-sided p values less than 0.05 were considered statistically significant. Patients without recorded body weight were assumed to be 70 kg if female and 80 kg if male in the calculation of hourly urine output. There were some additional missing data that were handled using only available data.
Results
Patient characteristics and event rates
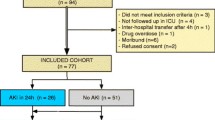
Of 300 OHCA patients eligible during the study period, 261were included in the NORCAST study. Altogether 66 patients were excluded from this substudy due to different reasons (Fig. 1).
In the total cohort of 195 included patients, 165 (85 %) were male and the mean age was 60 (±14) years. Overall 6-month outcome revealed that 88 (45 %) died and 96 (49 %) had PNO (Table 1). In total, 88 patients (45 %) developed AKI; 52 (27 %), 23 (12 %), and 13 (7 %) with stage 1, 2, and 3, respectively. Urine samples were collected from all 195 patients at admission and 164 (84 %) patients at day 3.
Risk factors for acute kidney injury
Many possible risk factors for AKI were identified in the univariate analysis (Table 1). Urine concentrations of cystatin C, NGAL, and TIMP-2 × IGFBP7 were significantly higher in patients with AKI compared with patients without kidney disease both at admission and day 3, except for TIMP-2 × IGFBP7 at day 3 (Table 1). Parameters excluded from the multivariate analysis were time to ROSC (because of 19 % missing data), as well as bicarbonate and lactate concentrations which were strongly correlated (r > 0.7) to base excess levels (in order to avoid co-linearity problems). In multiple logistic regression analysis, urine NGAL levels at day 3 (OR 5.46 (95 % CI 2.65–11.24)), SOFA score at admission day (OR 2.83 (95 % CI 1.24–6.50)) and serum urea concentration at admission day (OR 2.82 (95 % CI 1.12–4.66)) were independent risk factors for AKI. The Hosmer and Lemeshow goodness-of-fit test was not significant, indicating a satisfactory fit of the model (χ2 = 10.48, df = 6, p = 0.11). In the best predictive model, AuROC was 0.774 (95 % CI 0.700–0.848) indicating a good discriminative ability between patients with and without AKI (Model IV, Table 2).
Addition of biomarker measurements to clinical parameters significantly increased the discriminating power of AKI in Model II, but not in Model I (Table 2). Cystatin C and NGAL levels at day 3 were significantly better to predict AKI stage 2 or 3 than AKI stage 1 (Additional file 1). The ability to predict AKI was similar for urine NGAL and cystatin C concentrations (Additional file 2).
Risk factors for mortality and poor neurological outcome
Data from univariate analyses revealed many possible risk factors for mortality and PNO (Table 3 and Table 4). Urine cystatin C and NGAL levels at admission and day 3 were significantly higher in non-survivors compared with survivors (Table 3) and in patients with PNO compared with patients who had good neurological outcome (Table 4). In contrast, urine TIMP-2 × IGFBP7 concentrations were similar for both the considered outcomes at any time point. Time to ROSC, bicarbonate and lactate levels were excluded from multiple regression analyses for the same reasons as in the AKI analysis. Independent risk factors for mortality in multivariate analysis were high NGAL concentrations at admission (OR 2.87 (95 % CI 1.44–5.72)), initial non-shockable rhythm (OR 4.11 (95 % CI 1.99–8.53)), presence of AKI (OR 2.86 (95 % CI 1.43–5.74)) and high SOFA score at admission (OR 3.28 (95 % CI 1.43–7.50)). Independent risk factors for PNO in the multivariate analysis were high cystatin C levels at admission (OR 2.06 (95 % CI 1.00–4.25)), initial non-shockable rhythm (OR 5.07 (95 % CI 2.40–10.74)), presence of AKI (OR 2.67 (95 % CI 1.32–5.40)), low base excess levels at admission (OR 2.07 (95 % CI 1.00–4.26)) and high SOFA score at admission (OR 2.45 (95 % CI 1.08–5.57)). The Hosmer and Lemeshow goodness-of-fit tests were not significant, indicating satisfactory fit of the model for mortality (χ2 = 4.04, df = 7, p = 0.78) and PNO (χ2 = 5.84, df = 8, p = 0.67). In the best predictive models, the AuROCs were 0.812 (95 % CI 0.751–0.873) and 0.819 (95 % CI 0.759–0.878) indicating a good discriminative ability between survivors and non-survivors (Model VI, Table 2) in addition to patients with PNO and good neurological outcome (Model IX, Table 2), respectively.
Addition of biomarker measurements to clinical parameters did not significantly increase the discriminating power of mortality or PNO in Model V, VI, or IX, respectively (Table 2). The ability to predict mortality and PNO was not statistically different when comparing urine NGAL and cystatin C concentrations (Additional files 3 and 4).
Discussion
Our main finding in this prospective study on resuscitated comatose OHCA patients was that the urine concentrations of cystatin C and NGAL sampled at admission and on day 3 were independent risk factors for AKI, mortality, and PNO. In contrast, TIMP-2 × IGFBP7 levels only predicted AKI in urine samples collected at admission. The discriminating power was not uniformly improved in models combining biomarker concentrations and clinical parameters. Overall outcome was very good, with 51 % of the patients alive with good neurological outcome 6 months post-arrest.
The biomarkers in serum and urine that aim to predict AKI and prognosis have many shortcomings that limit their clinical use [9, 10, 22]. In addition to limited discriminating power they differ in organ specificity and time profile. Cystatin C is produced in all nucleated cells and may be used as a marker of glomerular filtration rate [11]. NGAL is expressed in epithelial cells in different organs, and is considered an inflammatory mediator upregulated in tubular injury [12]. TIMP-2 and IGFBP7 are markers of cell cycle arrest [13]; TIMP-2 probably has kidney-protective properties [13], whereas IGFBP7 may reflect renal haemodynamic alterations [13]. Although their time profiles in urine are not fully clarified, NGAL and cystatin C are elevated approximately 48 h prior to the development of the clinical syndrome of AKI, whereas TIMP-2 and IGFBP7 are thought to predict AKI developing within 12 h [23].
Among the most predictive biomarkers of AKI tested in general ICU patients are cystatin C, NGAL, and TIMP-2 × IGFBP7 measured in blood and/or urine samples [13, 23–26]. In CA patients, there are limited data showing that NGAL measured in blood within 4 h after ROSC is a predictor of AKI [15]. It is therefore not surprising that urine cystatin C, NGAL, and TIMP-2 × IGFBP7 were predictors of AKI in the present study. The finding that TIMP-2 × IGFBP7 was not significantly associated with the development of AKI at day 3 is probably caused by the short half-lives of these markers. Unfortunately, urine cystatin C, NGAL, and TIMP-2 × IGFBP7 levels do not discriminate between CA patients with and without AKI, thereby limiting their clinical utility. However, as shown in Additional file 1, our findings that cystatin C and NGAL at day 3 performed better in predicting moderate to severe AKI (stage 2 or 3) compared with mild AKI (stage 1) is interesting, since worsened AKI severity is associated with an increased need for RRT and reduced survival [27]. Because these biomarkers are good predictors of severe AKI they might be used to forecast the need for RRT after CA, as has been shown in general ICU patients [25].
The biomarkers cystatin C, NGAL, and TIMP-2 × IGFBP7 have been prognostic predictors of both renal recovery and mortality in general ICU patients [25, 28, 29]. Although data from CA patients are sparse, one recent study revealed that NGAL measured in blood within 4 h after ROSC was a predictor of mortality and neurological outcome [15]. In another study, enrolment serum NGAL concentrations in CA patients predicted mortality better than neuron-specific enolase and S100B [14]. In agreement with these findings, we found that urine cystatin C and NGAL, but not urine TIMP-2 × IGFBP7, were statistically associated with mortality and PNO. We might hypothesize that cystatin C and NGAL are influenced by whole-body ischemia and reperfusion injuries, whereas TIMP-2 and IGFBP7 may be more kidney-specific markers.
Several models to predict outcome after CA have been developed [14, 30], and addition of biomarker levels to clinical parameters have been suggested in order to improve the ability to predict [14, 31]. Although we found that urine cystatin C and NGAL levels on admission were risk factors of mortality and PNO, their AKI predictive accuracy was limited, and the prediction was not uniformly improved by adding biomarker concentrations to the clinical parameters. We therefore consider that these predictive models cannot be used in treatment allocation of patients, as no model had a perfect discriminating ability. This is in agreement with the European guidelines for post-resuscitation care that recommends a multimodal strategy with prolonged observation in cases with uncertain outcome [32]. However, the biomarkers might be useful in clinical research involving risk stratification of patients.
The present study has several important limitations. No “gold-standard” definition of AKI exists, and the occurrence of AKI in our study was not strictly according to the KDIGO criteria since we only assessed the first 3 days, and lacked data on body weight in 29 patients. There are also limitations in the measurement of urine biomarkers. Since time of urine sampling was not fully standardized, the variation in the time from CA and ROSC to urine sampling will affect biomarker concentrations. The urine collected as spot samples at admission and could potentially be diluted with urine present in the urinary bladder prior to arrest. This might affect the measured concentrations of biomarkers in our study, and peak values are most likely missed. Moreover, the urine was stored in a refrigerator longer than recommended (i.e., 24 h) and was centrifuged later than recommended (i.e., before freezing). However, as previous studies have revealed a good stability of AKI biomarkers independent of storage time [33, 34] and timing of centrifugation [33, 34], we consider the results to be valid. We also performed a pilot study in 10 ICU patients with and without AKI confirming the stability of urine cystatin C, NGAL, and TIMP-2 × IGFBP7 [21]. Furthermore, we were unable to compare the predictive ability of biomarkers at admission and day 3 since we did not have urine samples from day 3 in 31 patients. We were also unable to include time to ROSC in the multivariate analyses because data were missing in 37 patients; this might be an important co-variate among others not included in our analyses since time to ROSC is a strong predictor of PNO in most studies [32, 35, 36]. Additionally, we have not controlled for the development of AKI when assessing the ability of biomarkers to predict mortality and PNO. Finally, our study had a limited sample size and might also have restricted external validity.
Strengths of the study are that all patients came from the same cohort and time period and were treated according to a standardized treatment protocol documenting good and stable outcome over time [35, 37, 38]. We had clear definitions of risk factors and outcome variables, and tested the biomarkers in a population with a high pre-test probability of the considered outcomes.
Conclusions
In this observational study of resuscitated comatose OHCA patients, urine cystatin C and NGAL levels at admission and day 3 were independent risk factors for AKI, mortality, and PNO. In contrast, TIMP-2 × IGFBP7 levels only predicted AKI in urine samples collected at admission. Urine cystatin C and NGAL seem to be promising biomarkers that should be explored in future studies, but there are clear limitations in their clinical utility.
Abbreviations
- AKI:
-
Acute kidney injury
- AuROC:
-
Area under the receiver operating characteristics curve
- CA:
-
Cardiac arrest
- CI:
-
Confidence interval
- CKD:
-
Chronic kidney disease
- CPR:
-
Cardiopulmonary resuscitation
- ICU:
-
Intensive care unit
- IGFBP7:
-
Insulin-like growth factor-binding protein 7
- IQR:
-
Interquartile range
- KDIGO:
-
Kidney Disease Improving Global Outcomes
- NGAL:
-
Neutrophil gelatinase-associated lipocalin
- NORCAST:
-
Norwegian Cardiorespiratory Arrest Study
- OHCA:
-
Out-of-hospital cardiac arrest
- OR:
-
Odds ratio
- PNO:
-
Poor neurological outcome
- RCF:
-
Relative centrifugation force
- ROC:
-
Receiver operating characteristics curve
- ROSC:
-
Return of spontaneous circulation
- RRT:
-
Renal replacement therapy
- SAPS:
-
Simplified Acute Physiology Score
- SD:
-
Standard deviation
- SOFA:
-
Sequential Organ Failure Assessment
- SOP:
-
Standard operating procedure
- TIMP-2:
-
Tissue inhibitor of metalloproteinase-2
- TIMP-2 × IGFBP7:
-
Product of the concentrations of TIMP-2 and IGFBP7
- TTM:
-
Targeted temperature management
References
Grasner JT, Lefering R, Koster RW, Masterson S, Bottiger BW, Herlitz J, et al. EuReCa ONE-27 Nations, ONE Europe, ONE Registry: a prospective one month analysis of out-of-hospital cardiac arrest outcomes in 27 countries in Europe. Resuscitation. 2016;105:188-95.
Adrie C, Adib-Conquy M, Laurent I, Monchi M, Vinsonneau C, Fitting C, et al. Successful cardiopulmonary resuscitation after cardiac arrest as a “sepsis-like” syndrome. Circulation. 2002;106:562–8.
Herlitz J, Castren M, Friberg H, Nolan J, Skrifvars M, Sunde K, et al. Post resuscitation care: what are the therapeutic alternatives and what do we know? Resuscitation. 2006;69:15–22.
Rittenberger JC, Tisherman SA, Holm MB, Guyette FX, Callaway CW. An early, novel illness severity score to predict outcome after cardiac arrest. Resuscitation. 2011;82:1399–404.
Sandroni C, Dell’anna AM, Tujjar O, Geri G, Cariou A, Taccone FS. Acute kidney injury (AKI) after cardiac arrest: a systematic review and meta-analysis of clinical studies. Minerva Anestesiol. 2016;82:989-99.
Geri G, Guillemet L, Dumas F, Charpentier J, Antona M, Lemiale V, et al. Acute kidney injury after out-of-hospital cardiac arrest: risk factors and prognosis in a large cohort. Intensive Care Med. 2015;41:1273-80.
Jennett B, Bond M. Assessment of outcome after severe brain damage. Lancet. 1975;1:480–4.
Ostermann M. Diagnosis of acute kidney injury: Kidney Disease Improving Global Outcomes criteria and beyond. Curr Opin Crit Care. 2016;20:581–7.
Gonzalez F, Vincent F. Biomarkers for acute kidney injury in critically ill patients. Minerva Anestesiol. 2012;78:1394–403.
Prowle JR. Measurement of AKI biomarkers in the ICU: still striving for appropriate clinical indications. Intensive Care Med. 2016;41:541–3.
Delanaye P, Cavalier E, Morel J, Mehdi M, Maillard N, Claisse G, et al. Detection of decreased glomerular filtration rate in intensive care units: serum cystatin C versus serum creatinine. BMC Nephrol. 2014;15:9.
Martensson J, Bell M, Oldner A, Xu S, Venge P, Martling CR. Neutrophil gelatinase-associated lipocalin in adult septic patients with and without acute kidney injury. Intensive Care Med. 2010;36:1333–40.
Kashani K, Al-Khafaji A, Ardiles T, Artigas A, Bagshaw SM, et al. Discovery and validation of cell cycle arrest biomarkers in human acute kidney injury. Crit Care. 2013;17:R25.
Elmer J, Jeong K, Abebe KZ, Guyette FX, Murugan R, Callaway CW, et al. Serum neutrophil gelatinase-associated lipocalin predicts survival after resuscitation from cardiac arrest. Crit Care Med. 2016;44:111–9.
Park SO, Ahn JY, Lee YH, Kim YJ, Min YH, Ahn HC, et al. Plasma neutrophil gelatinase-associated lipocalin as an early predicting biomarker of acute kidney injury and clinical outcomes after recovery of spontaneous circulation in out-of-hospital cardiac arrest patients. Resuscitation. 2016;101:84–90.
Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group. KDIGO clinical practice guideline for acute kidney injury. Kidney Int Suppl. 2013;2:1–138.
Kidney Disease: Improving Global Outcomes (KDIGO) Chronic kidney disease work group. KDIGO clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl. 2013;3:1–150.
Le Gall JR, Lemeshow S, Saulnier F. A new Simplified Acute Physiology Score (SAPS II) based on a European/North American multicenter study. JAMA. 1993;270:2957–63.
Vincent JL, de Mendonca A, Cantraine F, Moreno R, Takala J, Suter PM, et al. Use of the SOFA score to assess the incidence of organ dysfunction/failure in intensive care units: results of a multicenter, prospective study. Working group on “sepsis-related problems” of the European Society of Intensive Care Medicine. Crit Care Med. 1998;26:1793–800.
Castren M, Karlsten R, Lippert F, Christensen EF, Bovim E, Kvam AM, et al. Recommended guidelines for reporting on emergency medical dispatch when conducting research in emergency medicine: the Utstein style. Resuscitation. 2008;2:193–7.
Beitland S, Trøseid AM, Brusletto BS, Berg JP, Waldum-Grevbo B, Sunde K. Stability of urinary biomarkers of acute kidney injury. Intensive Care Med Exp. 2016;4:S1. http://icm-experimental.springeropen.com/articles/10.1186/s40635-016-0099-9#Sec484.
Zhang A, Cai Y, Wang PF, Qu JN, Luo ZC, Chen XD, et al. Diagnosis and prognosis of neutrophil gelatinase-associated lipocalin for acute kidney injury with sepsis: a systematic review and meta-analysis. Crit Care. 2016. doi:10.1186/s13054-016-1212-x.
Wasung ME, Chawla LS, Madero M. Biomarkers of renal function, which and when? Clin Chim Acta. 2015;438:350–7.
Zhang Z, Lu B, Sheng X, Jin N. Cystatin C in prediction of acute kidney injury: a systemic review and meta-analysis. Am J Kidney Dis. 2011;58:356–65.
Herget-Rosenthal S, Poppen D, Husing J, Marggraf G, Pietruck F, Jakob HG, et al. Prognostic value of tubular proteinuria and enzymuria in nonoliguric acute tubular necrosis. Clin Chem. 2004;50:552–8.
Ronco C. Acute kidney injury: from clinical to molecular diagnosis. Crit Care. 2016;20:201.
Hoste EA, Bagshaw SM, Bellomo R, Cely CM, Colman R, Cruz DN, et al. Epidemiology of acute kidney injury in critically ill patients: the multinational AKI-EPI study. Intensive Care Med. 2015;41:1411–23.
Aregger F, Uehlinger DE, Witowski J, Brunisholz RA, Hunziker P, Frey FJ, et al. Identification of IGFBP-7 by urinary proteomics as a novel prognostic marker in early acute kidney injury. Kidney Int. 2014;85:909–19.
Dai X, Zeng Z, Fu C, Zhang S, Cai Y, Chen Z. Diagnostic value of neutrophil gelatinase-associated lipocalin, cystatin C, and soluble triggering receptor expressed on myeloid cells-1 in critically ill patients with sepsis-associated acute kidney injury. Crit Care. 2015;19:223.
Maupain C, Bougouin W, Lamhaut L, Deye N, Diehl JL, Geri G, et al. The CAHP (Cardiac Arrest Hospital Prognosis) score: a tool for risk stratification after out-of-hospital cardiac arrest. Eur Heart J. 2015.
Chen LX, Koyner JL. Biomarkers in acute kidney injury. Crit Care Clin. 2015;31:633–8.
Nolan JP, Soar J, Cariou A, Cronberg T, Moulaert VR, Deakin CD, et al. European Resuscitation Council and European Society of Intensive Care Medicine Guidelines for Post-resuscitation Care 2015: Section 5 of the European Resuscitation Council Resuscitation Guidelines 2015. Resuscitation. 2015;95:202-22.
Parikh CR, Butrymowicz I, Yu A, Chinchilli VM, Park M, Hsu CY, et al. Urine stability studies for novel biomarkers of acute kidney injury. Am J Kidney Dis. 2014;63:567–72.
van de Vrie M, Deegens JK, van der Vlag J, Hilbrands LB. Effect of long-term storage of urine samples on measurement of kidney injury molecule 1 (KIM-1) and neutrophil gelatinase-associated lipocalin (NGAL). Am J Kidney Dis. 2014;63:573–6.
Beitland S, Nakstad ER, Staer-Jensen H, Draegni T, Andersen GO, Jacobsen D, et al. Impact of acute kidney injury on patient outcome in out-of-hospital cardiac arrest: a prospective observational study. Acta Anaesthesiol Scand. 2016;60:1170-81.
Sivaraju A, Gilmore EJ, Wira CR, Stevens A, Rampal N, Moeller JJ, et al. Prognostication of post-cardiac arrest coma: early clinical and electroencephalographic predictors of outcome. Intensive Care Med. 2015;41:1264–72.
Sunde K, Pytte M, Jacobsen D, Mangschau A, Jensen LP, Smedsrud C, et al. Implementation of a standardised treatment protocol for post resuscitation care after out-of-hospital cardiac arrest. Resuscitation. 2007;73:29–39.
Tomte O, Andersen GO, Jacobsen D, Draegni T, Auestad B, Sunde K. Strong and weak aspects of an established post-resuscitation treatment protocol—a five-year observational study. Resuscitation. 2011;82:1186–93.
Funding
Financial support was provided solely from institutional sources.
Availability of data and material
The data that support the findings of this study are available from Oslo University Hospital, but restrictions apply to the availability of these data which were used under license for the current study and so are not publicly available. Data are, however, available from the authors upon reasonable request.
Authors’ contributions
SB, ERN, BEWG, GØA, and KS contributed to the conception and design of the study. ERN additionally recruited patients and collected patient data. AMST, BSB, and JPB were responsible for urine sample collection and analyses. CB contributed to the statistical analyses and data presentation. SB collected patient data and drafted the manuscript. All authors contributed to the interpretation of data and writing of the manuscript, and approved the final version.
Competing interests
KS received support for lectures and travel from Bard Medical. The other authors declare that they have no competing interest.
Ethics approval and consent to participate
The study was approved by the Regional Committee for Medical Ethics of South-East Norway (Approval number REK S-O A Ref 2010/1116a). Written informed consent was obtained from the nearest family relative after admission (n = 161) and later from all patients who regained consciousness and were considered competent to give consent within 6 months (n = 91). Patients were not considered for inclusion if the nearest family relative opposed it (n = 3). Relatives were not asked for consent if the patient did not meet the inclusion criteria (n = 36) (Fig. 1). Family relatives who were not present at the hospital were contacted by phone and had written study information sent by postal mail. The Regional Committee for Medical Ethics of South-East Norway approved the inclusion of thirteen patients whose relatives were unreachable or failed to return their consent forms.
Author information
Authors and Affiliations
Corresponding author
Additional files
Additional file 1:
Univariate analysis of risk factors for AKI in subgroups of resuscitated comatose out-of-hospital cardiac arrest patients. Consists of risk factors for AKI in subgroups of patients without AKI (KDIGO stage 0), with mild AKI (KDIGO stage 1) and with severe AKI (KDIGO stage 2–3). (DOCX 24 kb)
Additional file 2:
Comparisons of the ability to predict acute kidney injury in out-of-hospital cardiac arrest patients: cystatin C versus NGAL concentrations measured in spot urine. (DOCX 40 kb)
Additional file 3:
Comparisons of the ability to predict mortality in out-of-hospital cardiac arrest patients: cystatin C versus NGAL concentrations measured in spot urine. (DOCX 39 kb)
Additional file 4:
Comparisons of the ability to predict poor neurological outcome in out-of-hospital cardiac arrest patients: cystatin C versus NGAL concentrations measured in spot urine. (DOCX 27 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Beitland, S., Waldum-Grevbo, B.E., Nakstad, E.R. et al. Urine biomarkers give early prediction of acute kidney injury and outcome after out-of-hospital cardiac arrest. Crit Care 20, 314 (2016). https://doi.org/10.1186/s13054-016-1503-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13054-016-1503-2