Abstract
Background
The aims of this study were to identify the source and the transmission pathway for a Staphylococcal Scalded Skin Syndrome (SSSS) outbreak in a maternity setting in Italy over 2 months, during 2014; to implement appropriate control measures in order to prevent the epidemic spread within the maternity ward; and to identify the Methicillin-Resistant Staphylococcus aureus (MRSA) epidemic clone.
Methods
Epidemiological and microbiological investigations, based on phenotyping and genotyping methods, were performed. All neonates involved in the outbreak underwent clinical and microbiological investigations to detect the cause of illness. Parents and healthcare workers were screened for Staphylococcus aureus to identify asymptomatic carriers.
Results
The SSSS outbreak was due to the cross-transmission of a rare clone of ST5-CA-MRSA-SCCmecV-spa type t311, exfoliative toxin A-producer, isolated from three neonates, one mother (from her nose and from dermatological lesions due to pre-existing hand eczema) and from a nurse (colonized in her nose by this microorganism). The epidemiological and microbiological investigation confirmed these as two potential carriers.
Conclusions
A rapid containment of these infections was obtained only after implementation of robust swabbing of mothers and healthcare workers. The use of molecular methodologies for typing was able to identify all carriers and to trace the transmission.
Similar content being viewed by others
Background
Staphylococcal Scalded Skin Syndrome (SSSS) describes a spectrum of superficial blistering skin disorders caused by the exfoliative (or epidermolytic) toxins of Staphylococcus aureus, predominantly, but not exclusively, affecting neonates and children under the age of 5 years [1].
Clinically, SSSS in children usually starts within 24–48 h with fever, irritability and widespread redness of the skin, and fluid-filled blisters form that rupture easily, leaving an area that looks like a burn. Typical characteristics of the rash include: tissue-paper-like wrinkling of the skin, followed by the appearance of large fluid-filled blisters in the armpits, groin and body orifices such as the nose and ears; the rash quickly spreads to other parts of the body including the arms, legs and trunk. In newborns, lesions are often found in the diaper area, axillary folds and around the navel; the top layer of skin begins peeling off in sheets, leaving a moist, red and tender area exposed. Other symptoms may include weakness and dehydration [2–4].
In humans, SSSS is caused by the release of two exfoliative epidermolytic toxins, ETA or ETB, from Staphylococcus aureus toxigenic strains. These toxins induce epidermal blistering through the cleavage of the cell-cell adhesion molecule desmoglein-1, which is only expressed by keratinocytes in the granular cell layer, leading to a spectrum of illnesses ranging from mild localized blistering to extensive generalized lesions [2, 5]. ETs producing S. aureus strains have been associated with specific genetic backgrounds, on the basis of their phage types, accessory gene regulator (agr) groups and macrorestriction profiles [3–10].
SSSS in newborns has been mainly reported in clonal complexes (CC) 121 and 15, carrying eta and/or etb genes [2], but also in other less common genotypes, such as ETB-positive ST91 MRSA and ETA-positive ST2993-t211 (CC8) with and without SCCmecV [11, 12].
The prognosis in most cases is typically good if prompt antibiotic therapy is given. Approximately 5% of S. aureus strains produce exfoliative toxins, with a variable prevalence index in different countries. In Europe, the incidence of SSSS varies from 0.56 cases/million/year in France, to 2.53 cases/million/year in the Czech Republic [2, 3].
Recently, a nosocomial outbreak of SSSS has been described in neonates in England sustained by S. aureus isolates belonging to spa-type t346 [2].
Our study reports the experience of an SSSS outbreak, due to the cross transmission of a methicillin-resistant S.aureus isolate belonging to a rare clone of ST5-CA-MRSA-SCCmecV-spa type t311, ETA toxin producer, among three infants hospitalized in the maternity ward of an Italian hospital. Rapid containment of these infections was obtained only after implementation of robust swabbing of healthcare workers, and the use of molecular methodologies for typing that was able to identify carriers and to trace the transmission.
The aims of this study were: i) to identify the causative agent and the transmission pathway; ii) to enforce correct control measures avoiding the spread within the maternity ward; iii) to characterize the MRSA epidemic clone.
Methods
Setting
The outbreak occurred in the Maternity Unit of the hospital “Dell’Angelo” in Mestre (Venice), Italy, with 40 beds, about 1,800 delivers per year, 7 delivery rooms, 2 operating rooms and one nursery, where the neonates stay when they are not in the room with their mother. The Maternity Unit has 112 Health Care Workers (HCW).
Clinical investigations
During the cluster period, from November 20 to December 4, 2014, from the date of the first birth to the date of the discharge of the third newborn, 66 neonates were born in the unit. Umbilical swabs and other skin lesion swabs were performed on neonates presenting with clinical scalded skin syndrome signs. During the hospital admission, the neonates affected by SSSS were cared for in single rooms with their parents and swabs and digital photographs of skin lesions were taken.
Parent and staff screening investigations
Parents and relatives of the ill new-borns were screened by nasal swabs, and hand swabs only in cases of skin lesions.
The maternity staff screening for S. aureus carriers started on December 6 by nasal swabs. Visual hand inspection and anamnesis research for hand skin diseases were conducted among the operators involved in the sick neonates’ pathway from the delivery room to the nursery and mothers’ room. All staff voluntarily complied with the request for screening.
Infection control measures
During the progress of the investigation, the following control measures were applied: (i) immediate repetition to all the operators about contact measures for the prevention of infectious diseases and recommendation to the nursery operators to use a hand wash with a hydro-alcoholic solution before handling each neonate; (ii) immediate use of barrier measures (masks and gloves) for all the operators, until the identification of the index case. Operators usually used gloves only during delivery; (iii) immediate onset of screening to find S. aureus carriers; (iv) on December 9, extraordinary environmental sanitation in delivery rooms, nursery and ward rooms. In the nursery, in addition to the classic cleaning procedures, on December 18, a hydrogen peroxide and silver cation nebulization machine was used; (v) from December 12, topic mupirocin treatment was applied; (vi) on December 23 and January 8, two meetings with doctors, nurses, midwifes and support staff were held for clinical audit about the procedures followed from delivery to the neonates hospital discharge and discussion about the strategy used.
Microbiological investigations
Nose and hand swabs were collected from doctors, nurses, midwives and support staff involved in the sick newborns’ management, from the delivery room to the nursery, as well as from the neonates’ parents and from the skin lesions of the sick newborns. All samples were cultured on Columbia Blood Agar (CBA) and Mannitol Salt Agar (MSA) plates (Becton Dickinson GmbH, Heidelberg, Germany); colony identification was obtained using coagulase test and Vitek2 GP card (BioMerieux, Marcy-L’Etoile, France) and Aris Sensititre ITGPOSF was used for susceptibility tests (Trek Diagnostics, Cleveland, OH, USA).
All the culture plates positive for S. aureus were sent to the “Medical Molecular Microbiology and Antibiotic Resistance” reference laboratory (M.M.A.R.L. http://www.labmicrobiologia.unict.it), Department of Biomedical and Biotechnological Science (BIOMETEC), University of Catania, for further characterization.
Methicillin resistance was re-evaluated by the cefoxitin disk diffusion method and correlated with the presence of the mecA gene, as suggested by the European Committee on Antimicrobial Susceptibility Testing (EUCAST) and Clinical and Laboratory Standards Institute (CLSI) guidelines [13, 14]. The antimicrobial susceptibilities were evaluated in agreement with the EUCAST interpretation criteria [13]. The S.aureus isolates were also screened by population analysis (PAP/AUC) to verify the presence of vancomycin-intermediate (VISA) and heteroresistant vancomycin-intermediate S. aureus (hVISA) phenotypes [15].
Molecular typing, agr locus and the virulence gene content (adhesion and toxin genes) were performed as previously described [16–20]. Representative S. aureus control strains were included in the study for phenotypic and genotypic assays [16, 17].
Results
Clinical presentation of the outbreak and case series
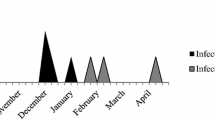
The outbreak of SSSS infections occurred in neonates from November to December 2014 in the Maternity Unit of the hospital “Dell’Angelo” in Mestre (Venice), Italy.
Three cases were identified over a 2-month period. Patients were born from 20 to 25 November 2014. All hospitalization periods overlapped, suggesting possible cross-transmission from one neonate to another.
In all cases, the skin symptoms began after 3–9 days from birth. After birth, they were discharged but returned to the hospital in 2–5 days. After four days from the admission of the third neonate, the Medical Chief of the pediatrics department reported this to the hospital Director of the Infectious Control Team (ICT) who then activated a Task Force for the epidemiological and microbiological investigation. All patient skin swabs were positive for MRSA. Blood cultures remained negative. None of the neonates required intensive care after delivery and none had an invasive procedure.
Screening investigations
During hospitalization, several control measures were implemented to prevent epidemic spread and an attempt was made to identify the source of pathogen transmission.
Multiple samples were collected from neonate mothers and from the hospital staff.
Among the mothers, case 1 mother had dermatological lesions due to pre-existing hand eczema. On December 12, she was found positive for MRSA strains by the rapid Vitek identification method, both in her nose and on her hands. Case 2 mother was not colonized by S.aureus. Case 3 mother was found positive for S.aureus by nasal swab.
Twenty-seven HCW (24.1%) were positives for S. aureus, 3 (2.7%) of them for MRSA. On December 12, the treatment with nasal mupirocin was started (twice a day, for 5 days) only for those operators that were MRSA positive, and those that were waiting for the microbiological response from the laboratory. Only for the carriers of the same cluster strain, was a new control swab taken after 5 days from the end of therapy. They were successfully decolonized. No further neonate or staff-member has been identified with MRSA strains to date; the last controls were made on 25 March, 2015.
Microbiological characteristics
In the course of the neonatal, parent and staff screening investigations, 9 S.aureus strains from affected skin regions, nasal swabs and carriers were isolated and sent to the reference lab (MMARL) of the BIOMETEC Department of the University of Catania, for further characterizations.
The microbiological and molecular characteristics of the sample in study are shown in Table 1.
Seven out of the 9 swab cultures yielded methicillin-resistant S.aureus, only resistant to beta-lactams and susceptible to levofloxacin, gentamicin, erythromycin, clindamycin, trimethoprim-sulfamethoxazole, tetracycline and rifampicin. The MIC values for the main anti-Gram positive drugs (linezolid, vancomycin, teicoplanin and daptomycin) were in the range of full susceptibility. Population analysis confirmed that all strains were vancomycin-susceptible, without glycopeptides heteroresistant sub-populations.
Two methicillin-susceptible S.aureus strains (MSSA), mecA gene negative and cefoxitin susceptible, and susceptible to all antimicrobials tested, were found in case 3 mother and in a staff-member.
All strains isolated from the three neonates, the case 1 mother (index-case) and one nurse showed a typical CA-MRSA phenotype and belonged to the same clone ST5-SCCmecV-spa type t311, with the same PFGE profile C (subtypes C1 and C2) and agr-locus II. The virulence gene content revealed that all isolates were positive for the eta gene, encoding for the epidermolytic toxin responsible for SSSS, and positive for lukE, icaA, fnbA, sej genes. No other toxin genes were present in these cases, including lukS-lukF (Panton-Valentine leukocidin), tst, sea, seb, sec, sek/q genes and the ACME-locus.
Two staff members were found to carry two non-outbreak MRSA strains, belonging to ST5-SCCmec IV-t1094 agrII (formerly pediatric or USA800 HA-MRSA clone) and ST8-SCCmec II-t3240 agrI (formerly Irish clone). They had different PFGE-types (A and B), and possessed the same toxin gene content with the addition of the Panton-Valentine Leukocidin gene.
Discussion
Our study describes an outbreak of SSSS in a maternity setting in Italy over 2 months during 2014.
Surveillance of the staff members by nasal swabs identified S.aureus carriage in 24.1% of HCW. This percentage is close to those reported by other authors from Ireland (30%) and England (21%) [2, 3, 21–23]. MRSA strains were found on three nurses (2.7%), of which only one showed the characteristics of the outbreak strain. It was possible to eradicate S. aureus and the nurse was still decolonized after 4 months. After an accurate analysis of the work shifts, it was possible to establish that the operator was always on duty during the care of the three ill newborns in the nursery.
The molecular characterization highlighted the spread of a rare CA-MRSA clone ST5-spa type t311, unusually associated with the SCCmecV cassette of veterinary origin. This association has rarely been described in the literature [24]. This clone (already known as USA800 or pediatric clone) is usually associated with the SCCmec IV element, and it is widely spread in both hospitals and the community [25]. From these data, it can be assumed that there was a possible horizontal transfer of the SCCmec V cassette in a different genetic background, commonly diffused in the hospital setting.
In this study, ST5-SCCmecV-spa-type t311 epidemic strains producing ETA toxin, isolated from the three neonates, case 1 mother and the nurse were indistinguishable.
Retrospectively, based on the microbiological and molecular data, two different routes can explain this neonate infection. The mother of one of the newborns was affected by chronic eczema, and was found to carry the epidemic strain; she might have been an asymptomatic carrier on admission to the unit, and she might have infected her child. Nasal, axillar and perianal carriage of S.aureus strains producing epidermolytic toxin have been reported in 3% of pregnant women [26, 27]. Moreover, atopic chronic dermatitis (also known as atopic eczema) has been recognized as an important source of skin infection, responsible for the dissemination of S.aureus in hospitals [28].
The other possibility is that the nurse was the source of the epidemic strain and might have infected the three neonates, one of which might have infected its own mother. In hospitals and in nurseries, outbreaks of staphylococcal infections are expected to originate from asymptomatically colonized care attendants rather than mothers [29, 30].
Conclusions
The validity of this survey was to promptly find the causative agent of SSSS, to prevent the spread of the pathogen within the hospital ward, and to identify the MRSA epidemic clone. In fact, the available mitigation measures, such as screening of parents and hospital staff that was immediately implemented, stopped the epidemic in less than a week after onset.
The immediate implementation of strong barrier measures was enough to break the spread of the infection. The nurse, decolonized after some months, is still employed in the same department.
References
Ladhani S. Recent developments in staphylococcal scalded skin syndrome. Clin Microbiol Infect. 2001;7(6):301–7.
Lamand V, et al. Epidemiological data of staphylococcal scalded skin syndrome in France from 1997 to 2007 and microbiological characteristics of Staphylococcus aureus associated strains. Clin Microbiol Infect. 2012;18(12):E514–521.
Paranthaman K, et al. Nosocomial ourbreak of staphylococcal scalded skin syndrome in neonates in England, December 2012 to March 2013. Euro Surveillance. 2014;19(33). Available online: http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=20880.
Occelli P, et al. Outbreak of staphylococcal bullous impetigo in a maternity ward linked to an asymptomatic healthcare worker. J Hosp Infect. 2007;67(3):264–70.
Saida K, et al. Exfoliative toxin a staphylococcal scalded skin syndrome in preterm infants. Eur J Pediatr. 2015;174(4):551–5.
El Helali N, et al. Nosocomial outbreak of staphylococcal scalded skin syndrome in neonates: epidemiological investigation and control. J Hosp Infect. 2005;61(2):130–8.
Kurlenda J, et al. Epidemiological investigation of nosocomial outbreak of staphylococcal skin diseases in neonatal ward. Antonie Van Leeuwenhoek. 2009;95(4):387–94.
Jarraud S, et al. Relationships between Staphylococcus aureus genetic background, virulence factors, agr groups (alleles), and human disease. Infect Immun. 2002;70(2):631–41.
Jarraud S, et al. Exfoliatin-producing strains define a fourth agr specificity group in Staphylococcus aureus. J Bacteriol. 2000;182(22):6517–22.
Neylon O, et al. Neonatal staphylococcal scalded skin syndrome: clinical and outbreak containment review. Eur J Pediatr. 2010;169(12):1503–9.
Shi D, et al. Staphylococcal scalded skin syndrome in an extremely low-birth-weight neonate: molecular characterization and rapid detection by multiplex and real-time PCR of methicillin-resistant Staphylococcus aureus. Pediatr Int. 2011;53(2):211–7.
Skryabin Y, et al. Niche expansion of Staphylococcus aureus clonal complex 8 by the acquisition of exfoliative toxin A gene. EV0876. 25th European Congress of Clinical Microbiology and Infectious Diseases (ECCMID). 25–28 April 2015 Copenhagen, Denmark.
European Committee on Antimicrobial Susceptibility Testing (EUCAST). [http://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Breakpoint_tables/v_6.0_Breakpoint_table.pdf]. Valid from 2016-01-01.
Clinical and Laboratory Standards Institute (CLSI). Methods for dilution antimicrobial susceptibility. Tests for bacteria guidelines CLSI 2014. Twenty-fourth informational supplement. CLSI document M100-S24. Wayne, Pennsylvania: Clinical and Laboratory Standards Institute, 2014.
Campanile F, et al. Heteroresistance to glycopeptides in Italian methicillin-resistance Sthaphylococcus aureus (MRSA) isolates. Int J Antimicrob. 2010;36(5):415–9.
Campanile F, et al. Hospital-associated methicillin-resistant Staphylococcus aureus (HA-MRSA) in Italy. Ann Clin Microbiol Antimicrob. 2009;8:22–32.
Cafiso V, et al. Modulating activity of vancomycin and daptomycin on the expression of autolysis cell-wall turnover and membrane charge genes in hVISA and VISA strains. PLoS One. 2012;7(1):e29573.
Mongelli G, et al. High Resolution Melting-Typing (HRMT) of methicillin-resistant Staphylococcus aureus (MRSA): The new frontier to replace multi-locus sequence typing (MLST) for epidemiological surveillance studies. J Microbiol Methods. 2015;117:136–8.
Sanchini A, et al. DNA microarray-based characterization of Panton-Valentine leukocidin-positive community-acquired methicillin-resistant Staphylococcus aureus from Italy. Eur J Clin Microbiol Infect Dis. 2011;30(11):1399–408.
Stefani S, et al. Pathotype and susceptibility profile of a community-acquired methicillin-resistant Staphylococcus aureus strain responsible for a case of severe pneumonia. Diagn Microbiol Infect Dis. 2009;63(1):100–4.
Li MY, et al. Staphylococcal scalded skin syndrome in neonates: an 8-year retrospective study in a single institution. Pediatr Dermatol J. 2014;31(1):43–7.
Layer F, et al. Molecular typing of toxic shock syndrome toxin-1- and enterotoxin a-producing methicillin-sensitive staphylococcus aureus isolates from an outbreak in a neonatal intensive care unit. Int J Med Microbiol. 2015;305(7):790–8.
Lipový B, et al. Staphylococcal scalded skin syndrome in the Czech Republic: an epidemiological study. Burns. 2012;38(2):296–300.
Urushibara N, et al. Two novel arginine catabolic mobile elements and staphylococcal chromosome cassette mec composite islands in community-acquired methicillin-resistant Staphylococcus aureus genotypes ST5-MRSA-V and ST5-MRSA-II. J Antimicrob Chemother. 2012;67(8):1828–34.
Inomata S, et al. Microbiological and molecular epidemiological analyses of community-associated methicillin-resistant Staphylococcus aureus at a tertiary care hospital in Japan. J Infect Chemother. 2015;21(10):729–36.
Dancer SJ, Noble WC. Nasal, axillary, and perineal carriage of Staphylococcus aureus among women: identification of strains producing epidermolytic toxin. J Clin Pathol. 1991;44(8):681–4.
Sollid JU, et al. Staphylococcus aureus: determinants of human carriage. Infect Genet Evol. 2014;21:531–41.
Brüssow H. Turning the inside out: the microbiology of atopic dermatitis. Environ Microbiol. 2015;16:1–14.
Haill C, et al. Prolonged outbreak of methicillin-resistant Staphylococcus aureus in a cardiac surgery unit linked to a single colonized healthcare worker. J Hosp Infect. 2013;83(3):219–25.
Vonberg RP, et al. How often do asymptomatic healthcare workers cause methicillin-resistant Staphylococcus aureus outbreaks? A systematic evaluation. Infect Control Hosp Epidemiol. 2006;27(10):1123–7.
Acknowledgements
We wish to thank the Scientific Bureau of the University of Catania for language support.
Funding
This work was partially supported by the National Operational Program for Research and Competitiveness 2007–2013 (project number PON01_02589).
Availability of data and materials
The authors agreed to make data and material promptly available to editors and peer-reviewers at the time of submission for the purposes of evaluating the manuscript.
Authors’ contributions
OL, LB, and PB conceived and organized the preventative measure under the prevention plan against the spread of the pathogen within the maternity ward. DB, VC, SS, and FC conceived and performed the microbiological and molecular experiments, and wrote the paper. SG, SM isolated and provided microbial pathogens and hospital data; GBP, MCu, MCh, and FB analyzed and interpreted the patient data. All authors read and agreed to the submission and are responsible for the content of the manuscript.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
Not applicable.
Ethics approval and consent to participate
Not applicable.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Lamanna, O., Bongiorno, D., Bertoncello, L. et al. Rapid containment of nosocomial transmission of a rare community-acquired methicillin-resistant Staphylococcus aureus (CA-MRSA) clone, responsible for the Staphylococcal Scalded Skin Syndrome (SSSS). Ital J Pediatr 43, 5 (2017). https://doi.org/10.1186/s13052-016-0323-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13052-016-0323-y