Abstract
Background
It is known that the risk of stroke in patients with traumatic brain injury might be increased. However, the relationship between mild traumatic brain injury and ischemic stroke has never been established. We conducted a study of patients in Taiwan with mild traumatic brain injury to evaluate if they had a higher risk of stroke compared with the general population.
Methods
We utilized a sampled National Health Insurance claims database containing one million beneficiaries. We followed all adult beneficiaries older than 18 years from January 1, 2007 to December 31, 2010 to determine if they were diagnosed with ischemic stroke. We further identified patients with mild traumatic brain injury and compared their risk of ischemic stroke with the general population.
Results
We identified 24,905 patients with mild traumatic brain injury and 719,811 patients without mild traumatic brain injury. After controlling for age, gender, urbanization level, socioeconomic status, diabetes, hypertension, coronary artery disease, hyperlipidemia, history of alcohol intoxication, malignancies, heart failure, atrial fibrillation, smoking, obesity, epilepsy, peripheral artery disease and Charlson Comorbidity Index score, the adjusted hazard ratio for ischemic stroke was 1.46 (95% confidence interval, 1.33—1.62).
Conclusion
Mild traumatic brain injury is an independent significant risk factor for ischemic stroke.
Similar content being viewed by others
Introduction
Each year, traumatic brain injury (TBI) accounts for 2.4 million emergency department visits, hospitalizations, or death in the United States, and the direct medical costs of TBI in the United States in 2010 were estimated to be $11.5 billion [1]. In many other countries, TBI also is one of the major causes of morbidity and mortality [2],[3]. TBI can induce damage of varying extent because of brain swelling, axonal injury, hypoxia, inflammatory responses, oxidative stress and neurodegeneration [4],[5]. Studies have also found that TBI may be associated with progression of some diseases, including epilepsy, Alzheimer’s disease, Parkinson’s disease, and psychiatric diseases [6]-[9].
Recently, in several studies TBI was found to be associated with increased risk of [10]-[12]. Because stroke results from disturbance of the blood supply to the brain, it has been hypothesized that vascular damage from TBI may also induce stroke in survivors [11]. It is well known that TBI can be classified into severe, moderate, and mild types based on the consciousness level, i.e., the Glasgow Coma Scale, and long-term sequelae are more common in patients with severe injuries [13],[14]. In previous studies, we found that even in mild TBI, victims may still have higher risks of developing dementia and mortality [15],[16]. However, the relationship between mild TBI and ischemic stroke has not yet been evaluated.
The aim of this study was to investigate the correlation between mild TBI and ischemic stroke by utilizing a large administration database. The results of this study might provide clinicians with further insights into this frequently encountered situation.
Methods
Ethics statement
This study was initiated after approval by the Institutional Review Board of Dalin Tzu Chi Hospital, Buddhist Tzu Chi Medical Foundation, Chiayi, Taiwan. Because all personal identification was stripped from the secondary files before analysis, the review board waived the requirement of obtaining written informed consent from the patients.
Database
The National Health Insurance (NHI) program in Taiwan was implemented in 1995 and provides compulsory universal health insurance. It enrolls up to 99% of the Taiwanese population and has contracts with 97% of all medical providers [17]. The database contains comprehensive information on all insured individuals, including sex, date of birth, residential or work location, dates of clinical visits, the International Classification of Diseases (Ninth Revision) Clinical Modification (ICD-9-CM) diagnostic codes, details of prescribed medications, expenditure amounts and outcome at hospital discharge (i.e., recovered, died, or transferred out). A random sample consisting of one million people based on the 2005 reimbursement data was established for public access. This group did not significantly differ statistically from the larger cohort in age, gender, or health care costs according to the Taiwan National Health Research Institute. A sampled group was used as our study cohort.
Study population
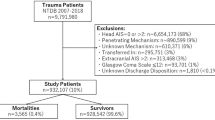
The sampled population was followed for 6 years from January 1, 2005 to December 31, 2010. We identified individuals for our study cohort who were still alive in 2007 and were older than 18 years. Mild TBI was defined by the ICD-9-CM code for head concussion (850.0, 850.1, 850.5, or 850.9), intracranial injury of other and unspecified nature (854.0), or head injury, unspecified (959.01) [16],[18]. Skull fracture (800.0, 800.5, 801.0, 801.5, 803.0, 803.5, 804.0, or 804.5) was not used as an alternative diagnosis because of the relative high impact of the injury [12]. Stroke was defined as either hemorrhagic (ICD-9-CM code 430–432) or ischemic (ICD-9-CM codes 433–437). To maximize case ascertainment, we only included patients hospitalized for stroke. We excluded patients with mild TBI and any type of stroke diagnosed before January 1, 2007. Since the ICD-9-CM codes for traumatic intracranial hemorrhage and hemorrhagic stroke may overlap, our study only focused on ischemic stroke. Patients who had been diagnosed with hemorrhagic stroke during the follow-up period were excluded. To avoid misclassification, we further excluded patients who had ever been hospitalized with TBI to ensure that enrolled patients with mild TBI were discharged directly after outpatient clinic or emergency departments visits. After exclusion of our cohort cases, we identified 24,905 patients with mild TBI and 719,811 without mild TBI. Each group was tracked from the date of mild TBI or January 1, 2007 (baseline) until December 31, 2010 (study end) to determine if ischemic stroke had been diagnosed during that period. Cases were censored for patients who were not beneficiaries anymore from the NHI Program (i.e., death or transfer out) or were still robust at the end of the follow-up period (Figure 1).
Covariates
To better understand the effect of mild TBI on the risk of ischemic stroke, we used several covariates including patient demographics such as age, sex, urbanization level (i.e., urban, suburban, and rural areas) and socioeconomic status (SES). Income-related insurance payment amounts were used as a proxy measure of individual SES at follow-up. People were classified into three groups: (1) low SES: payment lower than US$571 per month (New Taiwan Dollars [NT$] 20,000); (2) moderate SES: payment between US$571–1141 per month (NT$ 20,000–40,000); and (3) high SES: US$1142 or more payment per month (NT$40,001) or more [15]. Also, the prevalence of selected comorbid conditions (i.e., diabetes, hypertension, coronary artery disease, hyperlipidemia, history of alcohol intoxication, malignancies, heart failure, atrial fibrillation, smoking, obesity, epilepsy, peripheral artery disease) and the Charlson Cormobidity Index (CCI) score were determined according to the discharge diagnosis either during outpatient clinic visits or hospitalizations before January 1, 2007. The CCI is a scoring system that includes weighing factors of important concomitant diseases; it has been validated for use with ICD-9-CM coded administrative databases [19],[20].
Statistical analysis
The SAS statistical package, version 9.2 (SAS Institute, Inc., Cary, NC, USA), and STATA version 11.2 (StataCorp, College Station, TX, USA) were used for data analysis. All covariates were taken as categorical variables except age, which was treated as a continuous variable. Categorical variables were compared with Pearson’s chi-square test and continuous variables with the t test to reveal the baseline heterogeneity in the two groups. The Nelson-Aalen cumulative hazard estimates were first plotted to show the trend of ischemic stroke. Cox proportional hazard regression models were then used to calculate the hazard ratios (HRs) of ischemic stroke for individuals with mild TBI after adjustments for age, gender, urbanization level, SES, diabetes, hypertension, coronary artery disease, hyperlipidemia, history of alcohol intoxication, malignancies, heart failure, atrial fibrillation, smoking, obesity, epilepsy, peripheral artery disease, and the CCI. The reason we chose Cox regression analyses is because it is more statistically powerful and efficient [21],[22]. Adjusted HRs were analyzed both for 1) from mild TBI or baseline through study end, and 2) from 12 months after mild TBI or baseline through study end.
To further assess the robustness of our results, we performed a subgroup analysis to evaluate the risk of ischemic stroke in patients who had been hospitalized because of TBI to see if there is a `dose-response’ effect in the relationship between TBI and ischemic stroke. A two-tailed P value of <0.05 was considered significant.
Results
The distribution of both demographic characteristics and selected clinical characteristics is shown in Table 1. There were 24,905 patients in the mild TBI group and 719,811 in the control group. Total follow-up periods in the two groups were 48,371 and 2,793,892 person-years, respectively. In the mild TBI group 13.7% of patients underwent computed tomography (CT). Patients with mild TBI were significantly older and had significantly more comorbidities than the controls. By the end of follow-up, 9,654 patients had been diagnosed with ischemic stroke, including 412 in the mild TBI group and 9,242 in the control group. The average duration from mild TBI to ischemic stroke was 1.12 years (95% confidence interval [CI], 1.03–1.20). The crude HR for ischemic stroke between the two groups was 2.49 (95% CI, 2.25–2.74). Nelson-Aalen plot curves showed a higher trend for ischemic stroke in the mild TBI group (Figure 2).
Next, we performed the multivariate Cox regression models to evaluate the adjusted HRs of ischemic stroke. Patients with mild TBI still had higher HRs after controlling for age, gender, urbanization level, SES, diabetes, hypertension, coronary artery disease, hyperlipidemia, history of alcohol intoxication, malignancies, heart failure, atrial fibrillation, smoking, obesity, epilepsy, peripheral artery disease and CCI score (1.46; 95% CI, 1.33–1.62). Other independent risk factors of ischemic stroke included older age, male gender, living outside of an urban area, lower SES, diabetes, hypertension, coronary artery disease, history of alcohol intoxication, heart failure, atrial fibrillation, epilepsy and higher CCI. The statistical results are summarized in Table 2.
An analysis based on individuals who survived longer than 12 months was performed. There were 18,092 patients in the mild TBI group and 708,879 controls. The HR for mild TBI was slightly decreased but still statistically significant (1.38; 95% CI, 1.20–1.59) (Table 3).
A subgroup analysis based on patients admitted with TBI was conducted. There were 4,668 patients in the TBI group and 719,811 in the control group. After controlling for the same covariates, the HR for ischemic stroke in the hospitalized group was higher than for those discharged directly after visits (HR 3.43; 95% CI, 2.97–3.95). The statistical results of other covariates were similar to those of the primary study cohort and are summarized in Table 4.
Discussion
Studies have found that TBI is associated with increased risk of stroke [10]-[12]. Although the actual mechanism is still not completely understood, several hypotheses have been proposed. Impaired blood supply after damage to the cerebrovascular system caused by TBI might induce a stroke. Alterations in the coagulation cascade, clot formation and free radical generation after TBI may also be explanations [11],[23],[24]. Moreover, elevated intracranial pressure and blood pressure commonly noted among TBI patients may lead to stroke [25]. In our study, we further extended investigation of the impact of TBI to the mild type, utilizing a large administrative cohort and found that patients with a single mild TBI have a higher risk of ischemic stroke later in their lives compared to the general population. Our study had enough statistical power to provide a precise estimate of the HR (1.46; 95% CI, 1.33–1.62), which was statistically and clinically significant. The database corresponds well to the whole population; therefore, loss of follow-up or selection bias were not concerns. In Chen et al., the adjusted HR for stroke in all TBI patients who survived for more than one year was 4.61 (95% CI, 4.16–5.11) [11]. Compared to their results, HRs in our two mild TBI groups were lower, indicating that the degree of impact may be a factor in the occurrence of different sequelae.
Another advantage of our study is that we directly compared mild TBI patients with the general population simultaneously by survival analysis. Using this design, we were able to adjust extensively for possible confounding factors. Among the covariates, older age, male gender, living outside of an urban area, lower SES, diabetes, hypertension, coronary artery disease, history of alcohol intoxication, heart failure, atrial fibrillation, epilepsy and higher CCI scores were found to be associated with higher risks of ischemic stroke in patients with TBI, which is consistent with previous publications [12],[26]-[28].
This study had several limitations. First, our findings were derived from administrative data. Cases were collected using ICD-9-CM diagnosis codes, a relatively outdated system which is good for insurance reimbursement but is not a substitute for precise operative definition. Therefore, the validity of the diagnosis (i.e., sensitivity, specificity and accuracy) was not fully assessed. In Bazarian et al. [18], the sensitivity of ICD-9-CM codes for mild TBI was 45.9% with a specificity of 97.8%. In other words, patients in the mild TBI group were highly likely to have mild TBI, while some individuals in the control group may had mild TBI during the study period but the inclusion strategy failed to identify them. In this situation, the predicted effect of mild TBI on ischemic stroke should be toward the null, but we still found a significant risk of ischemic stroke in patients with mild TBI.
Second, we were unable to obtain the clinical information for patients with mild TBI, such as the Glasgow Coma Scale score, findings on cranial CT, the injury mechanism and the initial presentations. By definition, labeling our cases as `mild TBI’ may have been inappropriate. However, it has been validated that the ICD9-CM codes have high specificity regarding diagnosis of mild TBI [18]. Furthermore, we excluded those patients who were admitted to the hospitals to make sure that the patients enrolled were really in the “mild” category. Based on our inclusion criteria, although not totally precise, we think the cases in our study group were highly correlated with the definition of mild TBI [16].
Third, although we extensively adjusted for possible comorbidities, unmeasured cofounding is still an issue. However, we did a subgroup analysis and found a higher HR of ischemic stroke (3.43) in patients admitted with TBI and the unmeasured confounding could not fully explain the `dose-response’ of injury severity.
Conclusions
Mild TBI is an independent significant risk factor for ischemic stroke. The results indicate that more emphasis on head injury prevention and public awareness about long term sequelae of TBI would be worthwhile.
Authors’ contributions
Dr. Y-KL - study supervision and manuscript formation. Dr. C-WL – manuscript formation. Dr. M-YH – data analysis and study supervision. Dr. C-YH – data analysis. Dr. Y-CS- analysis and interpretation. All authors read and approved the final manuscript.
References
CDC grand rounds: reducing severe traumatic brain injury in the United States. MMWR Morb Mortal Wkly Rep. 2013, 62: 549-552.
Bruns J, Hauser WA: The epidemiology of traumatic brain injury: a review. Epilepsia. 2003, 44 (Suppl 10): 2-10. 10.1046/j.1528-1157.44.s10.3.x.
Tagliaferri F, Compagnone C, Korsic M, Servadei F, Kraus J: A systematic review of brain injury epidemiology in Europe. Acta Neurochir. 2006, 148: 255-268. 10.1007/s00701-005-0651-y. discussion 268
Sivanandam TM, Thakur MK: Traumatic brain injury: a risk factor for Alzheimer’s disease. Neurosci Biobehav Rev. 2012, 36: 1376-1381. 10.1016/j.neubiorev.2012.02.013.
Wright MJ, Wong AL, Obermeit LC, Woo E, Schmitter-Edgecombe M, Fuster JM: Memory for performed and observed activities following traumatic brain injury. J Clin Exp Neuropsychol. 2014, 36: 268-277. 10.1080/13803395.2014.884543.
Yasseen B, Colantonio A, Ratcliff G: Prescription medication use in persons many years following traumatic brain injury. Brain Inj. 2008, 22: 752-757. 10.1080/02699050802320132.
Fleminger S: Long-term psychiatric disorders after traumatic brain injury. Eur J Anaesthesiol Suppl. 2008, 42: 123-130. 10.1017/S0265021507003250.
Bower JH, Maraganore DM, Peterson BJ, McDonnell SK, Ahlskog JE, Rocca WA: Head trauma preceding PD: a case–control study. Neurology. 2003, 60: 1610-1615. 10.1212/01.WNL.0000068008.78394.2C.
Till C, Colella B, Verwegen J, Green RE: Postrecovery cognitive decline in adults with traumatic brain injury. Arch Phys Med Rehabil. 2008, 89: S25-S34. 10.1016/j.apmr.2008.07.004.
Burke JF, Stulc JL, Skolarus LE, Sears ED, Zahuranec DB, Morgenstern LB: Traumatic brain injury may be an independent risk factor for stroke. Neurology. 2013, 81: 33-39. 10.1212/WNL.0b013e318297eecf.
Chen YH, Kang JH, Lin HC: Patients with traumatic brain injury: population-based study suggests increased risk of stroke. Stroke. 2011, 42: 2733-2739. 10.1161/STROKEAHA.111.620112.
Liao CC, Chou YC, Yeh CC, Hu CJ, Chiu WT, Chen TL: Stroke risk and outcomes in patients with traumatic brain injury: 2 nationwide studies. Mayo Clin Proc. 2014, 89: 163-172. 10.1016/j.mayocp.2013.09.019.
Bazarian JJ, Cernak I, Noble-Haeusslein L, Potolicchio S, Temkin N: Long-term neurologic outcomes after traumatic brain injury. J Head Trauma Rehabil. 2009, 24: 439-451. 10.1097/HTR.0b013e3181c15600.
Brown AW, Leibson CL, Malec JF, Perkins PK, Diehl NN, Larson DR: Long-term survival after traumatic brain injury: a population-based analysis. NeuroRehabilitation. 2004, 19: 37-43.
Cheng PL, Lin HY, Lee YK, Hsu CY, Lee CC, Su YC: Higher mortality rates among the elderly with mild traumatic brain injury: a nationwide cohort study. Scand J Trauma Resusc Emerg Med. 2014, 22: 7-10.1186/1757-7241-22-7.
Lee YK, Hou SW, Lee CC, Hsu CY, Huang YS, Su YC: Increased risk of dementia in patients with mild traumatic brain injury: a nationwide cohort study. PLoS One. 2013, 8: e62422-10.1371/journal.pone.0062422.
Lee YK, Lee CC, Chen CC, Wong CH, Su YC: High risk of `failure’ among emergency physicians compared with other specialists: a nationwide cohort study. Emerg Med J. 2013, 30: 620-622. 10.1136/emermed-2012-201440.
Bazarian JJ, Veazie P, Mookerjee S, Lerner EB: Accuracy of mild traumatic brain injury case ascertainment using ICD-9 codes. Acad Emerg Med. 2006, 13: 31-38. 10.1111/j.1553-2712.2006.tb00981.x.
Charlson ME, Pompei P, Ales KL, MacKenzie CR: A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987, 40: 373-383. 10.1016/0021-9681(87)90171-8.
Deyo RA, Cherkin DC, Ciol MA: Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992, 45: 613-619. 10.1016/0895-4356(92)90133-8.
Ingram DD, Kleinman JC: Empirical comparisons of proportional hazards and logistic regression models. Stat Med. 1989, 8: 525-538. 10.1002/sim.4780080502.
van der Net JB, Janssens Ac Fau - Eijkemans MJC, Eijkemans Mj Fau - Kastelein JJP, Kastelein Jj Fau - Sijbrands EJG, Sijbrands Ej Fau - Steyerberg EW, Steyerberg EW: Cox proportional hazards models have more statistical power than logistic regression models in cross-sectional genetic association studies. Eur J Hum Genet. 2008, 16: 1111-1116. 10.1038/ejhg.2008.59.
Lu D, Mahmood A, Goussev A, Schallert T, Qu C, Zhang ZG, Li Y, Lu M, Chopp M: Atorvastatin reduction of intravascular thrombosis, increase in cerebral microvascular patency and integrity, and enhancement of spatial learning in rats subjected to traumatic brain injury. J Neurosurg. 2004, 101: 813-821. 10.3171/jns.2004.101.5.0813.
Thompson HJ, McCormick WC, Kagan SH: Traumatic brain injury in older adults: epidemiology, outcomes, and future implications. J Am Geriatr Soc. 2006, 54: 1590-1595. 10.1111/j.1532-5415.2006.00894.x.
Romero JR, Morris J, Pikula A: Stroke prevention: modifying risk factors. Ther Adv Cardiovasc Dis. 2008, 2: 287-303. 10.1177/1753944708093847.
Rodriguez-Sainz A, Pinedo-Brochado A, Sanchez-Menoyo JL, Ruiz-Ojeda J, Escalza-Cortina I, Garcia-Monco JC: Migraine, stroke and epilepsy: underlying and interrelated causes, diagnosis and treatment. Curr Treat Options Cardiovasc Med. 2013, 15: 322-334. 10.1007/s11936-013-0236-7.
Lazzarino AI, Hamer M, Stamatakis E, Steptoe A: Low socioeconomic status and psychological distress as synergistic predictors of mortality from stroke and coronary heart disease. Psychosom Med. 2013, 75: 311-316. 10.1097/PSY.0b013e3182898e6d.
Lip GY, Nieuwlaat R, Pisters R, Lane DA, Crijns HJ: Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the euro heart survey on atrial fibrillation. Chest. 2010, 137: 263-272. 10.1378/chest.09-1584.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Lee, YK., Lee, CW., Huang, MY. et al. Increased risk of ischemic stroke in patients with mild traumatic brain injury: a nationwide cohort study. Scand J Trauma Resusc Emerg Med 22, 66 (2014). https://doi.org/10.1186/s13049-014-0066-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13049-014-0066-y