Abstract
In the current study, we investigated the regenerative effects of amniotic fluid exosomes (AF-Exos) in a rat model for premature ovarian insufficiency (POI). POI is a condition characterized by a decrease in ovarian function that can lead to infertility. We induced POI by administering cyclophosphamide (CTX) for 15 consecutive days, and then transplanted AF-Exos directly into both ovarian tissues. Four weeks later, we measured the serum levels of follicle-stimulating hormone (FSH), luteinizing hormone (LH), and estradiol (E2), and performed histopathological evaluations using H & E and Masson’s trichrome staining. We also monitored the expression of genes related to the TGF-β signaling pathway using real-time PCR and examined the fertility rate of POI rats after AF-Exos therapy. Histological analysis showed an increase in atretic follicles and a decrease in healthy follicle count after POI induction. Four weeks post-AF-Exos intervention, the healthy follicle count increased (p < 0.01) while the atretic follicle count decreased (p < 0.001). In parallel, the deposition of collagen fibers also decreased following AF-Exos transplantation. The concentrations of FSH and LH hormones in sera remained unchanged after injection of AF-Exos, while E2 levels increased (p < 0.05). The expression of Smad-4 (p < 0.01) and Smad-6 (p < 0.05) was upregulated in POI rats that received AF-Exos, while Smad-2, TGF-β1, TNF-α, and IL-10 remained statistically unchanged. Our records showed a notable increase in litter number after AF-Exos compared to the non-treated POI rats. These results suggest that AF-Exos transplantation has the potential to restore ovarian function through the TGF-β/Smads signaling pathway in POI rats.
Similar content being viewed by others
Introduction
Premature ovarian insufficiency (POI), also known as premature ovarian failure (POF), is characterized by abnormal ovarian function in females under the age of 40 [1]. POI is marked by elevated levels of follicle-stimulating hormone (> 40 IU/mL), decreased levels of estradiol (< 30 pg/mL), and anti-Müllerian hormone (< 1 ng/mL) [2]. The prevalence of POI cases varies between 0.9% and 1.2% in different societies [3]. Several factors such as physiological conditions, genetic traits, autoimmune diseases, infections, surgical procedures, environmental effects, and idiopathic reasons have been linked to POI occurrence [4].
Histological and molecular studies have indicated that POI is characterized by fibrotic changes in ovarian tissue via the engagement of several signaling pathways [5, 6]. Among these, the TGF-β/Smads signaling axis regulates the growth of ovarian follicles at different developmental stages. Dysregulated TGF-β/Smads signaling pathway accounts for follicular atresia and inhibition of follicles, increasing the possibility of POI conditions [7, 8]. Local production of important inflammatory cytokines such as TNF-α and IL-10 has been shown to promote POI consequences [9, 10]. For example, TNF-α induces primary follicle apoptosis, vascular endothelial damage, and reduction of sex-related hormones [7].
Hormone replacement therapy (HRT) is the most common treatment for POI patients, but it lacks complete recovery of ovarian tissue activity and accounts for the occurrence of some female-related malignancies [11,12,13]. Oocyte and embryo cryopreservation and donation [14], as well as gonadotropin-releasing hormone (GnRH) therapy [15], have also been suggested for POI patients. Unfortunately, these modalities cannot restore normal ovarian tissue activity. Recently, new therapeutic strategies based on stem cells [16,17,18], and their secretome have been proposed as an effective treatment option for various complications such as infertility [19]. Numerous studies have shown that different cell types can produce and release nano-sized vesicles such as exosomes (Exos), microvesicles, and apoptotic bodies into the extracellular vesicles [20, 21]. Exos, with an average size of 40 to 150 nm, are abundant in several biofluids such as plasma, urine, and amniotic fluid [22, 23]. Exos harbor several signaling molecules that act in a paracrine manner to target cells, resulting in the regulation of specific molecular cascades [24,25,26]. Amniotic fluid (AF) is an easily accessible biological fluid with significant levels of Exos [27, 28]. AF-derived Exos (AF-Exos) act as mediators of paracrine communication within the intrauterine environment through the transfer of metabolic substances, including proteins, ions, carbohydrates, lipids, enzymes, and hormones, as well as genomic content (dsDNA, ssDNA, miRNA, mRNA), growth factors, and cytokines that support the growth of the placenta and fetus during pregnancy [29,30,31,32,33]. Emerging data have pointed to the fact that Exo-based treatments yield promising therapeutic outcomes for infertility, cancers, infectious diseases, on-target drug delivery, etc. [33,34,35,36,37]. Unlike whole-cell-based therapy, the application of Exos has several advantages such as low immunogenicity, non-toxicity, and ease of access to target cells [38,39,40,41]. In the current investigation, we studied the regenerative potential of AF-Exos in a rat model of POI concerning fibrotic changes and the TGF-β/Smads signaling.
Materials and methods
Animal issues
Female Wistar rats (n = 27; 7-8-week-old) weighing between 150 and 180 g were purchased from Tehran Medzist Company and maintained at the animal center of Tabriz University of Medical Sciences. All the experiments and interventions performed in this study were approved by the Animal Care and Ethics Committee (IR.TBZMED.VCR.REC.1398.060) and the Guide for the Care and Use of Laboratory Animals (NIH, 1986). Rats were kept in a normal environment with a temperature of 22 ± 2°C, a 12-hour light/dark cycle, and free access to standard chewing pellets and tap water.
Induction of POI rats
Seven days after acclimation, a POI rat model was induced by the administration of cyclophosphamide (CTX; Cat no: RHRI404, Supelco). In short, CTX was injected intraperitoneally (i.p.) at a dose of 200 mg/kg BW on day 1 and 8 mg/kg bw from days 2 to 14. To confirm the POI status, three rats from the CTX-injected and control groups were randomly selected and subjected to histopathological examination and hormonal evaluations. The remaining POI rats were randomly allocated into three groups as follows: Control (Control-matched POI group, n = 7), Sham (POI + normal saline, n = 7), and AF-Exos (POI + AF-Exos, n = 7) (Fig. 1A).
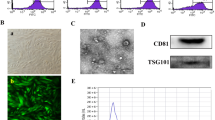
Schematic presentation experimental setup. Animal modeling procedure, exosome enrichment, intervention, sampling and mating (A), characterization of exosomes, size distribution (B) and zeta potential (C) assessment by dynamic light scattering method, scanning electron microscopy (D), transmission electron microscopy (E) and western blot analysis (F)
Isolation of AF-Exos
AF was obtained from a 3–4 months pregnant ewe without concomitant diseases and metabolic disorders at Tabriz local slaughterhouse under sterile conditions. AF-Exos were enriched by the ultracentrifugation method. To eliminate cells, samples were centrifuged at 300 g for 10 min, and the supernatant was then centrifuged at 2000 g for 15 min to remove dead cells and debris. The procedure was continued by centrifugation of samples at 10,000 g for 30 min. After passing through 0.22-micrometer microfilters, the collected supernatant was subjected to ultracentrifugation for 1 h at a speed of 100,000 g. To remove protein contaminants, a second round of ultracentrifugation was performed, and the pellet was resuspended in phosphate-buffered saline (PBS) and stored at ‒80 °C for subsequent analyses (Fig. 1A) [42, 43].
AF-Exos characterization
Dynamic light scattering (DLS)
To determine size distribution and zeta potential, the DLS approach was performed using a Zetasizer Nanoseries device (Malvern Nano ZS, Herrenberg, Germany), according to the manufacturer’s instructions [44].
Scanning electron microscopy (SEM)
Samples were fixed with 200 µL pre-cold paraformaldehyde solution (2.5% w/v) (Sigma-Aldrich GmbH, Germany) and lyophilized after ultracentrifugation. Subsequently, samples were gold-sputtered and visualized under a Mira-3 FEG SEM microscope (Tescan, Czech Republic) [45].
Transmission electron microscopy (TEM)
To further examine the morphology of the isolated AF-Exos, a drop of the exosomal suspension was placed on a 300-mesh copper grid, stained with 2% (w/v) uranyl acetate solution for 15 min, and subsequently covered with a carbon film to prevent degradation under the electron beam. The samples were observed using a TEM instrument (LEO 906, Zeiss, Germany) with a voltage of 100 kV [46].
Western blotting
Protein levels of exosomal markers were monitored using western blotting, according to our previously published paper [41].
Ovarian transplantation of AF-Exos
Twenty-one days after the last CTX injection, rats were deeply anesthetized using 90 mg/kg ketamine and 10 mg/kg xylazine. A one-centimeter incision was made in the supra flank region, and ovarian masses were exposed. Using a 25-G syringe, 10 µl of enriched AF-Exos (equal to 10 µg exosomal protein) was injected directly into the ovarian tissue. In the sham group, a similar volume of normal saline was administered.
Histological examination
Rats were euthanized using an overdose of ketamine and xylazine four weeks after AF-Exos injection (n = 4 per group). Left ovaries were sampled, fixed in 4% formaldehyde, and subsequently embedded in paraffin. Histological sections, with a thickness of 5 μm, were prepared using a microtome instrument. After dehydration in an alcohol series, sections were stained with Hematoxylin and Eosin (H&E) staining solution and evaluated under an Olympus BX51 light microscope. Parameters such as follicle number and quality at four stages of development (primary, secondary, and antral follicles), as well as the existence of corpus luteum (CL), were assessed [47]. To monitor fibrotic changes and collagen fiber deposition, Masson’s trichrome staining was performed [48].
ELISA analysis
Enzyme-linked immunosorbent assay (ELISA) was performed according to the kit manufacturer’s instructions to measure serum levels of FSH (Cat no: 334-096-4, Monobind), LH (Cat no: 0234 − 96, Monobind), and E2 (Cat no: 4925-300 A, Monobind). Blood samples were obtained from rats under deep anesthesia (90 mg/kg ketamine plus 10 mg/kg xylazine) directly from the heart (n = 4 per group). The samples were then centrifuged at 400 g for 10 min to harvest serum. The collected sera were transferred to cryovials and kept at -80 °C until use.
Real-time PCR analysis
The fibrosis rate was also assessed four weeks post-intervention by monitoring the expression of Smad-2, -4, -6, Tgf-β1, Tnf-α, and IL-10. To this end, the right ovaries were subjected to RNA extraction using TRIzol reagent (Cat No: 0000124; MaxZol) (n = 4 per group). After determining the quality and concentrations of RNA (NanoDrop Spectrophotometer), cDNA was synthesized using a cDNA synthesis kit (Cat no: YT4500, Yekta Tajhiz Azma). Specific primer pairs were designed using the Primer-Blast online software (https://www.ncbi.nlm.nih.gov/tools/primer-blast/) (Table 1), and specific annealing temperatures were optimized using gradient PCR. Quantitative real-time PCR reactions (qRT-PCR) were set up using SYBR Green 2X (Cat no: 5,000,850, Ampliqon) and cDNA using the Roche Light Cycler 96 system. The PCR reaction consisted of 45 cycles with three stages of denaturation, annealing, and extension at temperatures of (95, 60, and 72 °C, respectively). The specificity of reactions was monitored using melting and melting curves. Related quantifications were elaborated using 2−ΔΔct, with β-actin as a housekeeping gene.
Mating trial
To assess the fertility status of the rats, the remaining three rats per group were kept in separate cages with a 2:1 (male: female) ratio for a maximum of one week. After confirming successful mating by observing the vaginal plug, rats were placed in separate cages for three weeks. Finally, the number of offspring was recorded.
Statistical analysis
All mean ± SEM results were analyzed using GraphPad Prism 8.0.1 software. Data were analyzed using One-way analysis of variance with a post hoc Tukey test. A t-test was also used to find statistically significant differences between the two groups. Values below 0.05 were considered statistically significant.
Results
Induction of POI conditions
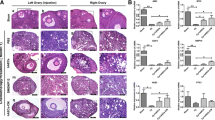
To confirm the POI condition, a histological evaluation was performed twenty-one days after the last dose of CTX injection, and follicular status was observed within the ovarian tissues (Fig. 2A-D). Bright-field imaging revealed a significant general follicular atresia with cumulus cell disintegration, vacuolation, and separation of granulosa cells recorded at all stages of development in the CTX-received rats compared to the control group (p < 0.05; Fig. 2A-D). On the contrary, a decline pattern was observed in the number of morphologically healthy follicles at all stages of development in the CTX-injected rats compared to the control group (p < 0.05; Fig. 2B). The data also revealed a significant reduction in the number of corpus luteum (CL) in POI rats compared to the non-treated control group (p < 0.05; Fig. 2D). These features confirmed that CTX administration led to POI conditions in rats.
Morphological assessment of ovarian tissue after animal modeling, total atretic and atretic follicles count at different developmental stages of primordial, primary, secondary and antral (A), total healthy and healthy follicles count at different developmental stages of primordial, primary, secondary and antral (B), H&E staining (C) and total corpus luteum count (D). (Scale bar = 200 µm), *p < 0.05; **p < 0.01; ***p < 0.001 and ****p < 0.0001 (n = 4)
AF-Exos characterization
We are currently performing several projects related to the application of AF-Exos for the restoration of injured ovarian tissue function. To this end, data from our previously published articles were presented here with permission to reproduce [41]. According to DLS data, the isolated AF-Exos exhibited an average zeta potential of − 7.16 mV and a mean diameter of 50 ± 7.521 nm (Fig. 1B-C). SEM and TEM images indicated typical round and cup-shaped particles, which are identical to Exos (Fig. 1D-E). The isolated Exos harbored specific tetraspanins such as CD63, CD9, and CD81 (Fig. 1F). These features indicated the efficiency of the current protocol to enrich the AF-Exos.
AF-Exos transplantation and ovarian follicles
To assess the regenerative potential of AF-Exos on ovarian tissue, H&E staining was performed four weeks after AF-Exos transplantation. The data revealed that the total number of atretic follicles significantly decreased after the administration of AF-Exos compared to the control POI group (p < 0.01; Fig. 3A-C). Along with this, the total number of morphologically healthy follicles increased in POI rats after the injection of AF-Exos (p < 0.01; Fig. 3A-C). General improvement was observed regarding the increase in the number of morphologically normal follicles and the decrease in the number of atretic follicles in all stages of growth (primary, secondary, and antral) in the AF-Exo-treated rats compared to the sham group (p < 0.05; Fig. 3A-C). The CL numbers were also significantly increased in POI rats after Exos transplantation compared to the sham group (p < 0.05; Fig. 3D). These data indicate the protective properties of AF-Exos on atretic follicles.
Morphological assessment of ovarian tissue after AF-Exos transplantation, total atretic and atretic follicles count at different developmental stages of primordial, primary, secondary and antral (A), total healthy and healthy follicles count at different developmental stages of primordial, primary, secondary and antral (B), H&E staining (C) and total corpus luteum count (D). (Scale bar = 200 µm), *p < 0.05; **p < 0.01 and ***p < 0.001 (n = 4)
AF-Exos transplantation and ovarian tissue fibrosis
The expression of fibrosis-related genes was monitored following AF-Exos administration in POI rats (Fig. 4). The data showed that the expression of Smad-4 and − 6 increased following intervention in the AF-Exos group compared to the control POI rats (p < 0.01 and p < 0.05 respectively; Fig. 4). Additionally, the expression of Tgf-β1, Tnf-α, and IL-10 was reduced after AF-Exos administration, but the differences were not statistically significant. Masson’s trichrome staining indicated the presence of blue-colored collagen fibers within the ovarian tissue parenchyma after CTX injection (Fig. 4). It was observed that AF-Exos administration reduced the fibrotic changes by reducing the number of blue-colored fibers. These data demonstrate that AF-Exos possess the regenerative potential to restore the function of POI ovaries by reducing fibrotic changes.
AF-Exos transplantation modulated the hormone levels in POI rats
The serum levels of FSH, LH, and E2 hormones were measured before and after intervention (Fig. 5A). An elevated pattern was noticed in the systemic levels of FSH and LH hormones in the POI rats compared to the control group, but the differences were not statistically significant (p > 0.05; Fig. 5A). Upon AF-Exos transplantation, the serum levels of FSH and LH hormones decreased; however, the differences were again not significant (p > 0.05; Fig. 5A). Concerning E2 levels, it was indicated that the injection of AF-Exos led to an increase in E2 levels compared to the control POI and POI + Normal Saline group (p < 0.05; Fig. 5A). These data showed that AF-Exos can restore the basal levels of certain sex-related hormones in POI rats after four weeks.
AF-Exos transplantation improved fertility status
Four weeks after AF-Exos treatment, three remaining rats from each group were mated with fertility-proven male rats. After the gestation period, the total number of births was recorded. Interestingly, all three rats in the AF-Exos group gave birth to 8, 9, and 8 litters, respectively. In the sham and POI-control groups, only one rat gave birth to 3 and one to 6 litters, respectively (p < 0.05; Fig. 5B). These data indicate that AF-Exos transplantation improved fertility status in POI rats.
Discussion
In dealing with POI patients, clinicians, and reproductive biotechnologists have been focusing on developing cell-free therapeutics with minimal side effects, due to the efficiency and safety concerns of available therapeutic modalities. However, few studies have analyzed the effects of Exos on the treatment of POI conditions [49]. In our experiment, we used AF as the source of Exos. It is well established that amniotic fluid is a rich source of stem cells and contains numerous factors with regenerative outcomes [50]. We assessed the regenerative potential of AF-Exos in terms of POI ovarian function in a rat model.
The data show that injection of CTX with the current protocol can induce POI-like conditions in rats that can be applied to human counterparts [47, 49, 51]. CTX is an alkylating agent with the potential to affect both resting and dividing cells in female reproductive organs, especially ovaries [11]. Consistent with this claim, histological examination revealed the reduction of healthy follicles and the progression of atretic changes in rats, leading to the development of POI conditions. Likewise, the levels of sex-related hormones such as FSH, LH, and E2 remained unchanged after the induction of POI. One reason for this could be that the current protocol only induced a non-overt POI model using the CTX injection, and the reduction of FSH, LH, and E2 levels can be compensated with the activity of the hypothalamic-pituitary-ovarian axis in response to the pathologies that occurred inside the ovaries [52, 53]. The levels of sex-related hormones can vary in POI models depending on the primordial follicle pool and rapid follicle exhaustion [52]. Despite the lack of significant differences in systemic levels of FSH, LH, and E2 in POI rats, Masson’s trichrome and H&E staining revealed progressive fibrotic changes and atretic follicles in all developmental stages.
The injection of AF-Exos prevented follicular atresia induced by CTX. Along with these changes, the number of morphologically healthy follicles increased compared to the control POI group. Consistent with the current data, Huang and colleagues reported that human adipose tissue mesenchymal stem cells (MSCs) Exos can increase the number of follicles to almost normal levels in four stages (primordial, primary, secondary, and antral) with the normalization of sex-related hormones [49]. It was suggested that Exos can reduce apoptotic changes via the inhibition of Fas, FasL, Caspase-8, and Caspase-3 [49]. Our data indicated the non-significant reduction of TNF-α and IL-10, which can lead to the reduction of follicular atresia. It is also possible that the protective effects of injected Exos are associated with the restoration of granulosa cell function under pathological conditions. Based on previous data, MSCs can increase the proliferation of granulosa cells in a paracrine manner in vitro conditions [49]. Likewise, Exos from other stem cell sources, such as human umbilical cord MSCs, can promote the activity and proliferation of granulosa cells via the regulation of the Hippo pathway, as indicated by ErdU and CCK-8 assays [54]. Zhang and co-workers found the induction of DAZL and FOXL2 in POI rat ovaries with the reduction of apoptosis that received menstrual blood-derived stem cell Exos [55]. The induction of the mTOR-dependent signaling pathway is another therapeutic effect associated with the administration of Exos. Yang and colleagues have indicated that the suitable uptake of human umbilical cord MSCs via primordial follicles further activates the PI3K/mTOR signaling pathway, which is crucial for the therapeutic effects of Exos [56]. The modulation of several signaling pathways in POI ovaries indicates the pleiotropic properties of Exos in the restoration of ovarian tissue function. Another interesting finding in this study is the reduction of fibrotic changes. Cen et al. have reported that after femustine treatment in POF model rats, the expression of SMAD2 and GDF-9 genes was increased [57]. In line with current data, Huang and co-workers have indicated a reduction in fibrotic changes in POI rats after the injection of adipose tissue MSC Exos, where in vitro and in vivo analyses have revealed reduced expression of SMAD2, SMAD3, and SMAD5 [49].
In this study, we have found a statistically significant up-regulation of the TGF-β signaling pathway inhibitory effector SMAD-6, while concurrently the expression of SMAD-4 has also increased. Despite the reduced expression of TGF-β and IL-10, these values did not reach statistically significant levels. One possible reason for the induction of SMAD-4 is that this factor acts as a stimulatory effector in TGF-β signaling pathway factors and concurrently can regulate lipogenesis activity. To be specific, studies have shown that SMAD-4 is a key regulator in androgen and estrogen production and FSH production via estrogen production [58, 59]. Regarding the data from Masson’s trichrome staining and real-time PCR analysis, we can hypothesize that AF-Exos can attenuate the fibrotic changes within the ovarian tissue with POI. It is also suggested that the increase in the stimulatory TGF-β signaling factor SMAD-4 is related to other biological effects of this factor. More investigations are mandatory to precisely address the pleiotropic effects of SMAD-4 under fibrotic changes. The lack of significant data in terms of TNF-α and IL-10 could be due to the short duration of the experiment, while in various studies, inflammatory factors were reduced after treatment in the POF model [47, 51, 60].
We have also noted an increase in E2 in POI ovaries after the injection of AF-Exos. In line with our data, Yang and co-workers have indicated improved ovarian function after transplantation of bone marrow MSC Exos in a rat model of POF [61]. This study reported normal levels of FSH, LH, E2, and AMH hormones after transplantation. Finally, the fertility of POI rats treated with AF-Exos was checked after one month. The data showed an enhanced fertility rate in POI rats that received AF-Exos compared to control-matched groups. In support of our data, previous studies have also reported that Exos can heal damaged follicles and increase the fertility rate [61,62,63,64,65].
The current experiment faces several limitations that need consideration for future investigations. Despite a sufficient amount of AF-Exos, it should be noted that the heterogeneity in isolated Exos is high in AF samples compared to the other cultured cell samples in laboratory settings. Enrichment of Exos should be carefully monitored in terms of insidious infections and genetic aberrancies before application in in vitro and in vivo conditions. Besides, the mucoid appearance and varied floating particles in AF make the Exo isolation more laborious compared to the other biofluids.
Conclusions
In conclusion, the administration of Exos derived from stem cells has shown promising therapeutic effects in the treatment of premature ovarian insufficiency (POI). Exos have been found to improve ovarian tissue function by modulating several signaling pathways, including the mTOR-dependent signaling pathway and the PI3K/mTOR signaling pathway. They have also been found to reduce fibrotic changes within the ovarian tissue, as evidenced by reduced expression of SMAD2, SMAD3, and SMAD5 genes.
In addition, Exos have been found to increase the expression of SMAD-6, an inhibitory effector of the TGF-β signaling pathway, which may contribute to the attenuation of fibrotic changes within the ovarian tissue. This effect may be related to other biological effects of SMAD-4, a stimulatory effector of TGF-β signaling, which has been found to play a key role in androgen and estrogen production, FSH production, and lipogenesis activity. These data indicate that Exos harbor distinct signaling cytokines and growth factors with the potential to promote the reparative mechanism in POI rats via the regulation of fibrotic changes and chronic inflammation, indicating the close relationship between the molecular biology and female fertilization rate [66, 67]. Whether and how the metabolic status of parent cells can affect the regenerative potential of isolated Exos in terms of fertility should be addressed in future studies.
Furthermore, Exos have been found to increase the levels of estrogen in POI ovaries and enhance fertility rates in POI rats. These findings suggest that Exos may be a promising therapeutic strategy for the treatment of POI, and further studies are warranted to fully elucidate their mechanisms of action and potential clinical applications. Overall, these findings have important implications for the development of novel treatments for POI and may offer hope to women suffering from this condition.
Data Availability
Data are contained within the article. Datasets related to this project can be obtained from the corresponding author based on a reasonable request.
References
Ishizuka B. Current understanding of the etiology, symptomatology, and treatment options in premature ovarian insufficiency (POI). Front Endocrinol. 2021;12:88.
Chon SJ, Umair Z, Yoon M-S. Premature ovarian insufficiency: past, present, and future. Front Cell Dev Biology, 2021. 9.
Huang Q-y, et al. Therapeutic options for premature ovarian insufficiency: an updated review. Reproductive Biology and Endocrinology. 2022;20(1):1–16.
Karska P, et al. Fresh insight into premature ovarian insufficiency. Ginekologia polska. 2021;92(7):518–24.
Zhang X, et al. Resumption of ovarian function after ovarian biopsy/scratch in patients with premature ovarian insufficiency. Reproductive Sci. 2019;26(2):207–13.
Ling L, et al. Human amnion-derived mesenchymal stem cell (hAD-MSC) transplantation improves ovarian function in rats with premature ovarian insufficiency (POI) at least partly through a paracrine mechanism. Stem Cell Res Ther. 2019;10:1–18.
Wang Y et al. Research Progress on the Effect of Traditional Chinese Medicine on Signal Pathway Related to Premature Ovarian Insufficiency 2022. 2022.
Gao Y et al. ANO1 inhibits cardiac fibrosis after myocardial infraction via TGF-β/smad3 pathway. Sci Rep. 7(1): 2355
Yang H, Pang H, Miao C. Ovarian IL-1α and IL-1β levels are associated with primary ovarian insufficiency. Int J Clin Exp Pathol. 2018;11(9):4711.
He Y, et al. The therapeutic potential of bone marrow mesenchymal stem cells in premature ovarian failure. Stem Cell Res Ther. 2018;9:1–7.
Ahmadian S, et al. Intra-ovarian injection of platelet-rich plasma into ovarian tissue promoted rejuvenation in the rat model of premature ovarian insufficiency and restored ovulation rate via angiogenesis modulation. Reproductive Biology and Endocrinology. 2020;18(1):1–13.
Sullivan SD, Sarrel PM, Nelson LM. Hormone replacement therapy in young women with primary ovarian insufficiency and early menopause. Fertil Steril. 2016;106(7):1588–99.
Bianco B, et al. Effects of FSHR and FSHB Variants on Hormonal Profile and Reproductive Outcomes of Infertile Women with Endometriosis. Front Endocrinol (Lausanne). 2021;12:760616.
Męczekalski B, Maciejewska-Jeske M, Podfigurna A. Reproduction in premature ovarian insufficiency patients–from latest studies to therapeutic approach. Przegląd Menopauzalny = Menopause Review. 2018;17(3):117.
Chen H, et al. Adjuvant gonadotropin-releasing hormone analogues for the prevention of chemotherapy-induced premature ovarian failure in premenopausal women. Cochrane Database of Systematic Reviews; 3(3):CD008018.
Yamchi NN, et al. Menstrual blood CD146 + mesenchymal stem cells reduced fibrosis rate in the rat model of premature ovarian failure. Cell Biochem Funct. 2021;39(8):998–1008.
Fu Y-X, et al. Human mesenchymal stem cell treatment of premature ovarian failure: new challenges and opportunities. Stem Cell Res Ther. 2021;12(1):1–13.
Chen C et al. Protective effects of puerarin on premature ovarian failure via regulation of Wnt/β-catenin signaling pathway and oxidative stress. 2021. 28(4): p. 982–90.
Esfandyari S et al. Mesenchymal stem cells as a bio organ for treatment of female infertility. 2020. 9(10): p. 2253.
Joshi BS et al. Endocytosis of extracellular vesicles and release of their cargo from endosomes. 2020. 14(4): p. 4444–55.
Mardi N, et al. Exosomal transmission of viruses, a two-edged biological sword. Cell Communication and Signaling. 2023;21(1):1–29.
Mardi N et al. Exosomes; multifaceted nanoplatform for targeting brain cancers. Cancer Lett, 2023: p. 216077.
Dezhakam E et al. Electrochemical biosensors in exosome analysis; a short journey to the present and future trends in early-stage evaluation of cancers Biosensors and Bioelectronics. 2022: 222: 114980
Kowalczyk A, et al. Exosomes–Spectacular role in reproduction. Biomed Pharmacother. 2022;148:112752.
Rezaie J et al. A review on exosomes application in clinical trials: perspective, questions, and challenges. 2022. 20(1): p. 1–13.
Pournaghi M, et al. Effect of melatonin on exosomal dynamics in bovine cumulus cells. Process Biochem. 2021;106:78–87.
Desrochers LM et al. Microvesicles provide a mechanism for intercellular communication by embryonic stem cells during embryo implantation 2016. 7(1): p. 1–11.
Dixon CL et al. Amniotic fluid exosome proteomic profile exhibits unique pathways of term and preterm labor. 2018. 159(5): p. 2229–40.
Bellio MA et al. Amniotic fluid-derived extracellular vesicles: characterization and therapeutic efficacy in an experimental model of bronchopulmonary dysplasia. 2021. 23(12): p. 1097–107.
Kalluri R, LeBleu VSJS. The biology, function, and biomedical applications of exosomes 2020. 367(6478): p. eaau6977.
Mehdizadeh M, Ghiasi MJJMM. Development and application of mesenchymal stem cell-derived Exosomes in Cartilage tissue repair. 2022. 24(5): p. 1319–29.
Bhartiya D et al. Very small embryonic-like stem cells (VSELs) regenerate whereas mesenchymal stromal cells (MSCs) rejuvenate diseased reproductive tissues 2021: p. 1–10.
Rahmati S, et al. Overv Curr Knowl Biol Funct potential theragnostic Appl exosomes. 2020;226:104836.
De Coppi P, et al. Isolation of amniotic stem cell lines with potential for therapy.Nat Biotechnol . 2007;25(1):100–6.
Zhang Y et al. Exosomes derived from mesenchymal stromal cells promote axonal growth of cortical neurons. Mol Neurobiol . 2017. 54(4): p. 2659–73.
Zhang J et al. Exosomes released from human induced pluripotent stem cells-derived MSCs facilitate cutaneous wound healing by promoting collagen synthesis and angiogenesis. J Transl Med. 2015. 13(1): p. 1–14.
Dezhakam E, et al. Electrochemical biosensors in exosome analysis; a short journey to the present and future trends in early-stage evaluation of cancers. Biosens Bioelectron. 2023;222:114980.
Elahi FM et al. Preclinical translation of exosomes derived from mesenchymal stem/stromal cells 2020. 38(1): p. 15–21.
Gomzikova MO, James V. J.F.i.i. Rizvanov. Therapeutic application of mesenchymal stem cells derived extracellular vesicles for immunomodulation. 2019;10:2663.
Basu J, J.W.J.E.O.o.B T, Ludlow. Exosomes for repair regeneration and rejuvenation. Expert Opin Biol Ther. 2016;16(4):489–506.
Mobarak H et al. Amniotic fluid-derived exosomes improved spermatogenesis in a rat model of azoospermia. Life Sci. 2021. 274: p. 119336.
Nojehdehi S, Hashemi SM, Hesampour A. Isolation and characterization of exosomes separated from stem cells by ultra-centrifuge method. Res Med. 2017;73:244–50.
Sheller-Miller S, Menon R. Isolation and characterization of human amniotic fluid-derived exosomes, in methods in Enzymology. Elsevier; 2020. pp. 181–94.
Bian D et al. The application of mesenchymal stromal cells (MSCs) and their derivative exosome in skin wound healing: a comprehensive review. 2022. 13(1): p. 1–17.
Wgealla MMAMA et al. Amniotic fluid derived stem cells promote skin regeneration and alleviate scar formation through exosomal miRNA-146a-5p via targeting CXCR4. J Cosmet Dermatol 2022;21(10):5026-5036.
Costa A, Quarto R, J.I.J.o.M S. Small Extracell Vesicles Hum Amniotic Fluid Samples as Promis Theranostics. Int J Mol Sci .2022;23(2):590.
Deng T et al. Human umbilical cord mesenchymal stem cells improve ovarian function in chemotherapy-induced premature ovarian failure mice through inhibiting apoptosis and inflammation via a paracrine mechanism. 2021. 28(6): p. 1718–32.
Chen X-Y, et al. Follicle loss and apoptosis in cyclophosphamide-treated mice: what’s the matter? Int J Mol Sci. 2016;17(6):836.
Huang B et al. Exosomes derived from human adipose mesenchymal stem cells improve ovary function of premature ovarian insufficiency by targeting SMAD. 2018. 9(1): p. 1–12.
Huang B, et al. Human amniotic fluid mesenchymal stem cells improve ovarian function during physiological aging resisting DNA damage. Front Pharmacol .2020;11:272.
hee Lee E et al. Establishment of effective mouse model of premature ovarian failure considering treatment duration of anticancer drugs and natural recovery time. 2018. 24(3): p. 196–203.
Jiao X, et al. Ovarian Reserve markers in premature ovarian insufficiency: within different clinical stages and different etiologies. Front Endocrinol (Lausanne). 2021;12:601752.
Chun S, Koo YH, Na YJ. Two cases of primary ovarian insufficiency accompanied by high serum Anti-Müllerian hormone levels and preservation of ovarian follicles. J Menopausal Med. 2020;26(2):143–6.
Li Z, et al. Human umbilical cord mesenchymal stem cell-derived Exosomes improve ovarian function and proliferation of premature ovarian insufficiency by regulating the Hippo Signaling Pathway. Frontiers in endocrinology; 2021. p. 12.
Zhang S, et al. Concentrated exosomes from menstrual blood-derived stromal cells improves ovarian activity in a rat model of premature ovarian insufficiency. Stem Cell Res Ther. 2021;12(1):178.
Yang W, et al. HucMSC-Derived Exosomes mitigate the age-related retardation of fertility in female mice. Mol Ther. 2020;28(4):1200–13.
Cen S et al. Efficacy and Clinical Significance of the Zuogui Pill on Premature Ovarian Failure via the GDF-9/Smad2 Pathway 2022: p. 1–10.
Kaivo-oja N, et al. Smad signalling in the ovary. Reproductive Biology and Endocrinology. 2006;4(1):1–13.
Qin G, et al. Deletion of Smad4 reduces hepatic inflammation and fibrogenesis during nonalcoholic steatohepatitis progression. J Dig Dis. 2018;19(5):301–13.
Lin J et al. The treatment of complementary and alternative medicine on premature ovarian failure 2021. 2021: 6677767
Yang M et al. Bone marrow mesenchymal stem cell-derived exosomal mir-144-5p improves rat ovarian function after chemotherapy-induced ovarian failure by targeting PTEN. 2020. 100(3): p. 342–52.
Naeem A et al. Amniotic stem cells as a source of regenerative medicine to treat female infertility 2022: p. 1–11.
Thabet E et al. Extracellular vesicles miRNA-21: a potential therapeutic tool in premature ovarian dysfunction. 2020. 26(12): p. 906–19.
Aygün EG. And G. Tümentemur, Effects of stem cells and amniotic fluid on uterus and ovaries in a rat model of abdominal adhesions: a controlled study. J Turk Ger Gynecol Assoc . 2022. 23(3): 154-166
Aygün EG. Effects of stem cells and amniotic fluid on uterus and ovaries on a rat model with abdominal adhesions: a controlled study Aygün and Tümentemur. Stem cells and amniotic fluid on uterus. 2022 23(3):154-166
Laganà AS et al. Molecular Biology of Human Fertility: stepping towards a tailored Approach. Int J Mol Sci, 2022. 23(14).
Mikuš M, et al. CTLA4-Linked autoimmunity in the pathogenesis of endometriosis and related infertility: a systematic review. Int J Mol Sci. 2022;23(18):10902.
Acknowledgements
The authors thank the Stem Cell Research Center of Tabriz University of Medical Sciences for supporting this work.
Funding
This study was supported by a grant number of 62832 and an ethical code of IR.TBZMED.VCR.REC.1398.060 from Tabriz University of Medical Sciences.
Author information
Authors and Affiliations
Contributions
Nahideh Nazdikbin Yamchi, Shahin Ahmadian, Halimeh mobarak, Farhad Amjadi, Rahim Beheshti, and Reza Rahbarghazi: Experiments, analyses, and manuscript preparation. Mahdi Mahdipour: Supervision and Funding.
Corresponding author
Ethics declarations
Supplementary Information
Not applicable.
Institutional Review Board Statement
Not applicable.
Informed consent statement
Not applicable.
Conflict of interest
The author Amin Tamadon was employed by PerciaVista R&D Co. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Nazdikbin Yamchi, N., Ahmadian, S., Mobarak, H. et al. Amniotic fluid-derived exosomes attenuated fibrotic changes in POI rats through modulation of the TGF-β/Smads signaling pathway. J Ovarian Res 16, 118 (2023). https://doi.org/10.1186/s13048-023-01214-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13048-023-01214-1