Abstract
Background
The intra-abdominal cavity, surrounded by adipocytes, is the main metastatic site of epithelial ovarian, fallopian tube, and peritoneal cancer. Epidemiological and molecular studies have demonstrated a link between adipose tissue and ovarian cancer. However, the clinical significance of fatty tissue has not been elucidated. Thus, we investigated the clinical significance of body composition in patients with epithelial ovarian, fallopian tube, and peritoneal cancer.
Methods
Fat and skeletal muscle areas were measured using software based on pretreatment computed tomography scans at the third lumbar vertebra. Fat-to-muscle ratios were calculated using the total (visceral and subcutaneous) fat area or visceral fat area. High fat-to-muscle ratios were defined by values greater than the mean. Sarcopenia was defined as a skeletal muscle index < 38.7 cm2/m2. The clinicopathological parameters and survival of 153 patients were analyzed.
Results
High visceral fat-to-muscle ratios and sarcopenia at the time of diagnosis were observed in 43.8% and 33.3% of the patients, respectively. Multivariate analysis showed that high visceral fat-to-muscle ratio (p = 0.014), advanced Federation of Gynecology and Obstetrics stage (p = 0.008), and chemoresistance (p = 0.027) were independent factors for worse overall survival. Patients with high visceral fat-to-muscle ratios were older, had higher body mass indexes, and were more likely to have diabetes/hypertension, serous cancer subtypes, and implementation of neoadjuvant chemotherapy than those with low visceral fat-to-muscle ratios. The platelet count was significantly higher in the high visceral fat-to-muscle ratio group than in the low visceral fat-to-muscle ratio group (p = 0.011).
Conclusions
Pretreatment visceral fat area could be an independent predictive factor of overall survival in patients with epithelial ovarian, fallopian tube, and peritoneal cancer and may be significantly associated with thrombocytosis.
Similar content being viewed by others
Background
Epithelial ovarian cancer is one of the most lethal gynecologic malignancies, and its incidence in Korea is gradually increasing [1]. Approximately 70% of patients are found to have advanced disease status at the time of diagnosis. Despite extensive surgery and chemotherapy, most patients experience disease recurrence in advanced cases [2, 3]. Attempts to improve survival have been made, and the modification of patient factors, including body composition, is essential for adequate treatment. Therefore, the identification of patient factors that affect survival is required.
Ovarian cancer is known to primarily spread into the abdominal cavity, which is largely surrounded by visceral fatty tissue, and rarely metastasizes outside the abdominal cavity. Compared to subcutaneous fatty tissue, visceral fatty tissue is metabolically active, producing hormones and inflammatory cytokines that are involved in tumor progression [4]. Moreover, intraabdominal adipocytes promote ovarian cancer metastasis by providing an energy source and adipokines [5]. Therefore, visceral fatty tissue may play an essential role in ovarian cancer progression.
Body composition, such as fat, muscle, and bone, is closely related to pathological conditions as well as normal physiological states [6]. The clinical significance of body composition has been studied in malignancy, and sarcopenia, the most common abnormality in body composition, has been identified as a prognostic factor for various malignancies. However, in ovarian cancer, sarcopenia is not associated with prognosis [7,8,9]. Instead, a high volume of fat tissue was correlated with an increased risk of 13 distinct types of cancer, including ovarian cancer, in an epidemiologic study [10], and visceral obesity in patients with advanced ovarian cancer was reported to be associated with a higher occurrence of postoperative complications [11]. However, the clinical significance of visceral fat in ovarian cancer has not been elucidated.
In this study, we aimed to investigate body composition, including visceral fat, subcutaneous fat, and skeletal muscle mass, at the time of diagnosis, and its association with prognosis in patients with epithelial ovarian, fallopian tube, and peritoneal cancer (EOFPC).
Results
Patient characteristics
The parameters of body composition at diagnosis and the clinicopathological characteristics are summarized in Table 1. The mean skeletal muscle index (SMI) and visceral (vFMR) and total fat-to-muscle ratio (tFMR) values were 41.53, 0.83, and 2.22, respectively. The majority of patients were under 60 years old (60.1%), had a body mass index (BMI) lower than 25 kg/m2 (66.7%), had serous-type carcinoma (65.4%), and underwent primary debulking surgery as a first-line therapy (88.2%). The median follow-up time after finishing the first-line treatment was 42.7 months (range, 0–150 months). Disease recurrence occurred in 65 patients (42.5%), and 32 patients died. Sarcopenia at diagnosis was observed in 51 patients (33.3%).
Higher visceral fat is an independent factor that predicts worse overall survival
The present study analyzed body composition and its relationship with the prognosis of patients with primary EOFPC. Univariate analysis showed that a higher BMI, serous subtype, implementation of neoadjuvant chemotherapy (NAC), higher Federation of Gynecology and Obstetrics (FIGO) stage, suboptimality, chemoresistance, and high vFMR were significantly associated with worse disease-free survival (DFS) (Table 2 and Fig. 1A). Multivariate analysis showed that higher BMI, serous subtype, implementation of NAC, higher FIGO stage, and chemoresistance were independent factors for DFS. In addition, older age, higher BMI, serous subtype, implementation of NAC, suboptimality, higher FIGO stage, chemoresistance, and high vFMR were significantly associated with worse overall survival (OS) in the univariate analysis (Table 2 and Fig. 1B). Multivariate analysis revealed that higher FIGO stage, chemoresistance, and high vFMR were independent factors for worse OS. In contrast to vFMR, tFMR and SMI were not significantly associated with survival.
Comparison between the low and high vFMR groups
To assess the characteristics of patients with high vFMR, we subdivided the patients into low and high vFMR groups according to the mean value (0.83) and compared the body composition, laboratory data, and clinicopathological parameters between the two groups (Table 3). In the high vFMR group, age, BMI, and subcutaneous fat levels were significantly higher than in the low vFMR group, whereas skeletal muscle area (SMA) and SMI did not differ between the two groups. Skeletal muscle attenuation was significantly lower in the high vFMR group than in the low vFMR group owing to the high lipid content in the muscles.
The comparison of the laboratory results at diagnosis showed that the mean platelet count was significantly higher in the high vFMR group than in the low vFMR group, while there were no differences in white blood cell (WBC) count, neutrophil or lymphocyte counts, hemoglobin, albumin, or prognostic nutrition index (PNI). We next compared the frequency of thrombocytosis, defined as > 400x103/μL, between the two groups. The results showed that thrombocytosis was significantly more frequent in patients in the high vFMR group (17 of 67, 25.4%), than in patients in the low vFMR group (9 of 86, 10.5%) (p < 0.02).
In the comparison of clinicopathological parameters, patients with high vFMR were older and more frequently had diabetes/hypertension (DM/HTN) or were menopausal than those with low vFMR. In addition, the presence of serous subtype and implementation of NAC were significantly associated with high vFMR.
Discussion
This study demonstrated that visceral fat at EOFPC diagnosis could be an independent prognostic factor by showing a significant association between vFMR and OS. Subcutaneous fat, total fat, and sarcopenia were not significant factors in the current analysis. Patients with a vFMR higher than the average value of this cohort were older, had a higher BMI, and more frequently had DM/HTN, serous subtype, and implementation of NAC compared to those with lower vFMR. These associated factors were unfavorable to the prognosis of patients, contributing to the significant association between high vFMR and low OS.
Three similar studies on the correlation between fat area and prognosis in epithelial ovarian cancer have been conducted. One report showed that higher tFMR was significantly associated with worse OS in patients with sarcopenia, but not in all patients; however, visceral fat was not analyzed, and only the serous ovarian subtype was included [7]. Two other reports analyzed the clinical significance of total fat area and showed no correlation with survival [12, 13]. In contrast to the visceral fat area at the level of the third lumbar vertebra (L3) on CT scans that was used in our study, one of the three reports measured the whole abdominal visceral fat area; however, it showed no clinical significance [13]. Consistent with previous reports, we did not find any clinical significance of the total fat area. Instead, we demonstrated that the visceral fat area at the L3 level on CT was a more reliable factor for predicting the survival of patients with EOFPC. Because previous reports did not analyze the visceral fat area at the L3 level on CT, this is the first report to demonstrate a significant association between the visceral fat area at the L3 level on CT and the prognosis of patients with EOFPC.
The clinical significance of visceral fat area depends on the type of malignancy. While higher visceral fat was significantly associated with better OS in gastric cancer [14], a higher visceral to subcutaneous fat ratio was significantly associated with worse OS in uterine cervical, pancreatic, and metastatic colorectal cancer [15,16,17]. Here, we provide additional evidence for ovarian cancer, supporting the significant association between a higher visceral fat area and worse OS.
There is evidence of a link between fat tissue and ovarian cancer. An epidemiological study showed that a high volume of fat tissue is correlated with an increased risk of 13 distinct types of cancer, including ovarian cancer [10]. The direct dissemination sites of epithelial ovarian cancer are the omentum, mesentery, and peritoneum, which are rich in adipocytes [18]. Molecular studies have demonstrated that epithelial ovarian cancer cells lead to phenotypic and metabolic alterations in adipocytes at the intraabdominal metastasis site and fatty acid production from adipocytes, resulting in epithelial ovarian cancer progression [5]. In addition, adipocytes are responsible for epithelial ovarian cancer treatment resistance through various mechanisms [19]. For example, adipocytes modulate survival genes by secreting leptin, interleukin (IL)-6, and IL-8, which activate the AKT and ERK survival pathways, and contribute to Taxol resistance [20, 21]. In addition, adipocyte-induced autophagy and adipocyte-derived mesenchymal stem cells also contribute to drug resistance [22, 23]. Taken together, visceral fat tissue may play an important role in epithelial ovarian cancer progression, and this is supported by our finding that high vFMR is an unfavorable prognostic factor in patients with EOFPC.
In this study, sarcopenia was not clinically significant. Sarcopenia is known to influence the survival of patients with various malignancies, such as gastric cancer [24], and breast cancer [25]. However, the results for ovarian cancer remain controversial. Most reports, including the current study, showed no association between sarcopenia and survival in patients with ovarian cancer [12, 26,27,28,29,30,31,32], whereas several studies reported a significant association [12, 32, 33]. Although a recent meta-analysis showed a significant correlation between sarcopenia and ovarian cancer survival, the authors carefully added that the source data were mainly retrospective in nature and of low quality [9]. Overall, further large-scale prospective studies with multiple centers are necessary to elucidate the clinical role of sarcopenia in patients with ovarian cancer.
Interestingly, the present study showed that platelet count was significantly higher and thrombocytosis was significantly more frequent in the high vFMR group than in the low vFMR group. The direct relationship between thrombocytosis and visceral fat area has never been assessed in population studies. Thrombocytosis is a paraneoplastic syndrome of ovarian cancer, and the mechanism is mainly explained by the higher plasma levels of thrombopoietin and IL-6 in patients with thrombocytosis compared to those without thrombocytosis [34]. In addition, platelet counts have been found to be higher in obese individuals than in non-obese controls [35]. This correlation is further supported by the observed reductions in platelet counts in the setting of weight loss following bariatric surgery [36]. Moreover, the secretion of thrombopoietin and IL-6 is enhanced in visceral adipose tissue, such as the omentum [21, 37]. Taken together, visceral adipose tissue may be an additional source of thrombopoietin and IL-6, which may explain thrombocytosis in patients with high vFMR; however, further studies are needed to prove this.
This study is the first to demonstrate the clinical significance of visceral fat in patients with EOFPC, whereas the previous studies that assessed the role of body composition did not focus on this factor. With this evidence, the role of visceral fat in ovarian carcinogenesis should be further addressed to achieve a better understanding of the cancer microenvironment, to evaluate the necessity of debulking visceral fat, and to integrate these findings into the prevention spectrum. Despite the present study’s important findings, it was limited by its retrospective nature and its small sample size from a single institution. Thus, further well-designed, prospective studies are required.
Conclusions
This study showed a novel finding that high vFMR, but not sarcopenia, was an independent predictor of worse OS and associated with older age, higher BMI, the implementation of NAC, and the presence of DM/HTN, serous subtype, and thrombocytosis. Therefore, high vFMR could be a reliable patient factor affecting survival, and its modification should be considered in patients with EOFPC.
Methods
Patients
A total of 216 patients diagnosed with primary EOFPC at Chung-Ang University Hospital between 2002 and 2017 were retrospectively identified. Of these, 63 were excluded from the study. Thirty-one patients were transferred immediately after diagnosis (N = 25) and after surgery (N = 6); five patients were foreigners (four Caucasian and one Black); seven patients had initial MRI but not CT scan data; 14 patients had received chemotherapy without operation at the hemato-oncology department; and six patients had coexisting cancers (one gastric, one pancreatic, two colon, one thyroid, and one gall bladder cancer). Therefore, 153 patients were included in this analysis (Fig. 2). Of these, 142 had ovarian cancers (91 serous, 20 mucinous, 14 clear cell, 12 endometrioid, 3 transitional, and 4 malignant mixed mesodermal tumors (MMMTs), 8 had primary peritoneal cancer, and 1 had fallopian tubal cancer. Nine cases of peritoneal and fallopian tubal cancers were serous. Patients underwent primary debulking surgery followed by adjuvant chemotherapy, which consisted of a minimum of six 21-day cycles of intravenous carboplatin (area under the curve 5) plus paclitaxel (175 mg/m2 body surface area). NAC was administered as in the adjuvant setting but only for two or three cycles, and interval debulking surgery followed after 3 or 4 weeks of rest. This study was reviewed and approved by the Institutional Review Board of Chung-Ang University Hospital (irb@caumc.or.kr; approval number: 2104-002-19360; date: April 29, 2021).
Body composition analysis using CT images
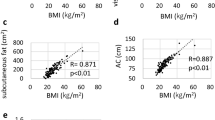
For body composition analysis, fat area and SMA were measured using commercial imaging software (TeraRecon Aquarius; TeraRecon, USA). A single slice at the L3 level of the CT scan at diagnosis, with both transverse processes visible, was selected, and the areas were calculated automatically. According to predefined Hounsfield units (HU) [38], the software calculated the SMA (-29 to +150 HU), visceral fat area (-150 to -50 HU), and subcutaneous fat area (-190 to -30 HU) (Fig. 3A and B). In this standard method, the fat of bowel feces was inevitably included in visceral fat. Total fat area was calculated as the sum of the visceral and subcutaneous fat areas. Skeletal muscle attenuation was assessed by calculating the average HU value of the total muscle area. The fat-to-muscle ratio (FMR) was defined as the ratio of fat area to lean SMA. tFMR and vFMR were calculated using the total and visceral fat areas, respectively. In this study, the FMR value was used in clinical analysis because our preliminarily data showed that massive ascites influenced the fat area and muscle area but not the FMR (Fig. S1). SMI was calculated by dividing the SMA (cm2) by the square of the patient’s height (m2). Sarcopenia was defined as an SMI < 38.7 cm2/m2 according to the proposed cut-off value for patients with ovarian cancer [31].
Data collection
Clinicopathological and laboratory data were collected via chart review. These included age, body weight, height, history of DM/HTN, menopausal status, histological type, serum cancer antigen (CA)-125 level, implementation of NAC, surgical optimality, FIGO stage, WBC count, neutrophil and lymphocyte counts, hemoglobin, platelet count, albumin, and PNI: 10x serum albumin (g/dL) + 0.005 × peripheral blood lymphocyte count (/μL). Comorbidities included in the NCI Comorbidity Index were screened [39]. One patient had stable angina, and three patients had asthma. These four patients had optimal conditions for surgery and underwent primary debulking surgery without complications. Due to the small number, we omitted comorbidity in the parameters. Surgical optimality was defined as a residual tumor < 1 cm after surgery. Chemoresistance was defined as recurrence within 6 months of first-line treatment.
Statistical analysis
DFS was defined as the time from the last therapy to the diagnosis of the first recurrence, and OS was defined as the time in months from the last therapy to disease-related death. Survival was estimated using Kaplan–Meier estimates and compared with a log-rank test, where indicated. Multivariate analysis was performed using Cox regression analysis. Mean counts were analyzed using the Student’s t-test because the distributions of both populations were equal. Dichotomous groupings were analyzed using chi-squared and Fisher’s exact tests, as appropriate. All p-values reported were two-sided, and statistical significance was defined as p < 0.05. Statistical analysis was performed using the Statistical Package for the Social Sciences (SPSS, version 15.0, Chicago, IL, USA).
Availability of data and materials
The datasets generated during the current study are available from the corresponding author on reasonable request.
References
Ha HI, Chang HK, Park SJ, Lim J, Won YJ, Lim MC. The incidence and survival of cervical, ovarian, and endometrial cancer in Korea, 1999-2017: Korea Central Cancer Registry. Obstet Gynecol Sci. 2021. https://doi.org/10.5468/ogs.21116.
Cho KR, Shih IM. Ovarian cancer. Annu Rev Pathol. 2009. https://doi.org/10.1146/annurev.pathol.4.110807.092246.
Dahm-Kähler P, Palmqvist C, Staf C, Holmberg E, Johannesson L. Centralized primary care of advanced ovarian cancer improves complete cytoreduction and survival-A population-based cohort study. Gynecol Oncol. 2016. https://doi.org/10.1016/j.ygyno.2016.05.025.
Quail DF, Dannenberg AJ. The obese adipose tissue microenvironment in cancer development and progression. Nat Rev Endocrinol. 2019. https://doi.org/10.1038/s41574-018-0126-x.
Nieman KM, Kenny HA, Penicka CV, Ladanyi A, Buell-Gutbrod R, Zillhardt MR, et al. Adipocytes promote ovarian cancer metastasis and provide energy for rapid tumor growth. Nat Med. 2011. https://doi.org/10.1038/nm.2492.
Müller M, Lagerpusch M, Enderle J, Schautz B, Heller M, Bosy-Westphal A. Beyond the body mass index: tracking body composition in the pathogenesis of obesity and the metabolic syndrome. Obes Rev. 2012. https://doi.org/10.1111/j.1467-789X.2012.01033.x.
Kim SI, Kim TM, Lee M, Kim HS, Chung HH, Cho JY, et al. Impact of CT-determined sarcopenia and body composition on survival outcome in patients with advanced-stage high-grade serous ovarian carcinoma. Cancers. 2020. https://doi.org/10.3390/cancers12030559.
Staley SA, Tucker K, Newton M, Ertel M, Oldan J, Doherty I, et al. Sarcopenia as a predictor of survival and chemotoxicity in patients with epithelial ovarian cancer receiving platinum and taxane-based chemotherapy. Gynecol Oncol. 2020. https://doi.org/10.1016/j.ygyno.2020.01.003.
Ubachs J, Ziemons J, Minis-Rutten IJ, Kruitwagen RF, Kleijnen J, Lambrechts S, et al. Sarcopenia and ovarian cancer survival: a systematic review and meta-analysis. J Cachexia Sarcopenia Muscle. 2019. https://doi.org/10.1002/jcsm.12468.
Lauby-Secretan B, Scoccianti C, Loomis D, Grosse Y, Bianchini F, Straif K. Body fatness and cancer—viewpoint of the IARC Working Group. N Engl J Med. 2016. https://doi.org/10.1056/NEJMsr1606602.
Heus C, Smorenburg A, Stoker J, Rutten M, Amant F, van Lonkhuijzen L. Visceral obesity and muscle mass determined by CT scan and surgical outcome in patients with advanced ovarian cancer. A retrospective cohort study. Gynecol Oncol. 2021. https://doi.org/10.1016/j.ygyno.2020.10.015.
Rutten IJ, van Dijk DP, Kruitwagen RF, Beets-Tan RG, Olde Damink SW, van Gorp T. Loss of skeletal muscle during neoadjuvant chemotherapy is related to decreased survival in ovarian cancer patients. J Cachexia Sarcopenia Muscle. 2016. https://doi.org/10.1002/jcsm.12107.
Zhang Y, Coletta AM, Allen PK, Parikh AM, Cox-Mattin M, Meyer LA, et al. Perirenal adiposity is associated with lower progression-free survival from ovarian cancer. Int J Gynecol Cancer. 2018. https://doi.org/10.1097/IGC.0000000000001165.
Harada K, Baba Y, Ishimoto T, Kosumi K, Tokunaga R, Izumi D, et al. Low visceral fat content is associated with poor prognosis in a database of 507 upper gastrointestinal cancers. Ann Surg Oncol. 2015. https://doi.org/10.1245/s10434-015-4432-4.
Basile D, Bartoletti M, Polano M, Bortot L, Gerratana L, Di Nardo P, et al. Prognostic role of visceral fat for overall survival in metastatic colorectal cancer: A pilot study. Clin Nutr. 2021. https://doi.org/10.1016/j.clnu.2020.05.019.
Bian X, Dai H, Feng J, Ji H, Fang Y, Jiang N, et al. Prognostic values of abdominal body compositions on survival in advanced pancreatic cancer. Medicine. 2018. https://doi.org/10.1097/MD.0000000000010988.
Chen HL, Shih CT, Lee YC, Tseng HC, Chou YH. Patients with cervical cancer without visceral obesity had better treatment outcomes. Ther Radiol Oncol. 2020. https://doi.org/10.21037/tro-20-22.
Lengyel E. Ovarian cancer development and metastasis. Am J Pathol. 2010. https://doi.org/10.2353/ajpath.2010.100105.
Dai L, Song K, Di W. Adipocytes: active facilitators in epithelial ovarian cancer progression? J Ovarian Res. 2020. https://doi.org/10.1186/s13048-020-00718-4.
Chen C, Chang YC, Lan MS, Breslin M. Leptin stimulates ovarian cancer cell growth and inhibits apoptosis by increasing cyclin D1 and Mcl-1 expression via the activation of the MEK/ERK1/2 and PI3K/Akt signaling pathways. Int J Oncol. 2013. https://doi.org/10.3892/ijo.2013.1789.
Wang Y, Li L, Guo X, Jin X, Sun W, Zhang X, et al. Interleukin-6 signaling regulates anchorage-independent growth, proliferation, adhesion and invasion in human ovarian cancer cells. Cytokine. 2012. https://doi.org/10.1016/j.cyto.2012.04.020.
Nowicka A, Marini FC, Solley TN, Elizondo PB, Zhang Y, Sharp HJ, et al. Human omental-derived adipose stem cells increase ovarian cancer proliferation, migration, and chemoresistance. PLoS One. 2013. https://doi.org/10.1371/journal.pone.0081859.
Wang J, Wu GS. Role of autophagy in cisplatin resistance in ovarian cancer cells. J Biol Chem. 2014. https://doi.org/10.1074/jbc.M114.558288.
Park SE, Choi JH, Park JY, Kim BJ, Kim JG, Kim JW, et al. Loss of skeletal muscle mass during palliative chemotherapy is a poor prognostic factor in patients with advanced gastric cancer. Sci Rep. 2020. https://doi.org/10.1038/s41598-020-74765-8.
Villaseñor A, Ballard-Barbash R, Baumgartner K, Baumgartner R, Bernstein L, McTiernan A, et al. Prevalence and prognostic effect of sarcopenia in breast cancer survivors: the HEAL Study. J Cancer Surviv. 2012. https://doi.org/10.1007/s11764-012-0234-x.
Ataseven B, Luengo TG, du Bois A, Waltering KU, Traut A, Heitz F, et al. Skeletal muscle attenuation (sarcopenia) predicts reduced overall survival in patients with advanced epithelial ovarian cancer undergoing primary debulking surgery. Ann Surg Oncol. 2018. https://doi.org/10.1245/s10434-018-6683-3.
Aust S, Knogler T, Pils D, Obermayr E, Reinthaller A, Zahn L, et al. Skeletal muscle depletion and markers for cancer cachexia are strong prognostic factors in epithelial ovarian cancer. PLoS One. 2015. https://doi.org/10.1371/journal.pone.0140403.
Conrad LB, Awdeh H, Acosta-Torres S, Conrad SA, Bailey AA, Miller DS, et al. Pre-operative core muscle index in combination with hypoalbuminemia is associated with poor prognosis in advanced ovarian cancer. J Surg Oncol. 2018. https://doi.org/10.1002/jso.24990.
Kumar A, Moynagh MR, Multinu F, Cliby WA, McGree ME, Weaver AL, et al. Muscle composition measured by CT scan is a measurable predictor of overall survival in advanced ovarian cancer. Gynecol Oncol. 2016. https://doi.org/10.1016/j.ygyno.2016.05.027.
Nakayama N, Nakayama K, Nakamura K, Razia S, Kyo S. Sarcopenic factors may have no impact on outcomes in ovarian cancer patients. Diagnostics. 2019. https://doi.org/10.3390/diagnostics9040206.
Rutten I, Ubachs J, Kruitwagen R, van Dijk D, Beets-Tan R, Massuger L, et al. The influence of sarcopenia on survival and surgical complications in ovarian cancer patients undergoing primary debulking surgery. Eur J Surg Oncol. 2017. https://doi.org/10.1016/j.ejso.2016.12.016.
Torres ML, Hartmann LC, Cliby WA, Kalli KR, Young PM, Weaver AL, et al. Nutritional status, CT body composition measures and survival in ovarian cancer. Gynecol Oncol. 2013. https://doi.org/10.1016/j.ygyno.2013.03.003.
Bronger H, Hederich P, Hapfelmeier A, Metz S, Noël PB, Kiechle M, et al. Sarcopenia in advanced serous ovarian cancer. Int J Gynecol Cancer. 2017. https://doi.org/10.1097/IGC.0000000000000867.
Stone RL, Nick AM, McNeish IA, Balkwill F, Han HD, Bottsford-Miller J, et al. Paraneoplastic thrombocytosis in ovarian cancer. N Engl J Med. 2012. https://doi.org/10.1056/NEJMoa1110352.
Samocha-Bonet D, Justo D, Rogowski O, Saar N, Abu-Abeid S, Shenkerman G, et al. Platelet counts and platelet activation markers in obese subjects. Mediators of inflamm. 2008. https://doi.org/10.1155/2008/834153.
Raoux L, Moszkowicz D, Vychnevskaia K, Poghosyan T, Beauchet A, Clauser S, et al. Effect of bariatric surgery-induced weight loss on platelet count and mean platelet volume: a 12-month follow-up study. Obes Surg. 2017. https://doi.org/10.1007/s11695-016-2292-z.
Maury E, Ehala-Aleksejev K, Guiot Y, Detry R, Vandenhooft A, Brichard SM. Adipokines oversecreted by omental adipose tissue in human obesity. Am J Physiol Endocrinol Metab. 2007. https://doi.org/10.1152/ajpendo.00127.2007.
Aubrey J, Esfandiari N, Baracos V, Buteau F, Frenette J, Putman C, et al. Measurement of skeletal muscle radiation attenuation and basis of its biological variation. Acta Physiol. 2014. https://doi.org/10.1111/apha.12224.
Klabunde C, Legler J, Warren J, Baldwin LM, Schrag D. A refined comorbidity measurement algorithm for claims-based studies of breast, prostate, colorectal, and lung cancer patients. Ann Epidemiol. 2007. https://doi.org/10.1016/j.annepidem.2007.03.011.
Funding
This study was supported by the Basic Science Research Program of the National Research Foundation of Korea (NRF) under grant number 2020R1A2C1003536.
Author information
Authors and Affiliations
Contributions
Eun-Ju Lee contributed to the study conception and design. Material preparation, data collection, and data analysis were performed by Sooji Ham, Jin Hwa Choi, Soo Gui Shin, and Eun-Ju Lee. The first draft of the manuscript was written by Sooji Ham and Jun Hwa Choi, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was reviewed and approved by the Institutional Review Board of Chung-Ang University Hospital (irb@caumc.or.kr; approval number: 2104-002-19360; date: April 29, 2021). No written informed consent of the participants was needed because this was a retrospective chart review of existing medical records.
Competing interests
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Figure S1.
Comparison of fat and muscle areas in patients with massive ascites before and after two cycles of neoadjuvant chemotherapy. Visceral fat and smooth muscle areas in CT images at L3 level marked in green and red, respectively, before treatment (A and B) and after treatment (C and D). Each value is shown (E), and no significant difference in vFMR was noted.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Ham, S., Choi, J., Shin, S. et al. High visceral fat-to-muscle ratio is an independent factor that predicts worse overall survival in patients with primary epithelial ovarian, fallopian tube, and peritoneal cancer. J Ovarian Res 16, 19 (2023). https://doi.org/10.1186/s13048-023-01098-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13048-023-01098-1