Abstract
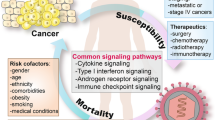
The outbreak and continued spread of the novel coronavirus disease 2019 (COVID-19) is a preeminent global health threat that has resulted in the infection of over 11.5 million people worldwide. In addition, the pandemic has claimed the lives of over 530,000 people worldwide. Age and the presence of underlying comorbid conditions have been found to be key determinants of patient mortality. One such comorbidity is the presence of an oncological malignancy, with cancer patients exhibiting an approximate two-fold increase in mortality rate. Due to a lack of data, no consensus has been reached about the best practices for the diagnosis and treatment of cancer patients. Interestingly, two independent research groups have discovered that Withaferin A (WFA), a steroidal lactone with anti-inflammatory and anti-tumorigenic properties, may bind to the viral spike (S-) protein of SARS-CoV-2. Further, preliminary data from our research group has demonstrated that WFA does not alter expression of ACE2 in the lungs of tumor-bearing female mice. Downregulation of ACE2 has recently been demonstrated to increase the severity of COVID-19. Therefore, WFA demonstrates real potential as a therapeutic agent to treat or prevent the spread of COVID-19 due to the reported interference in viral S-protein to host receptor binding and its lack of effect on ACE2 expression in the lungs.
Similar content being viewed by others
Introduction
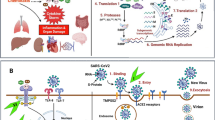
The novel coronavirus disease 2019 (COVID-19) has rapidly spread around the world since it was first reported in December 2019 within Wuhan, China as a pneumonia of unknown etiology [1]. Severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), termed by the World Health Organization (WHO), represents the third large-scale epidemic related to coronaviruses [1]. Although the disease was first reported within China, a retrospective study has subsequently found evidence that SARS-CoV-2 was spreading within France 4 days before it was first reported in Wuhan, China and 1 month before the first official case in the country [2]. Since its initial discovery, SARS-CoV-2 has spread worldwide, infecting over 11.5 million people and led to the death of more than 530,000 people as of July 6th, 2020 [3]. The severity of the disease widely ranges from an asymptomatic disease-state to patients exhibiting acute respiratory distress syndrome (ARDS), necessitating critical medical intervention to attempt to prevent patient death [4]. It was subsequently discovered that Angiotensin-converting enzyme 2 (ACE2) is a functional receptor for the SARS-CoV-2 spike (S-) protein, allowing the virus to enter cells [5]. ACE2 is a potent negative regulator of the renin angiotensin system (RAS), which is critical for maintaining the homeostasis of RAS.
The ACE2 gene is composed of 805 amino acids and is a type I integral membrane glycoprotein. ACE2 degrades angiotensin (Ang)-II, a potent vasoconstrictor (that is also pro-inflammatory and promotes fibrosis), and converts it into Ang (1–7) [6]. Ang (1–7) is a vasodilator, that also inhibits proliferation and apoptosis [6]. Beside the systemic effect on blood pressure regulation, ACE2 has local regulatory effects in the pathological changes of several organs, including the heart, kidney, and lungs [7]. ACE2 is highly expressed in lung alveolar cells, providing the main entry site for the virus into human host [8]. In addition to expression of ACE2 in lung alveolar cells, it is also expressed in various tissues, including: the vascular system (endothelial cells, migratory angiogenic cells and vascular smooth muscle cells), heart (cardiofibroblasts, cardiomyocytes, endothelial cells, pericytes, and epicardial adipose cells) and kidneys (glomerular endothelial cells, podocytes and proximal tubule epithelial cells), liver (cholangiocytes and hepatocytes), retina (pigmented epithelial cells, rod and cone photoreceptor cells, and Müller glial cells), enterocytes of the intestines, circumventricular organs of the central nervous system, and the upper airway (goblet and ciliated epithelial cells) [9].
There are two subunits of the SARS-CoV-2 S-protein: the S1 subunit has a receptor binding domain that engages with the host cell receptor ACE2, and the S2 subunit is involved in regulating fusion between the viral and the host membrane [10]. It has been reported that SARS-CoV-2 has a ten times higher affinity to ACE2 compared to SARS-CoV, which is consistent with the higher efficiency of infection of SARS-CoV-2 [11]. While no cure has currently been found, several clinical trials are being performed to determine what the most efficacious treatment regimen is for COVID-19, with an extensive list of potential therapies detailed in a review by Gosain et al. [12]. Currently, patient management involves supportive treatment and measures to prevent further spread of the virus [13]. Despite differences in patient population characteristics between Europe and China, two of the main determinants of patient mortality risk that were found in both groups are age and the presence of underlying comorbid conditions [14, 15]. One such underlying condition associated with an increase in COVID-19 patient mortality is the presence of cancer [16].
Cancer patients and the COVID-19 epidemic
Due to their potentially immune-compromised status, the proper treatment of cancer patients is a real and serious problem being faced by oncologists, regardless of if the patient is experiencing a SARS-CoV-2 infection [16]. Data from four SARS-CoV-2 hot spots (the United States, Italy, Spain and China) has shown that cancer patients infected with the novel coronavirus have a significantly increased risk of admission to an intensive care unit (ICU) and/or requiring mechanical ventilation, as well as an increase in patient mortality [15, 17,18,19]. In a retrospective study, the fatality rate for cancer patients in China infected with COVID-19 was found to be approximately 28% [20], compared to the overall symptomatic fatality rate of 1.4% or the crude mortality rate of 4.5% in China [21]. Perhaps unsurprisingly, the fatality rate of lung cancer patients with SARS-CoV-2 has been fairly grim, with a New York cohort study exhibiting a 55% fatality rate [19]. Cancer patients and their oncologists are currently facing the dilemma as to whether or not the patient should begin or continue therapy for their primary disease state due to the associated risks of contracting SARS-CoV-2 and the reduction in resources available to healthcare workers [22]. Information on the specific etiology of the cancer is scarce within several SARS-CoV-2 studies. However, lung, breast, gastrointestinal, and hematological cancers (ex. lymphoma) have been reported within COVID-19 cohort studies in the United States [19], Italy [23], and China [18]. Further, cervical cancer patients and patients with other unspecified gynecological malignancies have been reported in these studies [18, 19, 23].
While select literature sources provide glimpses of the oncological paradigms exhibited, the already small patient population assessed dwindles even further when stratified by oncological typing. This is a substantial limitation for assessing mortality risk and providing guidelines for management of COVID-19-positive cancer patients. Along similar lines, very little is known about COVID-19 infection in ovarian cancer patients. At the time of writing, there are 22 PubMed articles about the subject, of which 20 of them discuss potential changes to or challenges faced by cancer clinics to better serve ovarian cancer patients. The remaining two articles discuss a total of three ovarian cancer patients and how their treatment was modified due to the current pandemic [24, 25]. Only two of the three ovarian cancer patients were found to be positive for the novel coronavirus, requiring adjuvant treatment with platelets due to the development of chemotherapy-related thrombocytopenia [24]. The remaining ovarian cancer patient discussed tested negative for SARS-CoV-2 infection, but was presumed to be positive based upon patient symptoms and clinical findings (ex. abnormal CT scan findings consistent with pneumonia in COVID-19 patients) [25]. This patient’s cancer regiment was delayed until the resolution of the presenting atypical pneumonia, but otherwise did not receive any adjuvant therapy [25]. Currently, there are no globally accepted guidelines to address cancer patient management in the settings of a pandemic due to a lacking of data available [26]. Recently, an international collaboration has proposed a series of practical approaches for the diagnosis and treatment of cancer patients [26]. However, until more information or an effective therapeutic regimen against SARS-CoV-2 become available, cancer patients will continue to remain at a very high-risk of mortality due to the COVID-19 epidemic [26].
Withaferin a as a prospective treatment
Withaferin A (WFA) is a steroidal lactone isolated from the plant Withania somnifera, also known as Ashwagandha [27]. It is known for its anti-inflammatory properties, as well as its anti-tumorigenic properties [28,29,30]. Recent work has demonstrated that COVID-19 infections have a large immune component and can result in the development of cytokine storm, a potentially life-threatening immune reaction in which the body release too many cytokines into the blood at a rapid rate [31]. Work from our lab has demonstrated that WFA is capable of reducing the secretion of various proinflammatory cytokines (ex. TNFα, IL-6, IL-8, and IL-18) in a metastatic model of ovarian cancer [30]. It is within the realm of possibility that WFA treatment can abrogate the intensity of cytokine storm due to the reported anti-inflammatory properties. Interestingly, at least three independent research groups have suggested that phytochemicals found in the plant Withania somnifera could be developed as a therapeutic agent against COVID-19 infection using molecular docking approaches [32,33,34]. Two of the groups reported that various Withanolides, such as WFA, should be able to bind to the viral S-protein receptor binding domain, thereby blocking or reducing interactions with host ACE2 receptor [32, 33]. The third group reported that WFA and a separate withanolide, Withanone, are predicted to interact with the main protease of SARS-CoV-2, although WFA is predicted to have less of a binding affinity than an established N3 protease inhibitor used for baseline docking scores [34].
In an unrelated study, our group has been investigating WFA as a potential therapeutic to treat cancer, including the targeting of cancer stem cells and cancer-induced cachexia (a muscle wasting disorder). As Ang-II signaling is a known mediator of skeletal muscle atrophy [35], we investigated the effect of WFA on Ang-II signaling as it pertains to cachexia. Data (under publication) from our lab has indicated that WFA treatment can reduce circulating levels of Angiotensin II in an experimental model of cancer-induced cachexia. In this study, we xenografted the ovarian cancer cell line A2780 (8.0 × 105 low passage cells resuspended in 100 μl sterile PBS) intraperitoneally into 5 to 6-week old female NOD.Cg-PrkdcscidIl2rgtm1Wjl/SzJ (NSG; Jackson Lab Strain # 005557) mice. Tumor-free controls received an equivalent i.p. injection of sterile saline. After an 8-day of refractory period to allow engraftment of the ovarian cancer cells, tumor-free and tumor-bearing animals received i.p. injections of WFA (2 mg/kg) or vehicle (10% dimethyl sulfoxide, 90% glycerol trioctanoate) once every 3 days over the period of 4 weeks (post-xenografting).
Using qPCR and gene specific primers, we found that WFA treatment reduced the relative mRNA expression of AT1R (Angiotensin II Receptor Type 1) compared to the vehicle-treated group in tumor samples as determined by a two-way analysis of variance (ANOVA) followed by Tukey’s multiple comparison test post hoc analysis. Based upon our findings and the independently reported molecular docking studies, we investigated whether or not WFA treatment would alter ACE2 expression in the lungs under tumor-free and tumor-bearing conditions. Interestingly, we found no significant differences (NSD; p-values > 0.80 for all comparisons) in relative mRNA expression of ACE2 in response to WFA treatment as determined by a two-way ANOVA (Fig. 1). As we did not observe any significant differences in ACE2 mRNA expression in the lungs via qPCR, one of the primary regions where ACE2 is expressed, we did not investigate the expression of ACE2 in other organs. However, it was recently reported that, as a byproduct of SARS-CoV-2 infection, ACE2 expression is decreased as part of the disease process, which in turn facilitates the development of multiorgan damage [36]. Due to this effect, others have suggested that blocking the binding of SARS-CoV-2 to the ACE2 receptor may be a more beneficial strategy to combat the virus than augmenting ACE2 expression, due to its antagonistic effect on AT1R signaling [9]. In line with this rationale, it is within the realm of possibility that WFA can block or impede COVID-19 through interactions with the viral S-protein based upon the molecular docking studies [32, 33], without affecting ACE2 expression (as reported in our data) leading to a worsening of the pathological state.
Withaferin A’s effect on ACE2 mRNA expression. (A) Relative mRNA levels of ACE2 in lung samples of tumor-free and A2780 ovarian tumor-bearing female NSG mice treated with vehicle or WFA (2 mg/kg). N = 4–5 mice per group. Black circles indicate individual data points. NSD = No significant differences
Conclusion
The COVID-19 outbreak has become a significant clinical threat worldwide to both the general population and healthcare workers. Additionally, cancer patients and the elderly remain a very high-risk subpopulation that are more susceptible to disease-related fatality. While knowledge about this virus remains limited, over 100 clinical trials are currently being performed to help find a means to combat this epidemic. Due to their potentially immune-compromised position and associated rates of mortality, it would seem that special considerations should be taken in developing a potential therapeutic regimen for cancer patients and patients with other high-risk comorbidities. Withaferin A alone or in combination with drugs, such as: hydroxychloroquine, dexamethasone or other treatments (under clinical trials), could be developed into an attractive therapeutic agent for both the general population and cancer patients due to its anti-tumorigenic properties and the preliminary studies showing that it is capable of binding to the S-protein of SARS-CoV-2, thereby potentially inhibiting infection and/or spread of the disease.
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Abbreviations
- ACE2 :
-
Angiotensin-converting enzyme-2
- Ang :
-
Angiotensin
- ANOVA :
-
Analysis of variance
- AT1R :
-
Angiotensin II Receptor Type 1
- ARDS :
-
Acute respiratory distress syndrome
- COVID-19 :
-
Coronavirus disease 2019
- ICU :
-
Intensive care unit
- SARS-CoV-2 :
-
Severe acute respiratory syndrome coronavirus-2
- NSD :
-
No significant differences
- RAS :
-
Renin angiotensin system
- S-protein :
-
Spike protein
- WFA :
-
Withaferin A
- WHO :
-
World Health Organization
References
Bogoch II, Watts A, Thomas-Bachli A, Huber C, Kraemer MUG, Khan K. Pneumonia of unknown aetiology in Wuhan, China: potential for international spread via commercial air travel. J Travel Med. 2020;27:2.
Deslandes A, Berti V, Tandjaoui-Lambotte Y, Alloui C, Carbonnelle E, Zahar JR, Brichler S, Cohen Y. SARS-CoV-2 was already spreading in France in late December 2019. Int J Antimicrob Agents. 2020;106006.
COVID-19 Coronavirus Pandemic [https://www.worldometers.info/coronavirus/].
Hassan SA, Sheikh FN, Jamal S, Ezeh JK, Akhtar A. Coronavirus (COVID-19): a review of clinical features, diagnosis, and treatment. Cureus. 2020;12(3):e7355.
Li W, Moore MJ, Vasilieva N, Sui J, Wong SK, Berne MA, Somasundaran M, Sullivan JL, Luzuriaga K, Greenough TC, et al. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature. 2003;426(6965):450–4.
Hamming I, Cooper ME, Haagmans BL, Hooper NM, Korstanje R, Osterhaus AD, Timens W, Turner AJ, Navis G, van Goor H. The emerging role of ACE2 in physiology and disease. J Pathol. 2007;212(1):1–11.
Kuba K, Imai Y, Ohto-Nakanishi T, Penninger JM. Trilogy of ACE2: a peptidase in the renin-angiotensin system, a SARS receptor, and a partner for amino acid transporters. Pharmacol Ther. 2010;128(1):119–28.
Ge XY, Li JL, Yang XL, Chmura AA, Zhu G, Epstein JH, Mazet JK, Hu B, Zhang W, Peng C, et al. Isolation and characterization of a bat SARS-like coronavirus that uses the ACE2 receptor. Nature. 2013;503(7477):535–8.
Gheblawi M, Wang K, Viveiros A, Nguyen Q, Zhong JC, Turner AJ, Raizada MK, Grant MB, Oudit GY. Angiotensin-converting enzyme 2: SARS-CoV-2 receptor and regulator of the renin-angiotensin system: celebrating the 20th anniversary of the discovery of ACE2. Circ Res. 2020;126(10):1456–74.
Du L, He Y, Zhou Y, Liu S, Zheng BJ, Jiang S. The spike protein of SARS-CoV--a target for vaccine and therapeutic development. Nat Rev Microbiol. 2009;7(3):226–36.
Wrapp D, Wang N, Corbett KS, Goldsmith JA, Hsieh CL, Abiona O, Graham BS, McLellan JS. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science. 2020;367(6483):1260–3.
Gosain R, Abdou Y, Singh A, Rana N, Puzanov I, Ernstoff MS. COVID-19 and Cancer: a comprehensive review. Curr Oncol Rep. 2020;22(5):53.
Clinical management of severse acute respiratory infection (SARI) when COVID-19 desease is suspected. https://resourcecentre.savethechildren.net/node/17278/pdf/who_clinical-management-of-novel-cov_march_2020.pdf.
The epidemiological characteristics of an outbreak of 2019 Novel coronavirus diseases (COVID-19) in China. Zhonghua Liu Xing Bing Xue Za Zhi. 2020;41(2):145–51.
Fratino L, Procopio G, Di Maio M, Cinieri S, Leo S, Beretta G. Coronavirus: older persons with Cancer in Italy in the COVID-19 pandemic. Front Oncol. 2020;10:648.
Brunetti O, Derakhshani A, Baradaran B, Galvano A, Russo A, Silvestris N. COVID-19 infection in Cancer patients: how can oncologists Deal with these patients? Front Oncol. 2020;10:734.
Dai M, Liu D, Liu M, Zhou F, Li G, Chen Z, Zhang Z, You H, Wu M, Zheng Q, et al. Patients with Cancer appear more vulnerable to SARS-COV-2: a multicenter study during the COVID-19 outbreak. Cancer Discov. 2020.
Liang W, Guan W, Chen R, Wang W, Li J, Xu K, Li C, Ai Q, Lu W, Liang H, et al. Cancer patients in SARS-CoV-2 infection: a nationwide analysis in China. Lancet Oncol. 2020;21(3):335–7.
Mehta V, Goel S, Kabarriti R, Cole D, Goldfinger M, Acuna-Villaorduna A, Pradhan K, Thota R, Reissman S, Sparano JA, et al. Case fatality rate of Cancer patients with COVID-19 in a New York hospital system. Cancer Discov. 2020.
Zhang L, Zhu F, Xie L, Wang C, Wang J, Chen R, Jia P, Guan HQ, Peng L, Chen Y, et al. Clinical characteristics of COVID-19-infected cancer patients: a retrospective case study in three hospitals within Wuhan, China. Ann Oncol. 2020.
Wu JT, Leung K, Bushman M, Kishore N, Niehus R, de Salazar PM, Cowling BJ, Lipsitch M, Leung GM. Estimating clinical severity of COVID-19 from the transmission dynamics in Wuhan, China. Nat Med. 2020;26(4):506–10.
Disruptions in Cancer Care in the Era of COVID-19 [https://www.medscape.com/viewarticle/927215].
Stroppa EM, Toscani I, Citterio C, Anselmi E, Zaffignani E, Codeluppi M, Cavanna L. Coronavirus disease-2019 in cancer patients. A report of the first 25 cancer patients in a western country (Italy). Future Oncol. 2020;16(20):1425–32.
Chen Y, Li G. Gynecological malignancies with asymptomatic SARS-CoV-2 infection during the convalescence of outbreak. Gynecol Oncol. 2020;158(1):44–6.
Kobayashi Y, Suh DH, Aoki D, Kim JW. Management of ovarian cancer patients in affected areas during COVID-19 pandemic: Japan and Korea. J Gynecol Oncol. 2020;31(3):e65.
Al-Shamsi HO, Alhazzani W, Alhuraiji A, Coomes EA, Chemaly RF, Almuhanna M, Wolff RA, Ibrahim NK, Chua MLK, Hotte SJ, et al. A practical approach to the Management of Cancer Patients during the novel coronavirus disease 2019 (COVID-19) pandemic: an international collaborative group. Oncologist. 2020.
Dutta R, Khalil R, Green R, Mohapatra SS, Mohapatra S. Withania somnifera (Ashwagandha) and Withaferin A: Potential in Integrative Oncology. Int J Mol Sci. 2019;20:21.
Fugner A. Inhibition of immunologically induced inflammation by plant steroid WITHAFERIN-a. Arzneimittel-Forschung/Drug Research. 1973;23(7):932–5.
Kakar SS, Parte S, Carter K, Joshua IG, Worth C, Rameshwar P, Ratajczak MZ. Withaferin a (WFA) inhibits tumor growth and metastasis by targeting ovarian cancer stem cells. Oncotarget. 2017;8(43):74494–505.
Straughn AR, Kakar SS. Withaferin a ameliorates ovarian cancer-induced cachexia and proinflammatory signaling. J Ovarian Res. 2019;12(1):115.
Ye Q, Wang B, Mao J. The pathogenesis and treatment of the `cytokine Storm' in COVID-19. J Inf Secur. 2020;80(6):607–13.
Balkrishna A, Pokhrel S, Singh J, Varshney A: Withanone from Withania somnifera may inhibit novel coronavirus (COVID-19) entry by disrupting interactions between viral S-protein receptor binding domain and host ACE2 receptor. In. Research Square; 2020.
Maurya DK, Sharma D: Evaluation of traditional ayurvedic preparation for prevention and management of the novel coronavirus (SARS-CoV-2) using molecular docking approach. In. ChemRxiv; 2020.
Kumar V, Dhanjal JK, Kaul SC, Wadhwa R, Sundar D. Withanone and Caffeic acid Phenethyl Ester are predicted to interact with Main protease (M (pro)) of SARS-CoV-2 and inhibit its activity. J Biomol Struct Dyn. 2020:1–17.
Yoshida T, Tabony AM, Galvez S, Mitch WE, Higashi Y, Sukhanov S, Delafontaine P. Molecular mechanisms and signaling pathways of angiotensin II-induced muscle wasting: potential therapeutic targets for cardiac cachexia. Int J Biochem Cell Biol. 2013;45(10):2322–32.
Wang K, Gheblawi M, Oudit GY. Angiotensin converting enzyme 2: a double-edged sword. Circulation. 2020.
Acknowledgments
Not applicable.
Ethical approval
All procedures involving the usage of mice were carried out in strict accordance to the standards of the National Institute of Health guide for the care and use of laboratory animals. The Institutional Animal Care and Use Committee (IACUC, protocol # 15405) and Institutional Biosafety Committee (IBC, protocol # 18–208) of the University of Louisville approved all experimental protocols in mice in advance. No human data or tissue was used in this study.
Funding
This work was supported by NIH grants T32 HL134644 and R25GM133328 to SSK.
Author information
Authors and Affiliations
Contributions
SSK and ARS conceived the concept for the manuscript. ARS wrote the initial draft of the manuscript. All authors edited the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Consent for publication
Not applicable.
Competing interests
All authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Straughn, A.R., Kakar, S.S. Withaferin A: a potential therapeutic agent against COVID-19 infection. J Ovarian Res 13, 79 (2020). https://doi.org/10.1186/s13048-020-00684-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13048-020-00684-x