Abstract
Immune checkpoints include stimulatory and inhibitory checkpoint molecules. In recent years, inhibitory checkpoints, including cytotoxic T lymphocyte–associated antigen 4 (CTLA-4), programmed cell death protein-1 (PD-1), and programmed cell death ligand 1 (PD-L1), have been identified to suppress anti-tumor immune responses in solid tumors. Novel drugs targeting immune checkpoints have succeeded in cancer treatment. Specific PD-1 blockades were approved for treatment of melanoma in 2014 and for treatment of non-small-cell lung cancer in 2015 in the United States, European Union, and Japan. Preclinical and clinical studies show immune checkpoint therapy provides survival benefit for greater numbers of patients with liver cancer, including hepatocellular carcinoma and cholangiocarcinoma, two main primary liver cancers. The combination of anti-PD-1/PD-L1 with anti-CTLA-4 antibodies is being evaluated in phase 1, 2 or 3 trials, and the results suggest that an anti-PD-1 antibody combined with locoregional therapy or other molecular targeted agents is an effective treatment strategy for HCC. In addition, studies on activating co-stimulatory receptors to enhance anti-tumor immune responses have increased our understanding regarding this immunotherapy in liver cancer. Epigenetic modulations of checkpoints for improving the tumor microenvironment also expand our knowledge of potential therapeutic targets in improving the tumor microenvironment and restoring immune recognition and immunogenicity. In this review, we summarize current knowledge and recent developments in immune checkpoint-based therapies for the treatment of hepatocellular carcinoma and cholangiocarcinoma and attempt to clarify the mechanisms underlying its effects.
Similar content being viewed by others
Background
Globally, primary liver cancer accounts for 6% of all cancers and 9% of all death from cancer. It is the sixth most common cancer and the second leading cause of cancer death. The important primary liver cancers include hepatocellular carcinoma (HCC), accounting for approximately 75%, and cholangiocarcinoma, accounting for approximately 6%. Although either surgical resection or liver transplant can be used for the treatment of liver cancer, limitations are caused by high recurrence rates after resection and low-ratio eligibility for surgery and transplant because this cancer is often detected at a late stage [1, 2]. In the tumor microenvironment, cancer cells and host immune responses interact to promote or inhibit the pathologic progression of cancer. The immune system can identify cancer cells, and mobilizing the immune response is able to eliminate cancer [3]. Immunotherapy has emerged as a promising therapy and is being investigated in various tumors including liver cancer [4]. Emerging evidence supports that the blockade of immune checkpoints is among the most promising approaches in cancer immunotherapy [4,5,6].
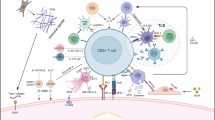
The activity of the immune system is mostly regulated by immune cells called T cells. In the tumor microenvironment, T cells can recognize tumor antigens, which are presented to T cell receptors by antigen-presenting cells (APCs). Besides signal via T cell receptors, T cell response is fine-tuned by a group of cell surface molecules, named immune checkpoints. They can be either stimulatory or inhibitory, and participate in various stages of T cell response (Fig. 1) [6,7,8,9,10,11]. Many cancers are able to evade the immune system, mainly by overexpressing inhibitory ligands to damp T cell attack. As a result, fewer, and damaged T cells were found in patients with HCC, which contributed to the progression of this cancer [12].
Illustration of stimulatory and inhibitory immune checkpoints between T-cells, APCs, and cancer cells. Blockade of inhibitory immune checkpoints can positively regulate T-cell activation and prevent immune escape of cancer cells within the tumor microenvironment. Activation of stimulatory immune check points can augment the effect of immune checkpoint inhibitors in cancer therapeutics. Red, inhibitory immune checkpoints; blue, stimulatory immune checkpoints
Recently, in vitro and in vivo results show histone deacetylase inhibitors (HDACi) and DNA methyltransferase inhibitors (DNMTi), two important epigenetic drugs, can up-regulate expression of inhibitory immune checkpoints in either immune or cancer cells [13,14,15]. Epigenetic modifiers function importantly in priming and enhancing the therapeutic effect of the host immune system on cancer [14, 15]. The purpose of this review is to give a brief overview of the role for immune checkpoints related to liver cancer progression. It also provides new insights into the epigenetic mechanism in checkpoint immunotherapy and checkpoint blocking – based therapeutic approaches for treatment of liver cancer.
Immune checkpoints and hepatocellular carcinoma
The most ex vivo studied and clinically relevant checkpoint proteins are CTLA-4, PD-1, and PD-L1 (Tables 1 and 2). The expression of inhibitory immune checkpoints can be dysregulated in a tumor microenvironment, which can lead to improvement of T cell-mediated immune response through cancer immunotherapy [16]. The PD-1 pathway is found to suppress T cell activation mainly within peripheral tissues at the later phase, whereas the CTLA-4 pathways are involved in regulation of T cell-mediated immune responses primarily in lymph nodes at the priming phase [17].
CTLA-4
CTLA-4 is a CD28 homolog and primarily located in intracellular compartments in resting naive T cells. CTLA-4 inhibits T cell response by directly delivering an inhibitory signal to T cell, and interfering with the binding between B7 and CD28 [18]. In 31 HCC patients, it was found the addition of anti-CTLA-4 antibody resulted in an increase in the frequency of tumor-associated antigens (TAA)-specific cytotoxic T cells in 60% of HCC patients, accompanied with enhanced antitumor effect of tumor-specific T cells [19]. In addition, CTLA-4 is shown to be important for regulatory T cell (Treg) function. Tregs control functions of the effector T cells, and thus crucially maintain peripheral tolerance [20]. Unlike effector T cells, Tregs constitutively express CTLA-4 to exert their immune suppression [21, 22]. Treg-specific CTLA-4 deficiency was shown to affect in vivo Treg suppressive function and promote tumor immunity [21, 22]. In a rat liver transplantation model with tumor recurrence, hepatic expressions of CTLA-4, TGF-β and PD-L1 were increased in the tumor tissues from small-for-size liver graft group compared to whole graft group. The results suggested that up-regulation of CTLA-4 may mediate the mobilization of Tregs by small-for-size graft injury, contributing to HCC recurrence after liver transplantation [23]. HCC-derived Tregs down-regulated CD80/86 expression on splenic DCs in a CTLA-4 dependent manner, and inhibition of CTLA-4 could prevent the Treg-mediated suppression in anti-tumor immune responses [24]. Thus, CTLA-4 could not only enhance the antitumor effect of effector T cells but also maintain self-tolerance and the suppressive function of Tregs in liver cancer immunity.
PD-1/PD-L1
PD-L1 is the main ligand for PD-1, which is crucial for tumor immunity. In addition, PD-L1 also interacts with B7-1 to inhibit T cell immunity, and the role of this interaction in cancer immunity is still unclear [25]. Binding of PD-L1 to its receptor can suppress T cell migration, proliferation, and secretion of cytotoxic mediators, and thus blocks the “cancer immunity cycle” [26]. In the HCC tumor microenvironment, PD-L1 expression is mainly expressed in Kupffer cells but is slightly expressed on other APCs or HCC tumor cells [27]. CD8+ T cells and Kupffer cells in human HCC tumor tissues expressed high levels of PD-1 and PD-L1, respectively. PD-L1+ Kupffer cells interact with PD-1 + CD8+ T cells and contribute to dysfunction of effector T cells in HCC. Elevated PD-L1 expression in HCC is indeed associated with poorer prognosis in HCC patients [27]. In 217 HCCs, PD-L1 was expressed by both neoplastic and intra-tumoral inflammatory cells, which are related to tumor aggressiveness. It also suggests that the PD-L1/PD-1 immune checkpoint could be targeted in the treatment of particular HCC variants [28]. More recently, 90 HCC patients with PD-L1 expression in peritumoral hepatocytes were demonstrated to have a significantly higher risk of cancer recurrence or metastasis and cancer-related death [29]. Immunohistochemistry data in 294 HCC tissue samples showed PD-1 and PD-L1 expression was significantly related to high CD8+ tumor-infiltrating lymphocytes (TILs). Only high Edmondson–Steiner grade was markedly related to high PD-1 expression. High PD-L1 expression was demonstrated as an independent poor prognostic factor for disease-free survival in the high CD8+ TILs group. Further, combined high expression of PD-L1 and CD8+ TIL is an important prognostic factor related to the immune checkpoint pathway in HCC. Also, this result would be helpful in evaluating the applicable group of PD-1/PD-L1 blocking agent for HCC patients [30]. PD-L1 expression was significantly increased in tumors with a high number of tumor-infiltrating lymphocytes (ρ = 0.533, p < 0.001). High PD-L1 expression was associated with significantly shorter overall survival [31]. These clinic data further support that PD-L1 is an important mediator in the progression and an important target in the anti-tumor therapy for liver cancer.
Other inhibitory checkpoints
Several other inhibitory receptors, including T-cell immunoglobulin- and mucin-domain-containing molecule-3 (Tim-3) and LAG-3, are also upregulated on TAA-specific CD8+ T-cells in various cancer types, and are also involved in progression of liver cancer. Tim-3 is strongly expressed on CD4+ and CD8+ T-cells obtained from HCC lesions in contrast to the surrounding liver tissue. Tim-3 is expressed on tumor-associated macrophages (TAM), which contributes to HCC growth [32]. Intriguingly, a high number of Tim3+ tumor infiltrating cells and Tim3+ TAM in HCC lesions are associated with a poor prognosis [33]. In 171 patients with hepatitis B virus (HBV)-related HCC, both PD-1 and Tim-3 expressions in liver infiltrating lymphocytes were significantly high in tumor tissues compared to tumor adjacent tissues. The up-regulation of PD-1 and Tim-3 were related to higher tumor grades [33]. There is a significant positive intercorrelation between the levels of PD-1 and Tim-3 expression in tumor tissues and tumor adjacent tissues. The expressions of PD-1 and Tim-3 in tumor tissues and tumor adjacent tissues were significantly associated with PD-1 and Tim-3 polymorphisms, with genotype AA of PD-1 rs10204525 and genotypes GT + TT of Tim-3 rs10053538 respectively [33]. LAG-3 is another important inhibitory immune check point and exerts synergistic effects with PD-1/PD-L1 on T cell activation in the tumor microenvironment. In HCC-vaccine-immunized mice, STAT3-blocked HCC vaccine downregulated expression of PD-1, TIGIT, and LAG-3, which could prevent cancer-induced dysfunction of CD8+ T and natural killer cells [34]. Recently, expression of LAG3 was found to be significantly higher on tumor-associated antigen (TAA)-specific CD8+ tumor-infiltrating T helper cells and CD8+ cytotoxic T cells in tumors than those in tumor-free liver tissues and blood of HCC patients [35]. Interestingly, blocking LAG-3 increased ex vivo proliferation of CD4+ and CD8+ TIL and effector cytokine production. Combination of LAG-3 blocking antibody with PD-L1 blockade further augmented TIL responses to polyclonal stimuli and TAA [35]. This suggests that LAG-3 plays an important role in T-cell suppression in the HCC microenvironment and might be a promising immunotherapeutic target for HCC. Further clinical trials about Tim-3, Lag-3 or TIGIT blockers should be performed in liver cancer treatment.
Co-stimulatory immune checkpoints
The best characterized co-stimulatory ligands that have been investigated in hepatocellular carcinoma are B7-1 and B7-2. These two important immune checkpoints are mainly expressed on professional antigen-presenting cells. B7-1 and B7-2 can bind to both CD28 and CTLA-4, and thus regulate T cell activation via selective interacting with either CD28 or CTLA-4 [36]. Expression of costimulatory molecules, including B7-1 and B7-2, have been found to be down-regulated in HCC cells [37]. This down-regulation may lead to suppression of activation of effector T-cells mediated by B7/CD28. The glucocorticoid-induced tumor necrosis factor receptor (GITR) and the inducible T-cell co-stimulator (ICOS) are co-stimulatory checkpoints and regulate the immunosuppressive Tregs function. Importantly, GITR and ICOS are up-regulated in Tregs infiltrating HCC and may function as potential targets for immunotherapeutic interventions for antitumor therapy [38].
Immune checkpoints and cholangiocarcinoma
Intrahepatic cholangiocarcinoma (ICC) represents the second most common primary liver malignancy, accounting for 10–20% of all primary liver cancers [39]. Although ICC is traditionally viewed as a rare cancer, its incidence has been steadily rising, with recent reports showing the incidence of ICC in the USA has increased from 0.44 to 1.18 cases/100,000 over the past three decades [40]. The prognosis for ICC continues to be poor, with surgery as the only definitive option for cure. Median survival rate is low because most patients are not eligible for curative resection. As such, there is an increasing need for the development of novel adjuvant therapies for patients with ICC.
PD-1/PD-L1
In contrast to HCC, immunotherapy in cholangiocarcinoma has been limited and mostly ineffective [41]. However, a high frequency of tumor-infiltrating lymphocytes and PD-L1 expression suggest that checkpoint inhibition may prove effective [42]. Expression of PD-L1 was found both in tumor-associated macrophages and in the tumor front. Patients with tumors exhibiting PD-L1 expression around the tumor front had a lower overall survival than tumor front-positive patients [43]. In 31 surgically resected ICC samples from Asian patients, PD-L1 expression was significantly higher in tumor tissue than that in adjacent tissue [44]. High levels of PD-L1 expression were also found in Western patients with ICC, which resulted in tumor poor differentiation, higher malignant tumor stage and higher levels of apoptotic CD8+ TILs, and therefore led to lower chance of survival [42]. More recently, in occupational cholangiocarcinoma, PD-L1 expression was found in biliary intraepithelial neoplasia and intraductal papillary neoplasm. Cholangiocarcinoma cells expressed PD-L1 in a low number of cases of occupational cholangiocarcinoma, while carcinoma cells expressed PD-L1 in all cases. Moreover, PD-L1 and PD-1 were also expressed in tumor-associated macrophages and tumor-infiltrating T cells expressed. The number of PD-L1-positive mononuclear cells, PD-1-positive lymphocytes, and CD8-positive lymphocytes infiltrating within the tumor was markedly high in occupational cholangiocarcinoma. Immunostaining with mAbs detected human leukocyte antigens (HLA) class I defects in 60% of ICC tumors and PD-L1 expression in 30%. Patients bearing tumors with HLA class I defects and PD-L1 expression had a significantly reduced survival rate. The results suggested PD-L1 up-regulation mediates immune escape in cholangiocarcinoma and could be potential biomarker of response to anti-PD-1/PDL1 immunotherapy [45]. The role of other immune checkpoints for cholangiocarcinoma is still not well established.
Epigenetic mechanism in checkpoint immunotherapy
In cancer, two important epigenetic mechanisms include hypermethylation, which is mediated by DNMTs, and histone deacetylation, which is mediated by HDACs. Epigenetic dysregulation is a crucial mechanism underlying the progression of cancer [46,47,48,49]. Some epigenetic regulators can act negatively and positively in immune responses and lead to immune evasion [50], which provides a novel mechanism in immune checkpoint therapy for treatment of cancers.
Recently, epigenetic modifications of the key immune checkpoints including PD-1, PD-L1, and CTLA-4 were analyzed in non-small cell lung cancer tissues from 39 patients [51]. It was shown that CTLA-4 and PD-1, but not PD-L1, are hypomethylated in human lung tumors. This hypomethylation also led to increased expression of these two genes as shown by transcriptome analysis [51]. In a phase 2 trial, hypomethylating agents such as vorinostat and azacitidine upregulated mRNA expression of PD-L1, PD-L2, PD-1 and CTLA-4 in 61 patients with acute myeloid leukemia [52]. More recently, profiling DNA methylation in peripheral blood mononuclear cells and T cells from HCC patients show that a broad signature of DNA methylation intensifies with progression of HCC [53]. Importantly, HCC DNA methylation is highly enriched in immune function-related gene PD-1 [53]. Interestingly, Liu et al. found highly upregulated DNA methyltransferase 1 (DNMT1) is positively correlated with PD-L1 overexpression in sorafenib-resistant HCC cells. PD-L1 further induced DNMT1-dependent DNA hypomethylation and restored the expression of methylation-silenced Cadherin 1, a metastasis suppressor in HCC [54].
Accumulating evidence also shows histone deacetylation regulates immune checkpoint expression and plays an important role in cancer progression. HDAC is have been shown to sensitize cancer cells to immune checkpoint therapy by upregulating the immune checkpoints CTLA-4, PD-1, PD-L1, and PD-L2 on tumor cells and TILs [55]. For example, inhibition of the class I HDAC1, HDAC2 and/or HDAC3 led to acetylation of the PD-L1 and PD-L2 promotors, which augmented up-regulation of PD-L1/L2 protein and RNA transcription in melanoma patients, in melanoma cell lines and in a syngeneic mouse model of melanoma [56]. Interestingly, Lienlaf et al. [57] found HDAC6i (ACY-241) reduced PD-L1 production and increased co-stimulatory checkpoint (CD28) levels, and thus suppressed tumor growth in vivo. In the WM164 HDAC6KD cells, the expression of PD-L2, B7-H4 and TRAIL-R1 were largely diminished, while B7-H3, Galectin-9 and TRAIL-R2 were moderately decreased. In breast cancer cells, CD137, a co-stimulatory checkpoint, was found to be up-regulated by HDACi (SAHA) treatment [58]. Therefore, inhibitory and co-stimulatory checkpoints can be up-regulated or down-regulated by different HDAC isoforms in different tumor types. To date, the immune modulatory activity of HDAC inhibitors on tumor-specific immunity including immune checkpoints has not been well demonstrated or characterized in HCC.
Recent evidence suggests that noncoding RNAs, such as microRNAs (miRNAs) and long noncoding RNAs (lncRNAs), may also have direct epigenetic functions by recruiting specific protein complexes to genomic DNA, and specifically to some promoters modulating the expression of the corresponding genes. MiRNAs and lncRNAs play important roles in regulating expression of immune checkpoints in various tumors [59]. In human malignant pleural mesothelioma, the levels of miR-15b, miR-16, miR-193a-3p, miR-195, and miR-200c were significantly lower in the immune checkpoint PD-L1-positive samples. Likewise, PD-L1 and miR-138-5p levels were inversely correlated in human colorectal cancer tumors, and miR-138-5p inhibited PD-L1 expression in tumor models in vivo [60]. In lung cancer, it was demonstrated that the p53/miR-34/PD-L1 and miR-200/ZEB1/PD-L1 axis are novel mechanisms in tumor immune evasion [61, 62]. Moreover, it is recently demonstrated that transfection of human CD4+ T cells with miR-138 suppressed expression of CTLA-4, PD-1, and Foxp3 in glioma preclinical models [63]. Whether the association between miRNA expression and immune checkpoint levels in tumors can be translated into a predictive marker of checkpoint inhibitor therapy in liver cancer requires further investigation. Interactions among three kinds of RNAs were revealed in the ‘lncRNA-miRNA-mRNA’ competing endogenous RNA network. Several biomarkers were identified for diagnosis of diabetic pancreatic cancer, such as lncRNAs (HOTAIR, CECR7 and UCA1), hsa-miR-214, hsa-miR-429, CCDC33 and CTLA-4. Notably, interactions of ‘CECR7-hsa-miR-429-CTLA4’ were highlighted in the endogenous RNA network, which is very important in enhancing the progression of pancreatic cancer [64]. Some miRNAs and lncRNAs might be involved in the “cancer immunity cycle” regulated by immune checkpoints such as CTLA-4 and PD-L1-PD-1 and could be the subject of future investigations in liver cancer.
Taken together, a wave of translational research highlights the mechanistic and functional link between epigenetic regulation and immune checkpoints in the development and progression of primary tumors including liver cancer.
Checkpoint-blocking based therapeutic approaches
Over the last decade, there has been significant progress in our understanding of the immune system which has led to development of numerous immune checkpoints blockades that have altered the management and prognosis in some cancers including liver cancer (Table 2). As more such drugs are developed, we will have multiple additional options and indications for these inhibitors in the near future. Among these pathways, the PD-1/PD-L1 and the B7-1/B7-2/CTLA-4 have been identified as clinically available inhibitors.
These immune checkpoint drugs such as nivolumab, pembrolizumab, and ipilimumab have already been FDA approved in non-small cell lung cancer, renal cell carcinoma, melanoma, Hodgkin lymphoma, and urothelial bladder cancer [65]. Trials investigating immune checkpoint blockades in HCC and cholangiocarcinoma are in progress and early signals of efficacy have recently been reported (Table 3). Encouraging clinical outcomes were reported from an ongoing phase I/II trial of the anti-PD-1 antibody nivolumab at the 2015 American Society of Clinical Oncology (ASCO) Annual Meeting held in Chicago [66]. Waterfall plots showed that the tumor size decreased to some extent in all cohorts including uninfected, HBV-infected, and hepatitis C virus-infected HCC patients. It was significant and stable in the response to the treatment of nivolumab in HCC patients. In another recent ongoing trial of nivolumab treatment in HCC patients, nivolumab showed a manageable safety profile, including acceptable tolerability. The objective response rate was 20% (95% CI 15–26) in patients treated with nivolumab 3 mg/kg in the dose-expansion phase and 15% (95% CI 6–28) in the dose-escalation phase [67]. Early data from the biliary tract cohort of Keynote-028 reported an objective response rate of 17% and a further 17% achieved stable disease in PD-L1 positive pretreated advanced cholangiocarcinoma [68].
Immunotherapy is promising for HCC and cholangiocarcinoma. However, even for those patients who respond to the single agent immunotherapy, combinational therapy may be more potent and lead to more durable response. At the 2016 ASCO meeting, an ongoing phase I trial showed trans catheter arterial chemoembolization. Radiofrequency, or cryoablation induced a peripheral immune response which may enhance the effect of anti-CTLA-4 treatment. This combination is safe and leads to the accumulation of intratumoral CD8+ T cells and activation of T cells in peripheral blood in responding patients. Encouraging clinical activity was seen with objective confirmed responses and a PFS of 5.7 months (NCT01853618) [65]. Another pilot study for the combined effect of immune checkpoint blocking and ablative therapies has been initiated in patients with advanced liver cancer (NCT02821754). Chemotherapy such as cisplatin can reduce PD-L2 expression on tumor cells [69, 70]. Both these studies show that chemotherapy can enhance antitumor immunity and thus may combine and augment immune checkpoint therapy for treatment of liver cancer.
As previously discussed, epigenetic modulators enhance cell surface expression of immune checkpoints. Several studies provided evidence to support increased expression of checkpoint inhibitors on tumor cells following epigenetic treatment, which enhances responses to immune checkpoint therapy [56, 71]. Recently, the role of HDACi and histone methyltransferases in tumor immunity and cancer therapy has been investigated. In melanoma-bearing mice, HDACi upregulated expression of PD-L1 and PD-L2 through increased histone acetylation. Further, combination of HDACi and PD-1 blockade led to higher efficiency in slowing tumor progression and improving survival rate than single agent therapy [56]. 3-Deazaneplanocin A and 5-aza-2′deoxycytidine, two important DNMTi, enhanced the therapeutic efficacy of PD-L1 blockade in reducing tumor volume, increasing tumor infiltrating CD8+ T cells and Th1-type chemokine expression in ovarian cancer in C57/BL6 mice [72]. Chiappinelli et al. demonstrated that 5-azacytidine, sensitized tumors to anti-CTLA-4 immune checkpoint therapy compared to 5-azacytidine or anti-CTLA-4 alone in a mouse model of melanoma [73]. Enhancer of zeste homolog 2 blockade led to reduced PD-L1 mRNA levels and a decrease in PD-L1+ Pax3+ in melanoma cells, which was maintained during concomitant IL-2cx or anti-CTLA-4 immunotherapy [74]. Taken together, these discoveries create a highly promising basis for combination studies using epigenetic and immune checkpoint therapy in patients with various cancers including liver cancer (Table 4).
Combination therapy with immunotherapy and chemotherapy or radiation therapy are being studied and reported to be synergistic through multiple mechanisms. As more data of these combinations is available, it will likely improve outcomes for patients with this rare aggressive group of cancers, and we will also be able to develop further trials to upgrade our understanding of therapies targeting liver cancers. Therefore, immunotherapy offers hope to liver cancer patients with a dismal prognosis that has not seen significant changes in therapy for a long time.
Limitations and perspectives of immune checkpoint therapy
Resistance to immune checkpoint blockades is still commonly observed in most cancer patients [75]. Failure of immune checkpoint inhibitors therapy can result from three categories: (1) mutations of the immunogenicity of cancer itself. The mutations influence expression of components of antigen-processing and presentation machinery (e.g., transporter associated with antigen processing, HLA class molecules, and β2 microglobulin), novel tumor-associated antigens (e.g., cancer-testis antigens, neoantigens), and cytokines; (2) expression of alternative immune checkpoint ligands on tumor cells (and/or immune cells). Expression of alternative co-inhibitory immune checkpoints (e.g., CTLA-4, TIM-3, LAG-3, and VISTA) has been associated with resistance to PD-1 blockade [76, 77]; or (3) defects in T cell infiltration. Diminished infiltration of T cells led to resistance to PD-1 blockade in melanoma patients [78]. However, epigenetic modifying agents including demethylating agents and histone deacetylase inhibitors may enable re-expression of immune related therapeutic genes, especially in combination of immunotherapy [79, 80]. They can also increase expression of immune checkpoints to synergize with immune checkpoint blockade therapy, leading to improving anti-tumor responses [81].
Conclusions
Most liver cancers are diagnosed at an advanced stage, while the therapy is limited. Immune checkpoint therapy provides survival benefit for liver cancer treatment. Epigenetic regulation mechanistically and functionally links with immune checkpoints. Epigenetic mechanisms of checkpoint blocking prove to be promising in treating liver cancers and determining patient prognosis. Further investigations are required to explore the clinical potential in combination with epigenetic and immune checkpoint therapy for liver cancer treatment.
Abbreviations
- APC:
-
Antigen presenting cell
- ASCO:
-
American Society of Clinical Oncology
- BTLA:
-
B- and T-lymphocyte attenuator
- CTLA-4:
-
Cytotoxic T lymphocyte–associated antigen 4
- DNMT1:
-
DNA methyltransferase 1
- DNMTi:
-
DNA methyltransferase inhibitors
- GITR:
-
Glucocorticoid-induced tumor necrosis factor receptor-related gene
- HBV:
-
Hepatitis B virus
- HCC:
-
Hepatocellular carcinoma
- HDACi:
-
Histone deacetylase inhibitors
- HLA:
-
Human leukocyte antigens
- HVEM:
-
Herpesvirus entry mediator
- ICC:
-
Intrahepatic cholangiocarcinoma
- IDO:
-
Indoleamine 2,3-dioxygenase
- KIRs:
-
Killer cell immunoglobulin-like receptors
- LAG-3:
-
Anti-lymphocyte activation gene-3
- lncRNAs:
-
long noncoding RNAs
- miRNAs:
-
microRNAs
- PD-1:
-
Programmed cell death protein-1
- PD-L1:
-
Programmed cell death ligand 1
- TAA:
-
Tumor-associated antigens
- TAM:
-
Tumor-associated macrophages
- TILs:
-
Tumor-infiltrating lymphocytes
- Tim-3:
-
T-cell immunoglobulin- and mucin-domain-containing molecule-3
- Tregs:
-
Regulatory T cells
- VISTA:
-
V-domain Ig suppressor of T-cell activation
References
Bruix J, Sherman M. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020–2.
Kuhlmann JB, Blum HE. Locoregional therapy for cholangiocarcinoma. Curr Opin Gastroenterol. 2013;29:324–8.
Lee S, Loecher M, Iyer R. Immunomodulation in hepatocellular cancer. J Gastrointest Oncol. 2018;9:208–19.
Sprinzl MF, Galle PR. Current progress in immunotherapy of hepatocellular carcinoma. J Hepatol. 2017;66:482–4.
Topalian SL, Drake CG, Pardoll DM. Immune checkpoint blockade: a common denominator approach to cancer therapy. Cancer Cell. 2015;27:450–61.
Rotte A, Jin JY, Lemaire V. Mechanistic overview of immune checkpoints to support the rational design of their combinations in cancer immunotherapy. Ann Oncol. 2018;29:71–83.
Bauman JE, Ferris RL. Integrating novel therapeutic monoclonal antibodies into the management of head and neck cancer. Cancer. 2016;120:624–32.
Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12:252–64.
Anderson AC, Joller N, Kuchroo VK. Lag-3, Tim-3, and TIGIT: co-inhibitory receptors with specialized functions in immune regulation. Immunity. 2016;44:989–1004.
Bhandaru M, Rotte A. Blockade of programmed cell death protein-1 pathway for the treatment of melanoma. J Dermatol Res Ther. 2017;1:1–11.
Granier C, De Guillebon E, Blanc C, Roussel H, Badoual C, Colin E, Saldmann A, Gey A, Oudard S, Tartour E. Mechanisms of action and rationale for the use of checkpoint inhibitors in cancer. ESMO Open. 2017;2:e000213.
Jia Y, Zeng Z, Li Y, Li Z, Jin L, Zhang Z, Wang L, Wang FS. Impaired function of CD4+ T follicular helper (Tfh) cells associated with hepatocellular carcinoma progression. PLoS One. 2015;10:e0117458.
Sigalotti L, Fratta E, Coral S, Maio M. Epigenetic drugs as immunomodulators for combination therapies in solid tumors. Pharmacol Ther. 2014;142:339–50.
Maio M, Covre A, Fratta E, Di Giacomo AM, Taverna P, Natali PG, Coral S, Sigalotti L. Molecular pathways: at the crossroads of Cancer epigenetics and immunotherapy. Clin Cancer Res. 2015;21:4040–7.
Chiappinelli KB, Zahnow CA, Ahuja N, Baylin SB. Combining epigenetic and immunotherapy to combat cancer. Cancer Res. 2016;76:1683–9.
Philips GK, Atkins M. Therapeutic uses of anti-PD-1 and anti-PD-L1 antibodies. Int Immunol. 2015;27:39–46.
Buchbinder EI, Desai A. CTLA-4 and PD-1 pathways: similarities, differences, and implications of their inhibition. Am J Clin Oncol. 2016;39:98–106.
Ramagopal UA, Liu W, Garrett-Thomson SC, Bonanno JB, Yan Q, Srinivasan M, Wong SC, Bell A, Mankikar S, Rangan VS, Deshpande S, Korman AJ, Almo SC. Structural basis for cancer immunotherapy by the first-in-class checkpoint inhibitor ipilimumab. Proc Natl Acad Sci U S A. 2017;114:E4223–32.
Mizukoshi E, Nakamoto Y, Arai K, Yamashita T, Sakai A, Sakai Y, Kagaya T, Yamashita T, Honda M, Kaneko S. Comparative analysis of various tumor-associated antigen-specific t-cell responses in patients with hepatocellular carcinoma. Hepatology. 2011;53:1206–16.
Duggleby R, Danby RD, Madrigal JA, Saudemont A. Clinical grade regulatory CD4(+) T cells (Tregs): moving toward cellular-based immunomodulatory therapies. Front Immunol. 2018;9:252.
Takahashi T, Tagami T, Yamazaki S, Uede T, Shimizu J, Sakaguchi N, Mak TW, Sakaguchi S. Immunologic self-tolerance maintained by CD25(+)CD4(+) regulatory T cells constitutively expressing cytotoxic T lymphocyte-associated antigen 4. J Exp Med. 2000;192:303–10.
Wing K, Onishi Y, Prieto-Martin P, Yamaguchi T, Miyara M, Fehervari Z, Nomura T, Sakaguchi S. CTLA-4 control over Foxp3+ regulatory T cell function. Science. 2008;322:271–5.
Li CX, Ling CC, Shao Y, Xu A, Li XC, Ng KT, Liu XB, Ma YY, Qi X, Liu H, Liu J, Yeung OW, Yang XX, et al. CXCL10/CXCR3 signaling mobilized-regulatory T cells promote liver tumor recurrence after transplantation. J Hepatol. 2016;65:944–52.
Chen X, Du Y, Hu Q, Huang Z. Tumor-derived CD4+CD25+regulatory T cells inhibit dendritic cells function by CTLA-4. Pathol Res Pract. 2017;213:245–9.
Butte MJ, Pena-Cruz V, Kim MJ, Freeman GJ, Sharpe AH. Interaction of human PD-L1 and B7-1. Mol Immunol. 2008;45:3567–72.
Butte MJ, Keir ME, Phamduy TB, Sharpe AH, Freeman GJ. Programmed death-1 ligand 1 interacts specifically with the B7-1 costimulatory molecule to inhibit T cell responses. Immunity. 2007;27:111–22.
Wu K, Kryczek I, Chen L, Zou W, Welling TH. Kupffer cell suppression of CD8+ T cells in human hepatocellular carcinoma is mediated by B7-H1/programmed death-1 interactions. Cancer Res. 2009;69:8067–75.
Calderaro J, Rousseau B, Amaddeo G, Mercey M, Charpy C, Costentin C, Luciani A, Zafrani ES, Laurent A, Azoulay D, Lafdil F, Pawlotsky JM. Programmed death ligand 1 expression in hepatocellular carcinoma: relationship with clinical and pathological features. Hepatology. 2016;64:2038–46.
Dai X, Xue J, Hu J, Yang SL, Chen GG, Lai PBS, Yu C, Zeng C, Fang X, Pan X, Zhang T. Positive expression of programmed death ligand 1 in Peritumoral liver tissue is associated with poor survival after curative resection of hepatocellular carcinoma. Transl Oncol. 2017;10:511–7.
Chang H, Jung W, Kim A, Kim HK, Kim WB, Kim JH, Kim BH. Expression and prognostic significance of programmed death protein 1 and programmed death ligand-1, and cytotoxic T lymphocyte-associated molecule-4 in hepatocellular carcinoma. APMIS. 2017;125:690–8.
Semaan A, Dietrich D, Bergheim D, Dietrich J, Kalff JC, Branchi V, Matthaei H, Kristiansen G, Fischer HP, Goltz D. CXCL12 expression and PD-L1 expression serve as prognostic biomarkers in HCC and are induced by hypoxia. Virchows Arch. 2017;470:185–96.
Yan W, Liu X, Ma H, Zhang H, Song X, Gao L, Liang X, Ma C. Tim-3 fosters HCC development by enhancing TGF-beta-mediated alternative activation of macrophages. Gut. 2015;64:1593–604.
Li Z, Li N, Li F, Zhou Z, Sang J, Chen Y, Han Q, Lv Y, Liu Z. Immune checkpoint proteins PD-1 and TIM-3 are both highly expressed in liver tissues and correlate with their gene polymorphisms in patients with HBV-related hepatocellular carcinoma. Medicine (Baltimore). 2016;95:e5749.
Han Q, Wang Y, Pang M, Zhang J. STAT3-blocked whole-cell hepatoma vaccine induces cellular and humoral immune response against HCC. J Exp Clin Cancer Res. 2017;36:156.
Zhou G, Sprengers D, Boor PPC, Doukas M, Schutz H, Mancham S, Pedroza-Gonzalez A, Polak WG, de Jonge J, Gaspersz M, Dong H, Thielemans K, Pan Q, et al. Antibodies against immune checkpoint molecules restore functions of tumor-infiltrating T cells in hepatocellular carcinomas. Gastroenterology. 2017;153:1107–1119.e10.
Kean LS, Turka LA, Blazar BR. Advances in targeting co-inhibitory and co-stimulatory pathways in transplantation settings: the yin to the Yang of cancer immunotherapy. Immunol Rev. 2017;276:192–212.
Fujiwara K, Higashi T, Nouso K, Nakatsukasa H, Kobayashi Y, Uemura M, Nakamura S, Sato S, Hanafusa T, Yumoto Y, Naito I, Shiratori Y. Decreased expression of B7 costimulatory molecules and major histocompatibility complex class-I in human hepatocellular carcinoma. J Gastroenterol Hepatol. 2004;19:1121–7.
Pedroza-Gonzalez A, Kwekkeboom J, Sprengers D. T-cell suppression mediated by regulatory T cells infiltrating hepatic tumors can be overcome by GITRL treatment. Oncoimmunology. 2013;2:e22450.
Gupta A, Dixon E. Epidemiology and risk factors: intrahepatic cholangiocarcinoma. Hepatobiliary Surg Nutr. 2017;6:101–4.
Saha SK, Zhu AX, Fuchs CS, Brooks GA. Forty-year trends in cholangiocarcinoma incidence in the U.S.: intrahepatic disease on the rise. Oncologist. 2016;21:594–9.
Kobayashi M, Sakabe T, Abe H, Tanii M, Takahashi H, Chiba A, Yanagida E, Shibamoto Y, Ogasawara M, Tsujitani S, Koido S, Nagai K, Shimodaira S, et al. Dendritic cell-based immunotherapy targeting synthesized peptides for advanced biliary tract cancer. J Gastrointest Surg. 2013;17:1609–17.
Sabbatino F, Villani V, Yearley JH, Deshpande V, Cai L, Konstantinidis IT, Moon C, Nota S, Wang Y, Al-Sukaini A, Zhu AX, Goyal L, Ting DT, et al. PD-L1 and HLA class I antigen expression and clinical course of the disease in intrahepatic cholangiocarcinoma. Clin Cancer Res. 2016;22:470–8.
Gani F, Nagarajan N, Kim Y, Zhu Q, Luan L, Bhaijjee F, Anders RA, Pawlik TM. Program death 1 immune checkpoint and tumor microenvironment: implications for patients with intrahepatic cholangiocarcinoma. Ann Surg Oncol. 2016;23:2610–7.
Ye Y, Zhou L, Xie X, Jiang G, Xie H, Zheng S. Interaction of B7-H1 on intrahepatic cholangiocarcinoma cells with PD-1 on tumor-infiltrating T cells as a mechanism of immune evasion. J Surg Oncol. 2009;100:500–4.
Sato Y, Kinoshita M, Takemura S, Tanaka S, Hamano G, Nakamori S, Fujikawa M, Sugawara Y, Yamamoto T, Arimoto A, Yamamura M, Sasaki M, Harada K, et al. The PD-1/PD-L1 axis may be aberrantly activated in occupational cholangiocarcinoma. Pathol Int. 2017;67:163–70.
Jones PA, Baylin SB. The fundamental role of epigenetic events in cancer. Nat Rev Genet. 2002;3:415–28.
Esteller M. Epigenetics in cancer. N Engl J Med. 2008;358:1148–59.
Karpathakis A, Dibra H, Pipinikas C, Feber A, Morris T, Francis J, Oukrif D, Mandair D, Pericleous M, Mohmaduvesh M, Serra S, Ogunbiyi O, Novelli M, et al. Progressive epigenetic dysregulation in neuroendocrine tumour liver metastases. Endocr Relat Cancer. 2017;24:L21–5.
Bennett RL, Licht JD. Targeting epigenetics in cancer. Annu Rev Pharmacol Toxicol. 2018;58:187–207.
Nelson HH, Kelsey KT. Epigenetic epidemiology as a tool to understand the role of immunity in chronic disease. Epigenomics. 2016;8:1007–9.
Marwitz S, Scheufele S, Perner S, Reck M, Ammerpohl O, Goldmann T. Epigenetic modifications of the immune-checkpoint genes CTLA4 and PDCD1 in non-small cell lung cancer results in increased expression. Clin Epigenetics. 2017;9:51.
Yang H, Bueso-Ramos C, DiNardo C, Estecio MR, Davanlou M, Geng QR, Fang Z, Nguyen M, Pierce S, Wei Y, Parmar S, Cortes J, Kantarjian H, et al. Expression of PD-L1, PD-L2, PD-1 and CTLA4 in myelodysplastic syndromes is enhanced by treatment with hypomethylating agents. Leukemia. 2014;28:1280–8.
Zhang Y, Petropoulos S, Liu J, Cheishvili D, Zhou R, Dymov S, Li K, Li N, Szyf M. The signature of liver cancer in immune cells DNA methylation. Clin Epigenetics. 2018;10:8.
Liu J, Liu Y, Meng L, Liu K, Ji B. Targeting the PD-L1/DNMT1 axis in acquired resistance to sorafenib in human hepatocellular carcinoma. Oncol Rep. 2017;38:899–907.
Dunn J, Rao S. Epigenetics and immunotherapy: the current state of play. Mol Immunol. 2017;87:227–39.
Woods DM, Sodre AL, Villagra A, Sarnaik A, Sotomayor EM, Weber J. HDAC inhibition upregulates PD-1 ligands in melanoma and augments immunotherapy with PD-1 blockade. Cancer Immunol Res. 2015;3:1375–85.
Lienlaf M, Perez-Villarroel P, Knox T, Pabon M, Sahakian E, Powers J, Woan KV, Lee C, Cheng F, Deng S, Smalley KS, Montecinoc M, Kozikowskid A, et al. Essential role of HDAC6 in the regulation of PD-L1 in melanoma. Mol Oncol. 2016;10:735–50.
Bellarosa D, Bressan A, Bigioni M, Parlani M, Maggi CA, Binaschi M. SAHA/Vorinostat induces the expression of the CD137 receptor/ligand system and enhances apoptosis mediated by soluble CD137 receptor in a human breast cancer cell line. Int J Oncol. 2012;41:1486–94.
Ali MA, Matboli M, Tarek M, Reda M, Kamal KM, Nouh M, Ashry AM, El-Bab AF, Mesalam HA, Shafei AE, Abdel-Rahman O. Epigenetic regulation of immune checkpoints: another target for cancer immunotherapy? Immunotherapy. 2017;9:99–108.
Zhao L, Yu H, Yi S, Peng X, Su P, Xiao Z, Liu R, Tang A, Li X, Liu F, Shen S. The tumor suppressor miR-138-5p targets PD-L1 in colorectal cancer. Oncotarget. 2016;7:45370–84.
Cortez MA, Ivan C, Valdecanas D, Wang X, Peltier HJ, Ye Y, Araujo L, Carbone DP, Shilo K, Giri DK, Kelnar K, Martin D, Komaki R, et al. PDL1 regulation by p53 via miR-34. J Natl Cancer Inst. 2015;108:djv303–djv303.
Chen L, Gibbons DL, Goswami S, Cortez MA, Ahn YH, Byers LA, Zhang X, Yi X, Dwyer D, Lin W, Diao L, Wang J, Roybal J, et al. Metastasis is regulated via microRNA-200/ZEB1 axis control of tumour cell PD-L1 expression and intratumoral immunosuppression. Nat Commun. 2014;5:5241.
Wei J, Nduom EK, Kong LY, Hashimoto Y, Xu S, Gabrusiewicz K, Ling X, Huang N, Qiao W, Zhou S, Ivan C, Fuller GN, Gilbert MR, et al. MiR-138 exerts anti-glioma efficacy by targeting immune checkpoints. Neuro-Oncology. 2016;18:639–48.
Yao K, Wang Q, Jia J, Zhao H. A competing endogenous RNA network identifies novel mRNA, miRNA and lncRNA markers for the prognosis of diabetic pancreatic cancer. Tumour Biol. 2017;39:1010428317707882.
Shah UA, Nandikolla AG, Rajdev L. Immunotherapeutic approaches to biliary Cancer. Curr Treat Options in Oncol. 2017;18:44.
Kudo M. Immune checkpoint blockade in hepatocellular carcinoma: 2017 update. Liver Cancer. 2017;6:1–12.
El-Khoueiry AB, Sangro B, Yau T, Crocenzi TS, Kudo M, Hsu C, Kim TY, Choo SP, Trojan J, Welling THR, Meyer T, Kang YK, Yeo W, et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet. 2017;389:2492–502.
Ott PA, Bang YJ, Berton-Rigaud D, Elez E, Pishvaian MJ, Rugo HS, Puzanov I, Mehnert JM, Aung KL, Lopez J, Carrigan M, Saraf S, Chen M, et al. Safety and antitumor activity of Pembrolizumab in advanced programmed death ligand 1-positive endometrial Cancer: results from the KEYNOTE-028 study. J Clin Oncol. 2017;35:2535–41.
Liu WM, Fowler DW, Smith P, Dalgleish AG. Pre-treatment with chemotherapy can enhance the antigenicity and immunogenicity of tumours by promoting adaptive immune responses. Br J Cancer. 2010;102:115–23.
Lesterhuis WJ, Punt CJ, Hato SV, Eleveld-Trancikova D, Jansen BJ, Nierkens S, Schreibelt G, de Boer A, Van Herpen CM, Kaanders JH, van Krieken JH, Adema GJ, Figdor CG, et al. Platinum-based drugs disrupt STAT6-mediated suppression of immune responses against cancer in humans and mice. J Clin Invest. 2010;121:3100–8.
Wrangle J, Wang W, Koch A, Easwaran H, Mohammad HP, Vendetti F, Vancriekinge W, Demeyer T, Du Z, Parsana P, Rodgers K, Yen RW, Zahnow CA, et al. Alterations of immune response of non-small cell lung Cancer with Azacytidine. Oncotarget. 2013;4:2067–79.
Peng D, Kryczek I, Nagarsheth N, Zhao L, Wei S, Wang W, Sun Y, Zhao E, Vatan L, Szeliga W, Kotarski J, Tarkowski R, Dou Y, et al. Epigenetic silencing of TH1-type chemokines shapes tumour immunity and immunotherapy. Nature. 2015;527:249–53.
Chiappinelli KB, Strissel PL, Desrichard A, Li H, Henke C, Akman B, Hein A, Rote NS, Cope LM, Snyder A, Makarov V, Budhu S, Slamon DJ, et al. Inhibiting DNA methylation causes an interferon response in cancer via dsRNA including endogenous retroviruses. Cell. 2016;169:361.
Zingg D, Arenas-Ramirez N, Sahin D, Rosalia RA, Antunes AT, Haeusel J, Sommer L, Boyman O. The histone methyltransferase Ezh2 controls mechanisms of adaptive resistance to tumor immunotherapy. Cell Rep. 2017;20:854–67.
Tang H, Wang Y, Chlewicki LK, Zhang Y, Guo J, Liang W, Wang J, Wang X, Fu YX. Facilitating T cell infiltration in tumor microenvironment overcomes resistance to PD-L1 blockade. Cancer Cell. 2016;30:500.
Koyama S, Akbay EA, Li YY, Herter-Sprie GS, Buczkowski KA, Richards WG, Gandhi L, Redig AJ, Rodig SJ, Asahina H, Jones RE, Kulkarni MM, Kuraguchi M, et al. Adaptive resistance to therapeutic PD-1 blockade is associated with upregulation of alternative immune checkpoints. Nat Commun. 2016;7:10501.
Thommen DS, Schreiner J, Muller P, Herzig P, Roller A, Belousov A, Umana P, Pisa P, Klein C, Bacac M, Fischer OS, Moersig W, Savic Prince S, et al. Progression of lung cancer is associated with increased dysfunction of T cells defined by coexpression of multiple inhibitory receptors. Cancer Immunol Res. 2015;3:1344–55.
Peng W, Chen JQ, Liu C, Malu S, Creasy C, Tetzlaff MT, Xu C, McKenzie JA, Zhang C, Liang X, Williams LJ, Deng W, Chen G, et al. Loss of PTEN promotes resistance to T cell-mediated immunotherapy. Cancer Discov. 2016;6:202–16.
Heninger E, Krueger TE, Lang JM. Augmenting antitumor immune responses with epigenetic modifying agents. Front Immunol. 2015;6:29.
Stone ML, Chiappinelli KB, Li H, Murphy LM, Travers ME, Topper MJ, Mathios D, Lim M, Shih IM, Wang TL, Hung CF, Bhargava V, Wiehagen KR, et al. Epigenetic therapy activates type I interferon signaling in murine ovarian cancer to reduce immunosuppression and tumor burden. Proc Natl Acad Sci U S A. 2017;114:E10981–90.
Patel SA, Minn AJ. Combination Cancer therapy with immune checkpoint blockade: mechanisms and strategies. Immunity. 2018;48:417–33.
Acknowledgements
The authors acknowledge the contribution of all investigators at the participating study sites.
Funding
This work was supported by Shenyang Science and Technology Project (No. 17-230-9-16).
Author information
Authors and Affiliations
Contributions
FX, YZ and CD contributed to study conception and design. FX and TJ wrote the main manuscript text and prepared the figures and Tables. YZ and CD provided advice regarding the paper. All authors reviewed the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Xu, F., Jin, T., Zhu, Y. et al. Immune checkpoint therapy in liver cancer. J Exp Clin Cancer Res 37, 110 (2018). https://doi.org/10.1186/s13046-018-0777-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13046-018-0777-4