Abstract
Background
Hepatocellular carcinoma (HCC) is a common malignancy worldwide, which is especially prevalent in Asia. Elucidating the molecular basis of HCC is crucial to develop targeted diagnostic tools and novel therapies. Recent studies have identified AT-rich interactive domain-containing protein 1A (ARID1A) as a broad-spectrum tumor suppressor. We evaluated the clinical implications of decreased ARID1A expression in HCC, and investigated the mechanisms of ARID1A-mediated tumor suppression.
Methods
Quantitative PCR, western blotting, immunohistochemical analysis of ARID1A mRNA and protein expression was conducted in 64 paired HCC and adjacent non-tumorous tissues. ARID1A function was evaluated in vitro in MHCC-97H and Huh7 HCC cell lines, and in vivo in a xenografted HCC tumor model.
Results
ARID1A mRNA and protein expression were significantly decreased in HCC tissues, and decreased expression was significantly associated with overall metastasis, including local lymph node and distant metastasis, and poor prognosis. ARID1A knockdown promoted HCC cell migration and invasion in vitro, whereas overexpression of ARID1A inhibited migration and invasion. E-cadherin levels were closely correlated with ARID1A expression, suggesting a role in migration and invasion. In addition, ARID1A and E-cadherin (CDH1) expression were found to be regulated in a coordinated fashion in HCC samples. Furthermore, ARID1A knockdown significantly increased HCC tumor growth and lung metastasis in vivo.
Conclusions
ARID1A served as an important tumor suppressor. Decreased expression of ARID1A was associated with tumor progression, metastasis, and reduced overall survival in mice and humans. ARID1A could represent a promising candidate therapeutic target for HCC.
Similar content being viewed by others
Background
Hepatocellular carcinoma (HCC) is one of the most common malignancies worldwide, and is especially prevalent in Asia [1]. Existing therapies are insufficient for complete tumor eradication. Elucidating the molecular basis of HCC is crucial to develop targeted diagnostic tools and therapies [2]. Genetic lesions play a major role in HCC tumorigenesis and progression. Recently, cancer genome sequencing has identified frequent mutations in epigenetic regulators, particularly chromatin remodeling proteins and histone modifiers, and aberrant chromatin regulation has emerged as a distinct mechanism that contributes to tumor development [3]. Genes encoding subunits of ATP-dependent chromatin remodelers, especially subunits of the SWItch/Sucrose NonFermentable (SWI/SNF) complex, are frequently mutated in a broad array of cancer types [4].
AT-rich interactive domain-containing protein 1A (ARID1A) is a key member of the SWI/SNF chromatin-remodeling complex. Also known as BAF250a, SMARCF1, or p270, ARID1A belongs to a family of proteins that contain a highly conserved, ~100 amino acid DNA binding domain termed ARID (AT-rich interacting domain) [5]. ARID1A has been implicated in numerous protein-protein interactions, and the most widely known and studied are those which make ARID1A a part of SWI/SNF chromatin remodeling complexes. As a member of SWI/SNF complexes, ARID1A is thought to contribute to specific recruitment of its chromatin remodeling activity by binding transcription factors and transcriptional coactivator/corepressor complexes [6, 7].
Several genome-wide sequencing studies have uncovered frequent ARID1A mutations in a multitude of human cancers including subtypes of ovarian [8, 9], endometrial [10], uterine cancers [11], gastric carcinoma [12, 13], esophageal adenocarcinoma [14], breast cancer [15] and transitional cell carcinoma of the bladder [16]. In liver cancer, ARID1A mutations were observed in 10–16.8 % of the studied tumors [17, 18] and in 13 % of hepatitis B virus-associated hepatocellular carcinomas [19]. Furthermore, many ARID1A mutations are insertion/deletion mutations, leading to downregulation of the encoded protein [20, 21]. Immunohistochemistry assays demonstrated that a substantial proportion of uterine endometrioid carcinomas, uterine clear-cell carcinomas, uterine serous carcinomas, and uterine carcinosarcomas also have loss of ARID1A protein (BAF250a) [10]. In two independent cohorts of >200 human breast cancer cases, low ARID1A protein expression was associated with more aggressive breast cancer phenotypes, such as those with a high tumor grade [15]. ARID1A protein loss also correlated with an advanced stage in non-small cell lung cancer [22]. However, the clinical significance of ARID1A and its biological function in HCC has not yet been clarified. In the present study, we investigated ARID1A protein expression in HCC tissues, and analyzed the correlation between the loss of ARID1A expression and the clinicopathological features of HCC. In addition, we explored the possible mechanisms by which ARID1A affects HCC metastases. Finally, we evaluated the role of ARID1A in HCC cell migration and invasion in vitro, and conducted HCC tumor xenograft studies to determine the biological functions of ARID1A in vivo.
Materials and methods
Tissue samples
Sixty-four patient-derived paired HCC and adjacent nontumorous tissue samples were collected at the Zhongshan Hospital of Xiamen University. Written informed consent was obtained from all patients, and the study was approved by the Clinical Research Ethics Committee of Zhongshan Hospital of Xiamen University.
RNA extraction and quantitative real-time reverse transcription polymerase chain reaction
Total RNA was isolated using TRIzol® Reagent (#15596-018, Life Technologies, New York, USA) according to manufacturers’ instructions. Subsequently, cDNA was generated using the PrimeScript™ RT reagent Kit with gDNA eraser (Takara Bio Inc., Dalian, China), and quantitative real-time reverse transcription polymerase chain reaction (qPCR) was performed using the Real-Time PCR detection system (#7500, Applied Biosystems, Shanghai, China) with 2× SYBR Green II/ROX qPCR Master Mix (Takara Bio Inc.). Relative mRNA expression was calculated using the delta threshold cycle (ΔΔCT) method and normalized to β-actin (ACTB) expression. The PCR primers are listed in Additional file 1: Table S1.
Immunohistochemical analysis of patient-derived HCC tissues
Surgically excised tumor specimens were fixed in 10 % neutral formalin and embedded in paraffin, and 4-μm thick sections were prepared using a microtome (#HM315, Thermo Scientific, Waltham, USA). Free-floating section immunostaining was performed using the avidin–biotin–peroxidase complex method (UltraSensitive™, Maixin, Fuzhou, China). Sections were deparaffinized in xylene, and rehydrated in a graded ethanol series. They were then placed in EDTA antigen retrieval buffer (pH 9.0, MVS-0099 Maixin, Fuzhou, China) and incubated at 121 °C for 3 min in an autoclave. Endogenous peroxidase activity was blocked by placing the specimens in 3 % hydrogen peroxide solution for 10 min. Sections were incubated overnight at 4 °C in an anti-human ARID1A antibody (rabbit polyclonal, 1:500, HPA005456, Sigma, USA). Sections were stained in parallel with non-immune immunoglobulin G as a negative control. Antibody binding was detected using an Elivision plus kit (Elivision™ super KIT9922, Maixin, Fuzhou, China), which uses 3, 3’-diaminobenzidine for visualization. Sections were counterstained with hematoxylin, then dehydrated, and coverslips were mounted onto slide-fixed specimens for microscopy. Slides were examined by 2 investigators in an independent and random manner. Five views per slide with 100 cells/view were evaluated at 400× magnification using a light microscope (#Axio Scope A1 pol, Carl Zeiss, Germany). Nuclear staining was considered as positive. Immunohistochemical grading was performed using the following scoring system: 0, 0–10 %; 1, 11–29 %; 2, 30–59 %; and 3, > 60 %. Samples had a higher score in HCC than in notumor tissue were defined as ARID1A high. Otherwise, they were be defined as ARID1A low. Hematoxylin-eosin (HE) stain was performed as previous described [23].
Clonal cell culture and small-interfering RNA
A panel of human HCC cell lines including MHCC-97H, LM3, SK-Hep1, SMMC-7721, HepG2, and Huh7 were purchased from the Cell Bank of Shanghai, Institutes for Biological Sciences, China. HEK293T and GP2-293 cells were generously gifted by the Medical College of Xiamen University. All clonal cells except SMMC-7721 cells were cultured in Dulbecco’s Modified Eagle’s Medium supplemented with 10 % fetal bovine serum in a humidified incubator at 37 °C and 5 % CO2. The short hairpin RNA (shRNA) retroviral plasmid (RNAi-Ready pSIREN-RetroQ), which contains a puromycin resistance gene, was purchased from Clontech. The ARID1A shRNA sequences cloned into this vector are shown in Additional file 1 Table S2. The full coding sequence of ARID1A was cloned into the lentiviral pLV-CS2.0 vector that contains an EF1α promoter to drive expression. All transfections were performed using Turbofect Transfection Reagent (#R0531, Thermo Scientific, Waltham, USA). Puromycin was used to generate cells with stable knockdown of ARID1A.
Western blotting
Total protein was extracted from cells in RIPA lysis buffer (#P0013B, Beyotime, Shanghai, China) and quantified using a Bradford assay. In total, 30 μg of protein was separated using 10 % sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and then transferred to a polyvinylidene difluoride (PVDF) membrane (Millipore, Bedford, MA, USA). The membrane was blocked in a 5 % powdered milk solution and incubated in primary antibody overnight at 4 °C. After washing, the membrane was incubated with a horseradish peroxidase–conjugated secondary antibody (Santa Cruz Biotechnology, Dallas, USA.) at 37 °C for 1 h. Protein bands were visualized using Western Bright ECL (#K-12049-D50, Advansta, CA, USA) and detected using ImageQuant LAS4000mini (General Electric, USA). Relative protein levels were calculated based on a glyceraldehyde 3-phosphate dehydrogenase (GAPDH) loading control. Antibodies used included E-cadherin (#3915S, Cell Signaling technology, Boston, USA), Vimentin (#5741S, Cell Signaling technology), Fibronectin (#610077, BD Bioscience, Bedford, USA), GAPDH (#AB-M-M 001, Hangzhou Xianzhi Biotechnology, Hangzhou, China), and ARID1A (#04-080, Millipore, Shanghai, China).
Cell migration and invasion assays
Migration of HCC cells was assessed using the 24-well polycarbonate membrane cell migration assay kit (#3422, Corning Incorporated Costar, Tewksbury, USA) according to the manufacturer’s instructions. Briefly, HCC cell lines were transfected with control and ARID1A shRNAs and were incubated in serum-free medium for 24 h. The cells were then transferred to the upper chamber of a Transwell plate by seeding 2 × 105 cells per well in 200 μL serum-free medium. Next, 0.5 mL of 10 % fetal bovine serum-containing medium was added to the lower chamber as a chemoattractant. Cells were incubated for 24–48 h (depending upon migration capability) at 37 °C. Non-migrating cells on the upper membrane surface were scraped off using cotton swabs. Cells that migrated to the bottom of the membrane were stained with 0.1 % crystal violet for 30 min, followed by washing with water for 30 s to remove residual dye. Four views were examined per transwell and cells/view were counted at 200× magnification. Each experiment was performed in triplicate. The invasion assay was performed in a similar fashion using BD BioCoat™ Matrigel™ Invasion Chambers (#354480, BD Biosciences), except that the upper chambers were precoated with ECMatrix™ gel.
Cell viability assay
Cell growth was determined using a Cell Counting Kit-8 (CCK-8) cell viability assay (#YB-K001, Yiyuan Biotechnologies, Guangzhou, China) according to the manufacturer’s instructions as described previously [24].
Cell apoptosis detection
Cell apoptosis detection was performed with Annexin V/PI (propidiumiodide) double staining (#KGA108KeyGEN Biotech, Nanjing, China). Briefly, 48 h after transfection and 12 h Cisplatin (20 μM) treatment, cells were harvested by 0.25 % trypsin (without EDTA), washed twice with chilled PBS, followed by resuspension in 200 μL of binding buffer. Staining solution containing Annexin V/FITC and PI was added to the cell suspension. After incubation in the dark for 30 min, the cells were analyzed by FACS Gallios flow cytometer (Beckman Coulter, USA).
Analysis of ARID1A function in HCC tumor xenografted mice
All animal protocols were approved by the Animal Care and Use Committee of Xiamen University. We purchased male BALB/c nude mice (4–5weeks old) from Shanghai Experimental Animal Center of Chinese Academic of Sciences (Shanghai, China). Animals were kept under standard pathogen-free conditions and allowed to acclimate for 1 week before use. MHCC-97H cells (5 × 106/0.2 mL of PBS) stably transfected with either control or ARID1A shRNA expression vectors were subcutaneously injected into right flank of each mouse (n =8 mice/group). Tumor growth was monitored once a week using a caliper, and the tumor volume was calculated using the following formula: volume = π/6 × length × width2. We monitored tumor growth over an 8-week period.
Statistical analyses
The statistical package SPSS 19.0 (SPSS, Chicago, IL, USA) was used for all analyses. All values are expressed as mean ± SEM. Correlations of ARID1A expression with clinicopathological characteristics were evaluated with a χ 2 test using R language. Survival analyses were conducted using the Kaplan-Meier method with the log-rank test. Other results were analyzed using a Student’s t test. All p-values were two-sided, and p <0.05 indicated statistical significance
Results
Reduced expression of ARID1A in HCC patients was associated with poor prognosis and an increased risk of metastasis
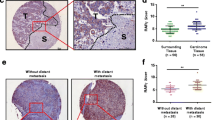
As revealed by qPCR, ARID1A mRNA levels were significantly downregulated in HCC tissues compared with nontumorous tissues (n = 64, Student’s t-test, p <0.01) (Fig. 1a). In the same 64 paired HCC samples, immunohistochemical analysis revealed that ARID1A protein expression was decreased in 41 out of 64 (64.1 %) tumor tissues when compared with adjacent non-tumorous counterparts. And 36 out of the 41 ARID1A protein downregulated samples also had reduced ARID1A mRNA expression. Strong ARID1A-positive nuclear staining was found in normal tissues (Fig. 1b, right panel), whereas ARID1A-negative staining (dark blue) can be seen in tumor tissues (Fig. 1b, the middle panel). Western blotting quantification of ARID1A was consistent with the immunohistochemical data, in which ARID1A expression in 61.7 %(29/47) HCC tissues was significantly lower than their adjacent nontumorous tissues counterparts (Fig. 1c and Additional file 2: Figure S1).
Recurrent reduction of ARID1A expression in hepatocellular carcinoma was associated with poor prognosis. a mRNA level of ARID1A was quantified with qPCR in 64 paired HCC (T) and nontumorous tissues (NT). Horizontal lines indicate the median of biological replicates. Significant differences between normal and cancer tissue were calculated by Student’s t-test (p < 0.01). b Immunohistochemistry of ARID1A (BAF250a) protein expression in HCC surgical specimens. Strong ARID1A-positive staining in normal tissue is shown in the right panel, whereas ARID1A-negative staining in HCC is presented in the middle panel. Red boxes in the left panel indicate the area enlarged in the middle and right panels. c The summary of the differences in the expression of ARID1A protein and mRNA between paired tumor and non-tumor liver tissues (right panel). d Kaplan–Meier survival curves of HCC patients (n = 64, all from Zhongshan Hospital of Xiamen University) after hepatectomy. Patients with low ARID1A expression had a significantly worse prognosis than those with high ARID1A expression (log-rank test, p = 0.042). e Endogenous expression of ARID1A was examined in HepG2, SK-Hep1, SMMC-7721, Huh7, LM3 and MHCC-97H cell lines by western blot. f Western blot analysis showed that ARID1A shRNA treatment markedly decreases its protein levels in Huh7 cells while plv cs2.0 plasmid transfection significantly enhanced ARID1A expression in MHCC-97H cells
After surgical resection, patients with tumors with low ARID1A expression showed a significantly worse prognosis compared with those with high ARID1A expression (log-rank test, n = 64, p = 0.042) (Fig. 1d). In addition, low ARID1A expression in tumors was significantly correlated with a higher metastatic rate including local lymph node and distant metastases (Additional file 1: Table S3). Univariate analyses of the 64 paired HCC cases indicated that there was no significant difference in ARID1A expression according to age, sex, liver cirrhosis, hepatitis virus B infection, or serum alfa-fetoprotein levels.
ARID1A knockdown promotes HCC cell migration and invasion, whereas overexpression of ARID1A inhibits HCC cell migration and invasion
In order to investigate the function of ARID1A in vitro, we used a panel of HCC cell lines. Western blot analysis indicated relatively high ARID1A expression in Huh7 cells, whereas MHCC-97H cells lacked ARID1A expression (Fig. 1e), so these cell lines were chosen for subsequent knockdown and overexpression analyses, respectively. The efficiencies of knockdown and overexpression in these cells are shown in Fig. 1f.
shRNA-mediated knockdown of ARID1A significantly promoted migration and invasion of Huh7 cells (sh1 = 124.40 % and sh3 = 37.7 %; and sh1 = 200.2 %, and sh3 = 100.8 %, respectively; Fig. 2a-b). To investigate the molecular mechanism underlying the role of ARID1A in migration and invasion, we investigated the association of ARID1A with proteins that regulate epithelial–mesenchymal transition. E-cadherin was significantly downregulated in ARID1A-silenced cells (Fig. 2c), whereas Fibronectin, N-cadherin and Vimentin expressions were not significantly affected (Fig. 2c). In addition, there was a strong correlation between ARID1A and E-cadherin (CDH1) expression in HCC tissue samples (Fig. 2d). Conversely, overexpression of ARID1A in MHCC-97H cells significantly inhibited migration and invasion (Fig. 3a-b). Furthermore, E-cadherin expression was significantly increased in ARID1A overexpressing cells (Fig. 3c).
Silencing of ARID1A promotes HCC cell migration and invasion. a shows cell migration ability was increased in ARID1A knockdown Huh7 cell line, while (b) exhibits its effect on invasion. For migration/invasion assay cells were cultured in the upper chamber of transwell for 24 h/48 h respectively. c We examined epithelial–mesenchymal transition (EMT) associated proteins, including E-Cadherin, N-Cadherin, Fibronectin and Vimentin in ARID1A knockdown Huh7 cells. d The expression level of both ARID1A and CDH1 (E-Cadherin) was evaluated by qPCR in 36 paired HCC specimens collected from Zhongshan Hospital, Xiamen, China. Correlation coefficient (R 2) of both proteins was calculated as log2-transformed expression between normal and cancer tissues
Overexpression of ARID1A inhibits HCC cells migration and invasion. a Left panel shows cell migration ability was decreased in ARID1A overexpressed MHCC-97H cells, right panel exhibits statistical description of result A. b Cell invasive capacity was inhibited in MHCC-97H cells with forced expression of ARID1A. c Western blot analysis showed that ARID1A transfection increased E-cadherin expression, but Vimentin was not significantly affected
ARID1A impaired in vivo xenograft tumor growth and HCC lung metastasis
In addition to examine the biological functions of ARID1A in vivo, we also assessed the functions of ARID1A by using a xenograft transplantation model in nude mice. We subcutaneously transplanted the same amount of Control shRNA and ARID1A shRNA cells into nude mice respectively; thereafter we monitored the tumor growth over a 8-week period. As shown in Fig. 4a, 8 weeks after transplantation, tumor growth in ARID1A knockdown transplanted mice was significantly greater compared with that in control-shRNA transplanted mice (p < 0.01). Our results exhibited the tumour growth curve on the time course after tumor cell injection (Fig. 4.b), and the difference between Control group and kockdown group can easily be witnessed. Three out of 6 ARID1A knockdown mice developed lung metastasis of HCC (Fig. 4c), whereas none of the mice in the control shRNA group showed any lung metastases.
Downregulation of ARID1A increases in vivo xenograft tumor growth. a Images of xenografts in ARID1A knockdown group (bottom) and control group (top) at the end of the experiment. Control shRNA and ARID1A shRNA cells were injected subcutaneously into nude mice (n = 8 for each group), and the tumors were isolated 8 weeks later. b ARID1A knockdown promotes xenograft growth of MHCC-97H cells in nude mice (n = 6, P < 0.005). c Silencing ARID1A in HCC cells promotes its lung metastasis, left panel shows the HE image of mouse lung tissue in control group, right panel exhibits the HE image of mouse lung tissue with HCC metastasis, indicated by the black arrow
Discussion
The ARID1A gene has been classified as a novel tumor suppressor, as evidenced by associations between ARID1A mRNA or protein expression and several cancers including ovarian, endometrial, gastric, and breast cancers [10, 25]. Based on the results of a previous whole-exome sequencing study [26], we evaluated the comprehensive role of ARID1A in HCC.
Reduced ARID1A expression was associated with lymph node metastasis, tumor infiltration, and poor prognosis in patients with gastric carcinoma [27, 28]. Similarly, the present study found that ARID1A protein expression was decreased in patient-derived HCC tumor tissues, and that decreased expression was significantly correlated with lymph node and distant metastasis, and poor prognosis.
Previous studies demonstrated that ARID1A also served as a regulator of cell proliferation and survival [22, 29]. So, we checked its role in HCC cell proliferation and apoptosis as well. Here, ARID1A knockdown promoted HCC cell proliferation (Additional file 3: Figure S2-A), while overexpression of ARID1A inhibited proliferation and impaired clonal formation in HCC cells (Additional file 3: Figure S2-B). These results are consistant with the previous findings that Yi Zhang.et al. did in non-small cell lung cancer [22]. We also evaluated a putative role of ARID1A in mediating cisplatin-induced apoptosis in HCC cell lines, and found that overexpression of ARID1A promoted cisplatin-induced apoptosis (Additional file 3: Figure S2-C). This was congruent with the findings from a previous study that evaluated decreased ARID1A expression in a leukemia cell line with conferred resistance to Fas-mediated apoptosis [29].
Since epithelial-mesenchymal transition (EMT) is one of the crucial events regulating hepatocellular carcinoma, prostate cancer invasion and metastasis [30, 31], we checked EMT associated proteins in ARID1A knockdown and overexpression cells. In our study, the expression of E-cadherin was strongly correlated with that of ARID1A, suggesting that the two interact in some way to regulate migration and invasion. E-cadherin is a core protein mediated cell-cell adhesion to hold the epithelial cells tight together. Loss of E-cadherin decreases the cellular adhesion, resulting in an increase of cell motility [32]. However, ARID1A silencing did not induce epithelial–mesenchymal transition in HCC cells, as evidenced by a lack of any changes in cell morphology in HCC cell lines subjected to ARID1A knockdown. Considering that E-cadherin is essential for cell adhesion, it is possible that decreased ARID1A expression in HCC tissues might loosen cell-cell junctions, promoting the migration and invasive capacity of tumor cells. Additional studies are needed to elucidate how ARID1A interacts with E-cadherin in HCC.
In nature, HCC is an invasive tumor that metastasizes hematogenously and lymphogenously to other organs, even after local recurrence. The most common organs of distant metastases include the lungs, lymph nodes, bone, and brain, with the lung metastasis occurring in 18–60 % of HCC cases [33]. This was reflected in the present study with the development of lung metastasis in 50 % of the mice bearing HCC tumors with ARID1A knockdown, further implicating decreased ARID1A expression with the development of metastasis in HCC.
Our results,together with previous mutational and functional studies, suggest ARID1A is a bona fide tumor suppressor. Therefore, it will be of interest to determine whether depletion of ARID1A can be therapeutically exploited by targeting downstream and potentially reversible epigenetic consequences of remodeler mutation [34].
Conclusion
In conclusion, our results have shown that ARID1A is frequently downregulated in hepatocellular carcinoma and is related to the aggressive phenotype of HCCs. We explored comprehensive role of the gene in HCC, which might provide direction for future studies on the molecular mechanisms of ARID1A. ARID1A represents a potential drug candidate for molecular-targeted therapy for HCC.
Abbreviations
- ARID1A:
-
AT-rich interactive domain-containing protein 1A
- BAF250a:
-
BRG1-associated factor 250a
- CDH1:
-
Cadherin 1
- IHC:
-
Immunohistochemistry
- qPCR:
-
Quantitative reverse transcription PCR
- shRNA:
-
Short hairpin RNA
- SWI/SNF:
-
SWItch/Sucrose non-fermentable
References
Schütte K, Bornschein J, Malfertheiner P. Hepatocellular carcinoma-epidemiological trends and risk factors. Dig Dis. 2009;27(2):80–92.
Fernandez-Banet J, Lee NP, Chan KT, Gao H, Liu X, Sung WK, et al. Decoding complex patterns of genomic rearrangement in hepatocellular carcinoma. Genomics. 2014;103(2–3):189–203.
Wu JN, Roberts CW. ARID1A mutations in cancer: another epigenetic tumor suppressor? Cancer Discovery. 2013;3(1):35–43.
Wilson BG, Roberts CW. SWI/SNF nucleosome remodellers and cancer. Nat Rev Cancer. 2011;11:481–92.
Wu RC, Wang TL, Shih IM. The emerging roles of ARID1A in tumor suppression. Cancer Biol Ther. 2014;15(6):655–64.
Nie Z, Xue Y, Yang D, Zhou S, Deroo BJ, Archer TK, et al. A specificity and targeting subunit of a human SWI/SNF family-related chromatin-remodeling complex. Mol Cell Biol. 2000;20(23):8879–88.
Trotter KW, Archer TK. Reconstitution of glucocorticoid receptor-dependent transcription in vivo. Mol Cell Biol. 2004;24(8):3347–58.
Jones S, Wang TL, Shih IM, Mao TL, Nakayama K, Roden R, et al. Frequent mutations of chromatin remodeling gene ARID1A in ovarian clear cell carcinoma. Science. 2010;330(6001):228–31.
Wiegand KC, Shah SP, Al-Agha OM, Zhao Y, Tse K, Zeng T, et al. ARID1A mutations in endometriosis-associated ovarian carcinomas. N Engl J Med. 2010;363(16):1532–43.
Wiegand KC, Lee AF, Al-Agha OM, Chow C, Kalloger SE, Scott DW, et al. Loss of BAF250a (ARID1A) is frequent in high-grade endometrial carcinomas. J Pathol. 2011;224(3):328–33.
Guan B, Mao TL, Panuganti PK, Kuhn E, Kurman RJ, Maeda D, et al. Mutation and loss of expression of ARID1A in uterine low-grade endometrioid carcinoma. Am J Surg Pathol. 2011;35(5):625–32.
Wang K, Kan J, Yuen ST, Shi ST, Chu KM, Law S, et al. Exome sequencing identifies frequent mutation of ARID1A in molecular subtypes of gastric cancer. Nat Genet. 2011;43(12):1219–23.
Zang ZJ, Cutcutache I, Poon SL, Zhang SL, McPherson JR, Tao J, et al. Exome sequencing of gastric adenocarcinoma identifies recurrent somatic mutations in cell adhesion and chromatin remodeling genes. Nat Genet. 2012;44(5):570–4.
Dulak AM, Stojanov P, Peng S, Lawrence MS, Fox C, Stewart C, et al. Exome and whole-genome sequencing of esophageal adenocarcinoma identifies recurrent driver events and mutational complexity. Nat Genet. 2013;45(5):478–86.
Mamo A, Cavallone L, Tuzmen S, Chabot C, Ferrario C, Hassan S, et al. An integrated genomic approach identifies ARID1A as a candidate tumor-suppressor gene in breast cancer. Oncogene. 2012;31(16):2090–100.
Gui Y, Guo G, Huang Y, Hu X, Tang A, Gao S, et al. Frequent mutations of chromatin remodeling genes in transitional cell carcinoma of the bladder. Nat Genet. 2011;43(9):875–8.
Guichard C, Amaddeo G, Imbeaud S, Ladeiro Y, Pelletier L, Maad IB, et al. Integrated analysis of somatic mutations and focal copy-number changes identifies key genes and pathways in hepatocellular carcinoma. Nat Genet. 2012;44(6):694–8.
Fujimoto A, Totoki Y, Abe T, Boroevich KA, Hosoda F, Nguyen HH, et al. Whole-genome sequencing of liver cancers identifies etiological influences on mutation patterns and recurrent mutations in chromatin regulators. Nat Genet. 2012;44(7):760–4.
Huang J, Deng Q, Wang Q, Li KY, Dai JH, Li N, et al. Exome sequencingof hepatitis B virus-associated hepatocellular carcinoma. Nat Genet. 2012;44(10):1117–21. doi:10.1038/ng.2391.
Tornesello ML, Buonaguro L, Tatangelo F, Botti G, Izzo F, Buonaguro FM. Mutations in TP53, CTNNB1 and PIK3CA genes in hepatocellular carcinoma associated with hepatitis B and hepatitis C virus infections. Genomics. 2013;102(2):74–83.
Maeda D, Mao TL, Fukayama M, Nakagawa S, Yano T, Taketani Y, et al. Clinicopathological significance of loss of ARID1A immunoreactivity in ovarian clear cell carcinoma. Int J Mol Sci. 2010;11:5120–8.
Zhang Y, Xu X, Zhang M, Bai X, Li H, Kan L, et al. ARID1A is downregulated in non-small cell lung cancer and regulates cell proliferation and apoptosis. Tumour Biol. 2014;35(6):5701–7.
Zhang S, Li J, He F, Wang XM. Abnormal nuclear expression of Pygopus-2 in human primary hepatocellular carcinoma correlates with a poor prognosis. Histopathology. 2014. doi: 10.1111/his.12637. [Epub ahead of print]
Wu LM, Yang Z, Zhou L, Zhang F, Xie HY, Feng XW, et al. Identification of histone deacetylase 3 as a biomarker for tumor recurrence following liver transplantation in HBV-associated hepatocellular carcinoma. PLoS One. 2010;5:e14460.
Zhang X, Zhang Y, Yang Y, Niu M, Sun S, Ji H, et al. Frequent low expression of chromatin remodeling gene ARID1A in breast cancer and its clinical significance. Cancer Epidemiol. 2012;36(3):288–93.
Amaddeo G, Cao Q, Ladeiro Y, Imbeaud S, Nault JC, Jaoui D, et al. Integration of tumour and viral genomic characterisations in HBV-related hepatocellular carcinomas. Gut. 2015;64(5):820–9.
Yan HB, Wang XF, Zhang Q, Tang ZQ, Jiang YH, Fan HZ, et al. Reduced expression of the chromatin remodeling gene ARID1A enhances gastric cancer cell migration and invasion via downregulation of E-cadherin transcription. Carcinogenesis. 2014;35(4):867–76.
Wang DD, Chen YB, Pan K, Wang W, Chen SP, Chen JG, et al. Decreased expression of the ARID1A gene is associated with poor prognosis in primary gastric cancer. PLoS One. 2012;7(7):e40364.
Luo B, Cheung HW, Subramanian A, Sharifnia T, Okamoto M, Yang X, et al. Highly parallel identification of essential genes in cancer cells. Proc Natl Acad Sci. 2008;105(51):20380–5.
Ma CQ, Yang Y, Wang JM, Du GS, Shen Q, Liu Y, et al. The aPKCiota blocking agent ATM negatively regulates EMT and invasion of hepatocellular carcinoma. Cell Death Dis. 2014;5:e1129.
Vicente CM, Lima MA, Nader HB, Toma L. SULF2 overexpression positively regulates tumorigenicity of human prostate cancer cells. J Exp Clin Cancer Res. 2015;34(1):25.
Nelson WJ, Nusse R. Convergence of Wnt, beta-catenin, and cadherin pathways. Science. 2004;303(5663):1483–7.
Natsuizaka M, Omura T, Akaike T, Kuwata Y, Yamazaki K, Sato T, et al. Clinical features of hepatocellular carcinoma with extrahepatic metastases. J Gastroenterol Hepatol. 2005;20(11):1781–7.
Popovic R, Licht JD. Emerging epigenetic targets and therapies in cancer medicine. Cancer Discov. 2012;2(5):405–13.
Acknowledgments
This work was supported by grants from the National Key Basic Research Program of China (2013CB933900), National Natural Science Foundation of China (Grant No. 81472231), the Natural Science Foundation of Fujian Province, China (Grant No. 2014D012), and the Middle-aged and Young Key Talents Program of Fujian Province, China (No.2014-ZQN-JC-43)
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
Conceived and designed the study: XMW and FH Performed the laboratory analysis: FH, JL, and WXZ. Performed the animal model experiment: JFX. Performed the histopathological analysis: SZ and ZYY Contributed reagents, materials, and analysis tools: FH, JL, and YPX. Wrote the manuscript: FH. All authors read and approved the final manuscript.
Additional files
Additional file 1: Table S1.
Information of qPCR Primers; Table S2. ARID1A shRNA sequence list; Table S3. Correlation between the clinicopathological characteristics and expression of the ARID1A (BAF250a) protein in hepatocellular carcinoma.
Additional file 2: Figure S1.
Western blot analysis of ARID1A protein expression in 47 human HCC samples and their adjacent non-tumorous liver tissue samples. N: non-tumorous liver tissue, T: tumor tissue.
Additional file 3: Figure S2.
ARID1A depletion facilitates cell proliferation and inhibits apoptosis. (A) CCK8 assay in Huh7 cells transfected with ARID1A shRNA. A time dependent increase was witnessed in cell proliferation after ARID1A shRNA transfection compared with control, but ARID1A overexpression in MHCC-97H cells caused a decrease in cell proliferation. (B) Colony formation assay was performed in Huh7 cells with ARID1A knockdown and MHCC-97H cells with forced expression of ARID1A. A marked increase in colony formation is seen in the groups with ARID1A depletion. (C) AnnexinV/PI analysis showed that ARID1A depletion inhibited early apoptosis induced by Cisplatin, while cells with overexpression of ARID1A are more sensitive to Cisplatin-induced early apoptosis. (* p < 0.05, ** p < 0.01).
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
He, F., Li, J., Xu, J. et al. Decreased expression of ARID1A associates with poor prognosis and promotes metastases of hepatocellular carcinoma. J Exp Clin Cancer Res 34, 47 (2015). https://doi.org/10.1186/s13046-015-0164-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13046-015-0164-3