Abstract
BRAF and KRAS are two key oncogenes in the RAS/RAF/MEK/MAPK signaling pathway. Concomitant mutations in both KRAS and BRAF genes have been identified in non-small cell lung cancer (NSCLC). They lead to the proliferation, differentiation, and apoptosis of tumor cells by activating the RAS/RAF/MEK/ERK signaling pathway. To date, agents that target RAS/RAF/MEK/ERK signaling pathway have been investigated in NSCLC patients harboring BRAF mutations. BRAF and MEK inhibitors have gained approval for the treatment of patients with NSCLC. According to the reported findings, the combination of MEK inhibitors with chemotherapy, immune checkpoint inhibitors, epidermal growth factor receptor-tyrosine kinase inhibitors or BRAF inhibitors is highly significant for improving clinical efficacy and causing delay in the occurrence of drug resistance. This review summarized the existing experimental results and presented ongoing clinical studies as well. However, further researches need to be conducted to indicate how we can combine other drugs with MEK inhibitors to significantly increase therapeutic effects on patients with lung cancer.
Similar content being viewed by others
Introduction
Lung cancer is the most common cause of cancer-related death worldwide, with over 1.8 million lung cancer deaths annually [1]. Over the past decades, the treatment of non-small cell lung cancer (NSCLC) has changed dramatically with the development of molecular profiling, targeted therapeutic agents, and precision medicine, while the overall prognosis of lung cancer is still poor with a 5-year overall survival (OS) rate of 18% across all stages [2]. NSCLC accounts for about 80–85% of lung cancer cases and almost 70% of NSCLC patients presenting with locally advanced or metastatic disease at initial diagnosis [3]. NSCLC comprises several histologic subtypes, such as squamous cell carcinoma, adenocarcinoma, large cell or undifferentiated carcinoma. Non-squamous carcinoma (70–75%) and squamous cell carcinoma (25–30%) are two major subtypes [4]. In NSCLC somatic mutations in epidermal growth factor receptor (EGFR) and rearrangements in anaplastic lymphoma kinase gene (ALK) and ROS proto-oncogene1 (ROS1) have been validated as strong predictive biomarkers and attractive drug targets. However, the mitogen-activated protein kinase (MAPK) pathway, comprising the kinases RAS, RAF, MEK, and ERK, is also implicated in the tumorigenesis of NSCLC. Thus, MEK inhibitors’ monotherapy or combination with other targeted drugs harboring MAPK pathway become a promising strategy for NSCLC patients with B-Raf proto-oncogene (BRAF) or Kirsten rat sarcoma viral oncogene homolog (KRAS) mutations. Currently, the prevalence of BRAF mutations is 3–5% in NSCLC patients, of which BRAF V600E mutations constitute approximately 50% [5]. To date, BRAF plus MEK inhibitors have shown a remarkable survival and response rate in advanced and unresectable melanoma patients, compared with single-agent BRAF inhibition [6, 7]. Moreover, concomitant inhibition of both BRAF and MEK has been validated to overcome acquired resistance to BRAF inhibitors alone [8, 9]. Besides, the prevalence of KRAS mutations is ~ 25% and ~ 15% in Western and Asian populations with lung adenocarcinoma, respectively [10]. Although the unprecedented challenge of effective KRAS targeting is evidenced by disappointing results to date, MEK inhibitors plus other targeted agents are actively exploring the potential effect in some clinical trials right now.
The present study aimed to review researches concentrated on the effects of MEK inhibitors on NSCLC patients to facilitate the clinical management of such patients.
Structures and functions of MEK proteins
MEK proteins are mitogen-activated protein kinase kinase, a dual specificity Tyr/Thr protein kinase that selectively phosphorylates serine/threonine and tyrosine residues in the activation loop of ERK1 and ERK2. MEK proteins are coded by 7 different genes, among which MEK1 and MEK2 are of significance. MEK1 gene exists in human chromosome 15q22.31, and MEK2 gene exists in chromosome 9q13.3 [11]. The MEK1/2 proteins have three crucial domains (Fig. 1): a core protein kinase domain, an N-terminal domain (approximately 80 amino acids), and a shorter C-terminal region (within 30 amino acids) [11, 12]. The protein kinase domain contains the ATP site and catalytic segment; besides, a pocket structure near the ATP-binding site is an ideal target for small target agents that can change the molecule to an inactive state. The N-terminal region plays a regulatory role in signal transduction, including the D-domain (docking site) binding to the ERK substrate. Additionally, mitogen-activated protein kinase (MAPK) is localized to the cytoplasm through its specific association with the N-terminal 1–32 residues of MAPKK in unstimulated cells [13]. The C-terminal region contains the domain for versatile docking (DVD), a critical binding site for the upstream apparatus of the MAPK signaling pathway [14].
Molecular pathways and MEK inhibitors
MEK is the downstream of RAS/RAF/MEK/ERK signaling pathway, highly regulating and playing an important role in cell proliferation, differentiation, apoptosis, and stress responses [15]. It transmits mitogenic signals from outside the cell to the nucleus through multistage phosphorylation [16]. In tumor cells, certain growth factors are combined with transmembrane receptors on the cell surface, leading to the increase in RAS guanosine triphosphate-binding protein in the cell [17]. Once RAS is activated, the plasma membrane of the cell secretes and activates the downstream molecule RAF kinase, stimulates a series of protein kinases, and forms the RAS/RAF/MEK/ERK signaling pathway [18] (Fig. 2).
RAS/RAF/MEK/ERK signaling pathway. RTK: receptor tyrosine kinase; GRB: growth factor receptor bound protein; SOS: Son of Sevenless homolog; GDP: guanosine diphosphate; GTP: guanosine triphosphate; RAS: rat sarcoma viral oncogene; RAF: v-raf murine sarcoma viral oncogene; MEK: mitogen-activated protein kinase kinase; ERK: extracellular signal-regulated kinase; PI3K: phosphatidylinositol 3-kinase; AKT: protein kinase B; mTOR: mammalian target of rapamycin; NF-kB: nuclear factor-kB
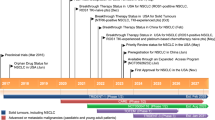
To date, four MEK inhibitors have been approved by the United States Food and Drug Administration (FDA), including trametinib, binimetinib, selumetinib, and cobimetinib [19,20,21,22]. They are oral, allosteric, selective, ATP-non-competitive MEK1/2 inhibitors that are not easy to produce cross-inhibition to other targets [23,24,25,26,27]. Notably, trametinib is the only MEK inhibitor approved for the treatment of NSCLC patients with BRAF V600E mutation in combination with dabrafenib till now (Table 1).
Evidence for MEK monotherapy for NSCLC patients
Several trials have explored the function of single-agent MEK inhibition in early clinical development. An initial phase II study evaluated the efficacy and safety of AZD6244 versus pemetrexed as second- or third-line treatment in patients with advanced NSCLC. In this trial, 84 patients were enrolled, and 5% and 4.5% of patients achieved an objective response in AZD6244 group and pemetrexed group, respectively. However, there was no significant difference in median progression-free survival (PFS) between the two groups (90 days vs 67 days, HR:1.08, P = 0.79). The incidence of treatment-related serious adverse events appeared more commonly in the pemetrexed group (6.8% vs 2.5%) than in the AZD6244 group. Most frequently, toxicities were primarily dermatitis acneiform (43%), diarrhea (30%), nausea (18%), and vomiting (18%) with AZD6244 [36]. Another single-arm phase II study was conducted to test PD-0325901 in two administration schedules. This study enrolled 34 patients. Thirteen patients were administered intermittently (3 weeks on/1 week off), while 21 patients were administered adjusting schedule (5 days on/2 days off for 3 weeks, followed by 1 week off). No objective responses were observed in two schedules, while 7 patients had stable disease. Median PFS was 1.8 months (95% CI 1.5–1.9), and overall survival was 7.8 months (95% CI 4.5–13.9). The most common treatment-related toxicities (incidence in schedule A/incidence in schedule B) were diarrhea (54%/76%), fatigue (31%/48%), rash (46%/33%), vomiting (38%/33%), and nausea (38%/29%) [37]. Another phase II study evaluated the safety and efficacy of trametinib versus docetaxel for patients with KRAS-mutant NSCLC patients. In this trial, 129 were enrolled. However, there was no significant difference in median PFS in trametinib and docetaxel arm (12 weeks vs 11 weeks, HR:1.14, P = 0.5197) and in median OS (8 months vs not reached, HR:0.97, P = 0.934). Partial responses (PRs) for these two groups were 12% and 12% (P = 1.0000). The most frequent grade 3 or higher toxicities were primarily hypertension (9%), rash (9%), diarrhea (5%), sepsis (5%), and asthenia (5%) vs. neutropenia (35%) in trametinib and docetaxel arms, respectively. One treatment-related death occurred with trametinib and none with docetaxel [38]. An initial phase II basket trial evaluated the efficacy of selumetinib in NSCLC patients with molecular profiling. In this trial, 110 patients presented RAS/RAF mutations with KRAS mutations (24.9%), BRAF mutations (2%), HRAS and NRAS mutations (0.7%), and 10 patients were enrolled onto the selumetinib arm. However, 9 patients failed to achieve selumetinib monotherapy primary end point, with only one partial response (ORR 11%, 95% CI 0–48%), a median PFS time of 2.3 months, and median OS time of 6.5 months [39]. The results of these phase II studies indicated that MEK inhibitors’ monotherapy seemed to have poor clinical outcomes and more toxicities for NSCLC patients compared with chemotherapy alone.
Evidences for combination of BRAF and MEK inhibitors for NSCLC patients
The combination of BRAF and MEK inhibitors has been proved to be clinically effective for NSCLC patients to date. An initial phase II trial evaluated the combination of dabrafenib and trametinib in previously treated BRAF(V600E)-mutant NSCLC patients. Fifty-seven patients were enrolled in this study. The overall response was 63.2% (95% CI 49.3–75.6%), the median PFS was 9.7 months (95% CI 6.9–19.6), and median duration of response (DOR) was 9.0 months (95% CI 6.9–18.3). Common grade 3/4 AEs were neutropenia (9%), hyponatremia (7%), and anemia (5%) [40]. Besides, the same research team developed another phase II study to assess the efficacy and safety of dabrafenib plus trametinib treatment in previously untreated patients with BRAF(V600E)-mutant metastatic NSCLC. In this study, 36 patients were enrolled and treated with first-line dabrafenib plus trametinib. The ORR was 64% (95% CI 46–79%), median DOR was 10.4 months (95%CI 8.3–17.9), and PFS was 10.9 months (95% CI 7.0–16.6). Grade 3 or 4 AEs were pyrexia (11%), alanine aminotransferase increase (11%), hypertension (11%), and vomiting (8%) [41]. The NCI-MATCH Trial Subprotocol H evaluated the combination of dabrafenib and trametinib in solid tumor patients, 5 lung adenocarcinoma patients included. One patient was progression-free at 32.5 months, and 1 patient who was considered unevaluable, with an 81% reduction in the sum of measured lesions, had a PFS of 12.7 months. Three patients had SD for 15.6, 6.6, and 3.6 months which is sought to investigate the selective BRAF inhibitor [42]. The clinical data showed the efficacy of combination of MEK and BRAF inhibitors with untreated or treated BRAF V600E-mutant metastatic NSCLC, indicating that physicians can flexibly treat patients with this targeted therapy combination in either the first-line or following chemotherapy and provide strategies to accommodate the individual patient needs.
Evidence for combination of chemotherapy and MEK inhibitors for NSCLC patients
Chemotherapy is no longer the most efficacious treatment, and targeted agents have been rationally designed to inhibit particular mutations, leading to a more streamlined clinical trial process. Ten years ago, numerous clinical trials have concentrated on exploration of the combination of chemotherapy plus MEK inhibitors for NSCLC patients (Table 2). In the early stage, a phase II study evaluated selumetinib plus docetaxel versus docetaxel plus placebo for patients with KRAS-mutant advanced NSCLC. Forty-four and 43 patients were enrolled in selumetinib and placebo groups, respectively. The median OS was 5.2, 9.4 months (HR: 0.80, P = 0.21) in selumetinib and placebo group, respectively. However, the median PFS in the selumetinib group was significant longer than the placebo group (5.3 months vs. 2.1 months, HR: 0.58, P = 0.014). Similarly, the ORR was 37% and none (P < 0.0001) in selumetinib and placebo groups, respectively. Grade 3 or higher AEs occurred in 82% patients in selumetinib group and 67% patients in the placebo group (Table 3) [43]. Another phase II study of selumetinib in combination with chemotherapy was conducted in patients with advanced or metastatic non-squamous NSCLC. A total of 63 enrolled patients were randomly assigned 1:1:1 to intermittent selumetinib + chemotherapy (arm A) or continuous selumetinib + chemotherapy (arm B) or chemotherapy alone (arm C). The ORR was 35%, 62%, and 24% in arm A/B/C, respectively. Similarly, the PFS was 7.5, 6.7, 4.0 respectively. Skin and gastrointestinal adverse events were more common with the addition of selumetinib (Table 3) [44]. A phase II trial evaluating the combination of selumetinib plus docetaxel in KRAS-mutant advanced NSCLC patients also demonstrated modest improved efficacy. The retrospective analysis indicated that OS for the selumetinib + docetaxel arm vs. placebo + docetaxel arm in KRAS mutation group (MG1) and MG2 was 9.6 vs 4.4 months and 8.6 vs 7.1 months, respectively. Similarly, PFS for selumetinib and placebo groups in KRAS MG1 and MG2 was 5.7 vs 1.4 months and 4.9 vs 2.6 months, respectively. The ORR showed a numerically higher rate in MG1 compared with MG2 (46% vs 26%, respectively). Thus, for patients receiving selumetinib + docetaxel and harboring KRAS G12C or G12V mutations, there were trends toward greater improvement in OS, PFS, and ORR compared with other KRAS mutations [45]. A phase 1/1b study evaluated the efficacy and safety of trametinib plus docetaxel or pemetrexed in advanced NSCLC. In this trial, 95 patients were enrolled. In trametinib plus docetaxel group, the ORR was 18% versus 24% in KRAS-WT and KRAS-mutant, respectively. In trametinib plus pemetrexed group, the ORR was 17% versus 11% in KRAS-WT and KRAS-mutant, respectively. Most common AEs were diarrhea, nausea, and fatigue (Table 3) [46]. SELECT-1 was designed to assess the efficacy and safety of selumetinib plus docetaxel in patients with KRAS-mutant locally advanced or metastatic NSCLC. In total, 510 patients were enrolled and randomized. PFS was 3.9, 1.1 months in selumetinib and placebo groups, respectively (HR:0.93, P = 0.44). OS was 8.7, 0.9 months (HR:1.05, P = 0.64), respectively. ORR was 20.1% and 13.7% in selumetinib and placebo groups, respectively. Grade 3 or higher AEs were more frequent with selumetinib group than placebo (67% vs 45%) (Table 3) [47]. However, Jacob Kaufman et al. from Duke University questioned whether other mutations are related to the response to MEK inhibition, such as the concurrent loss of tumor-suppressor genes in LKB1, which may also affect the results of the trial [48]. The SELECT-2 trial assessed the efficacy of selumetinib plus docetaxel as a second-line treatment for patients with advanced metastatic NSCLC. A total of 212 patients were randomized. There were no statistically significant improvements in PFS or OS for overall or KRAS-WT in either selumetinib or placebo group. PFS for selumetinib + docetaxel 60 mg/m2, selumetinib + docetaxel 75 mg/m2 compared with placebo + docetaxel 75 mg/m2 was 3.0, 4.2, and 4.3 months. The most commonly reported grade 3 or higher AE was neutropenia (Table 3) [49]. SELECT-3 trial was designed a phase I study to assess the efficacy of selumetinib in combination with platinum-doublet chemotherapy for NSCLC patients in first-line setting. Fifty-five patients were enrolled. Most frequent adverse events (AEs) were fatigue, nausea, diarrhea, and vomiting (Table 3) [50]. Another phase I study evaluated the safety and tolerability of selumetinib as a monotherapy, or in combination with docetaxel as a second-line therapy for Japanese patients with advanced NSCLC. Thirty-three patients were enrolled and 25 assigned to treatment. Grade 3 dose-limiting toxicities were febrile neutropenia and pneumonitis (Table 3) [51]. Current clinical data showed that MEK inhibitor combined with chemotherapy can improve the outcomes while some not. One possibility is that clinical benefit may occur in a specific subset of tumors that exhibits a favorable genetic of signaling environment. So effective drug candidates of MEK inhibitors and proper special patients should be detected for this combination therapy.
Evidence for combination of immune checkpoint inhibitors and MEK inhibitors for NSCLC patients
Immune checkpoint inhibitors (ICIs) have opened up a new era for lung cancer treatment in recent years. However, even when patients with 50% or higher positivity for PD-L1 expression are selected, overall response rates still do not exceed 31% [52, 53]. Thus, different combination treatments have been proposed. Preclinical data suggested an improved T cell activation and increased CTLA-4 expression for selumetinib and trametinib. Besides, pulsatile MEKi treatment combined with CTLA-4 blockade prolonged survival in mice-bearing tumors with mutant KRAS [54, 55]. An initial phase Ib study was conducted to investigate the safety and efficacy of cobimetinib plus atezolizumab for patients with solid tumors (n = 152), 28 NSCLC patients included. The median OS time was 13.2 months, and ORR was 18% with NSCLC. 12-month PFS and OS rates were 29% and 57% for NSCLC patients, respectively. The most common AEs were diarrhea (67%), skin rash (48%), and fatigue (40%) [56]. Another phase I/II trial evaluated immunotherapy with durvalumab and tremelimumab with continuous or intermittent administration of selumetinib in NSCLC patients. The trial is actively screening and enrolling patients, and the estimated study completion is scheduled for April 2021 [57]. Currently, the clinical data about ICI-combined MEK inhibitors are still not efficient enough to validate the most proper way to treat NSCLC. More clinical outcomes are worthy being awaited furthermore.
Evidence for combination of EGFR tyrosine kinase inhibitors (TKIs) and MEK inhibitors for NSCLC patients
To our knowledge, acquired resistance has become a major clinical problem for advanced NSCLC patients with the increasing administration of EGFR-TKIs. The combination strategy of MEK inhibitors plus EGFR-TKIs has been proposed in certain clinical trials. Preclinical data suggested the stronger inhibitory effect of the cell proliferation of EGFR-TKIs-resistant cells for MEK inhibitors plus EGFR-TKIs [58]. A phase II study was concentrated on administration of selumetinib with and without erlotinib for KRAS-mutant and KRAS wild-type (WT) advanced NSCLC patients. Forty-one KARS-mutant and 38 KRAS-WT patients were enrolled. In KRAS-WT cohort, the median PFS was 2.1 and 2.4 months for erlotinib + selumetinib and erlotinib, respectively. Similarly, OS was 12.9 and 6.3 months, respectively. In KRAS-mutant cohort, the median PFS was 2.3 and 4.0 months for erlotinib + selumetinib and selumetinib, respectively. Similarly, OS was 21.8 and 10.5 months, respectively. In terms of safety, grade 3 and 4 toxicities were also increased in combination therapy, with diarrhea, dehydration, and fatigue all occurring in > 20% of patients [59]. TATTON was initially designed as a phase Ib trial to assess the safety and tolerability of osimertinib in combination with selumetinib, savolitinib, or durvalumab for EGFR-mutant lung cancer patients. Seventy-seven patients were enrolled in this study. The ORR was 42%, 44%, and 43% in selumetinib + osimertinib, savolitinib + osimertinib, and durvalumab + osimertinib arms, respectively. The most common AEs in selumetinib plus osimertinib group were diarrhea (75%), skin rash (58%), nausea (47%) [60]. Another phase I study evaluated the efficacy of afatinib plus selumetinib in patients with KRAS-mutant-positive solid tumors, 6 NSCLC patients included. Dose-limiting toxicities (DLTs) consisted of grade 3 diarrhea, decreased appetite, nausea/vomiting, dehydration, and mucositis. Stable disease for 221 days in a NSCLC patient was the best response [61]. In ESMO 2019 Congress, a phase I study evaluated the combination of lapatinib and trametinib for patients with KRAS-mutant solid tumors, 15 NSCLC patients included. One patient was confirmed partial response. Grade 3 AEs were diarrhea, rash, and nausea [62]. The clinical data showed that a number of trials were focused on detecting the strong rationale supporting combination therapy with MEK inhibitors for overcoming or delaying drug resistance in EGFR-mutant NSCLC. However, there are no EGFR-based combination therapies with global adoption, and therapies for patients with acquired resistance to EGFR-TKIs remain to be detected.
Mechanisms of resistance to MEK inhibitors
RAS/RAF/MEK/ERK signaling pathway-associated inhibitors have proven to be effective in treatment of various types of cancer, but have presented drug resistance in clinical application and MEK inhibitors as well. The resistance mechanisms to MEK inhibitors have not been detected clearly to date. However, studies concentrated on metastatic melanoma and other tumors showed some underlying mechanisms expected to be overlapped. A large number of MEK-acquired drug resistance mutations have been detected, such as the acquired concurrent MEK2-Q60P mutation and BRAF V600E amplification, which conferred resistance to MEK and BRAF inhibitors [63]. MEK1P124 and MEK1Q56P mutations were evaluated to be the mechanism of cross-resistance to PLX4720 (a selective BRAF inhibitor) and selumetinib [64]. Moreover, RAS can simultaneously induce ERK/MAPK and PI3K/AKT signaling pathways to induce drug resistance to MEK inhibitors. In preclinical studies [65,66,67,68,69], the combination of inhibitors, such as mTOR, PI3K, AKT/Raf, and dual inhibitors of RTK/MAPK and PI3K/AKT signaling pathways was proved to be effective to overcome drug resistance of MEK inhibitors. Besides, tumor microenvironment (TME) has been detected to play a pivotal role in promotion of the targeted therapy resistance as well [70].
Other combined therapies and ongoing studies
As acquired resistance becomes a frequent problem for all the target agents, a number of clinical trials have been designed to evaluate the efficacy and safety of combination of two different types of targets plus MEK inhibitors, according to the probable resistance mechanisms in the former part. A preclinical experiment revealed that selumetinib combined with BEZ235 (PI3K/mTOR inhibitor) markedly enhanced their antitumor effects and inhibited the tumor growth of NCI-H1993 in gefitinib-resistant NSCLC xenograft models [71]. Other ongoing clinical trials on administration of MEK inhibitors for NSCLC patients have been summarized (Table 4). To date, a variety of MEK1/2 inhibitors have been applied for different types of cancer, including NSCLC at various stages of clinical testing. The publication of the final results of these studies is still awaited.
Conclusions/expectations
The functions of EGFR-TKIs, checkpoint inhibitors, and traditional chemotherapy have been widely studied in NSCLC patients, while the role of MEK inhibitors in the treatment of lung cancer has not been clearly described. A number of clinical trials explored the clinical application of MEK inhibitors, and combination therapy has demonstrated promising outcomes. The brief summarization of MEK inhibitors in the selected clinical trials with NSCLC can be found in Table 5.
At the early stage, MEK inhibitors’ monotherapy had been detected a lot but seemed not to be effective for NSCLC patients for its poor efficacy and higher toxicities. No matter compared with pemetrexed or docetaxel, no significant difference in median PFS or OS was observed and dermatitis acneiform, hypertension, and diarrhea toxicities were more common [36,37,38,39].
MEK inhibitors in combination with BRAF inhibitors as a treatment demonstrated an improved efficacy for NSCLC patients. Currently, trametinib combined with dabrafenib has been the only therapy approved by the United States Food and Drug Administration (FDA) and European Medicines Agency (EMA) for the treatment of BRAF V600E-mutant NSCLC patients, which has been written into the National Comprehensive Cancer Network (NCCN) guidelines as well. The phase II trials in previously treated and untreated BRAF V600E-mutant NSCLC demonstrated that PFS and OS were longer and ORR was higher, which were much better than the outcomes of single-agent BRAF inhibitor in the previous study [40, 41, 72]. According to these trials, NCCN guidelines recommend that dabrafenib combined with trametinib be the first-line and subsequent therapy for BRAF V600E mutation-positive NSCLC patients. However, the challenge with this combination is the emergence of drug resistance and no effective treatment strategy to overcome it yet. Another challenge in targeted therapy for non-V600E mutation patients is still lacking.
Chemotherapy plus MEK inhibitors have showed obscure clinical outcomes to date. Some trials demonstrated that this combination therapy had the trend of longer PFS, OS, and higher ORR, but with no significant difference, especially in the SELECT series trials [47, 49, 50]. Other trails [44,45,46] tried to do some exploration in subgroup NSCLC patients, such as KRAS-mutant and KRAS-WT patients. Regrettably, different chemotherapy drugs seemed to influence the outcomes as well. A phase 1/1b study showed that ORR was 18% versus 24% in KRAS-WT and KRAS-mutant patients in trametinib plus docetaxel group, while ORR was 17% versus 11% KRAS-WT and KRAS-mutant patients in trametinib plus pemetrexed group [46]. Chemotherapy applied concurrently with MEK inhibitors requires further specific validation including the different chemotherapy agents, KRAS or other gene mutations and different MEK inhibitors before this combination strategy can become a standard treatment option for NSCLC patients.
Based on the preclinical studies, MEK inhibitors could improved T cell activation, conditioned the tumor microenvironment to facilitate improved response to anti-CTLA-4 treatment and prolonged survival in KRAS-mutant mice in combination with CTLA-4 blockade [54, 55]. However, the current relevant clinical trials of ICIs plus MEK inhibitors were not sufficient to draw the conclusion yet. Since only a phase Ib study [56] investigated the safety and efficacy of cobimetinib plus atezolizumab in a single arm for few NSCLC patients and several PD-1/L1 inhibitors plus MEK inhibitors clinical trials [57] (Table 4) are still ongoing, the finial clinical outcomes are worthy being looking forward to furthermore.
Although targeted therapy has dramatically changed our approach to treating NSCLC, the emergency of drug resistance and the lack of effective treatments to some special target such as KRAS still affect the prognosis of NSCLC patients. Preclinical data showed that MEK inhibitors plus EGFR-TKIs could inhibit cell proliferation significantly of EGFR-TKIs-resistant cells, while similar clinical trials have not been designed yet [58]. Current clinical trials [59,60,61,62] focused on EGFR-TKIs, including erlotinib, osimertinib, and afatinib, in combination with MEK inhibitors appearing somewhat illusory for OS, PFS or ORR. These outcomes seemed not to be improved under this strategy and more obvious toxicities were revealed. Further researches should be designed more on the administration way to combine two or three drugs together to optimize the therapeutic effect in appropriate subset patients.
In addition to the emerging drugs and clinical studies mentioned above, there are still many more new treatment combinations that have conducted in early stages of clinical development. Novel combination drugs can be broadly classified as BRAF inhibitors, EGFR-TKIs, multi-target tyrosine kinase inhibitors, CDK4/6 inhibitors, ALK inhibitors, platin-based chemotherapy, and ICIs. Additionally, many treatment combinations being explored in early-stage clinical studies, such as PI3K and AKT inhibitors should be further detected in a more rational way with MEK inhibitors in human bodies [73,74,75,76,77] (Fig. 2). The preclinical data indicated that the combined therapy of MEK and PI3K inhibitors has presented promising outcomes for NSCLC patients with the acquired resistance to EGFR-TKIs [78], but more clinical effects should be validated in the future. Overall, there seems to be hope on the horizon for NSCLC patients administrated with MEK inhibitors combined with other promising agents to improve patient outcomes finally.
Availability of data and materials
Not applicable as no datasets were generated or analyzed.
Abbreviations
- NSCLC:
-
Non-small cell lung cancer
- RTK:
-
Receptor tyrosine kinase
- GRB:
-
Growth factor receptor bound protein
- SOS:
-
Son of Sevenless homolog
- GDP:
-
Guanosine diphosphate
- GTP:
-
Guanosine triphosphate
- RAS:
-
Rat sarcoma viral oncogene
- RAF:
-
V-raf murine sarcoma viral oncogene
- MEK:
-
Mitogen-activated protein kinase kinase
- ERK:
-
Extracellular signal-regulated kinase
- PI3K:
-
Phosphatidylinositol 3-kinase
- AKT:
-
Protein kinase B
- mTOR:
-
Mammalian target of rapamycin
- NF-kB:
-
Nuclear factor-kB
- NA:
-
Non-available
- ORR:
-
Objective response rate
- OS:
-
Overall survival
- PFS:
-
Progression-free survival
- HR:
-
Hazard ratio
- MEKi:
-
MEK inhibitors
- BRAFi:
-
BRAF inhibitors
- CT:
-
Chemotherapy
- ICI:
-
Immune checkpoint inhibitors
- EGFR-TKI:
-
Epidermal growth factor receptor tyrosine kinase inhibitors
- DLTs:
-
Dose-limiting toxicities
- NCCN:
-
National Comprehensive Cancer Network
- FDA:
-
The United States Food and Drug Administration
- EMA:
-
European Medicines Agency
References
Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424.
Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin. 2017;67(1):7–30.
Molina JR, Yang P, Cassivi SD, et al. Non-small cell lung cancer: epidemiology, risk factors, treatment, and survivorship. Mayo Clin Proc. 2008;83(5):584–94.
N A Howlader N, Krapcho M, Miller D, Bishop K, Kosary Cl, Yu M, Ruhl J, Tatalovich Z, Mariotto A, Lewis DR, Chen HS, Feuer EJ, Cronin KA (Eds). SEER Cancer Statistics Review, 1975–2014. National Cancer Institute. 2016.
Marchetti A, Felicioni L, Malatesta S, et al. Clinical features and outcome of patients with non-small-cell lung cancer harboring BRAF mutations. J Clin Oncol. 2011;29(26):3574–9.
Larkin J, Ascierto PA, Dreno B, et al. Combined vemurafenib and cobimetinib in BRAF-mutated melanoma. N Engl J Med. 2014;371(20):1867–76.
Robert C, Karaszewska B, Schachter J, et al. Improved overall survival in melanoma with combined dabrafenib and trametinib. N Engl J Med. 2015;372(1):30–9.
Martinelli E, Morgillo F, Troiani T, et al. Cancer resistance to therapies against the EGFR-RAS-RAF pathway: the role of MEK. Cancer Treat Rev. 2017;53:61–9.
Sanchez JN, Wang T, Cohen MS. BRAF and MEK inhibitors: use and resistance in BRAF-mutated cancers. Drugs. 2018;78(5):549–66.
Ricciuti B, Leonardi GC, Metro G, et al. Targeting the KRAS variant for treatment of non-small cell lung cancer: potential therapeutic applications. Expert Rev Respir Med. 2016;10(1):53–68.
Fischmann TO, Smith CK, Mayhood TW, et al. Crystal structures of MEK1 binary and ternary complexes with nucleotides and inhibitors. Biochemistry. 2009;48(12):2661–74.
Shaul YD, Seger R. The MEK/ERK cascade: from signaling specificity to diverse functions. Biochim Biophys Acta. 2007;1773(8):1213–26.
Fukuda M, Gotoh Y, Nishida E. Interaction of MAP kinase with MAP kinase kinase: its possible role in the control of nucleocytoplasmic transport of MAP kinase. EMBO J. 1997;16(8):1901–8.
Takekawa M, Tatebayashi K, Saito H. Conserved docking site is essential for activation of mammalian MAP kinase kinases by specific MAP kinase kinase kinases. Mol Cell. 2005;18(3):295–306.
Guo YJ, Pan WW, Liu SB, et al. ERK/MAPK signalling pathway and tumorigenesis. Exp Ther Med. 2020;19(3):1997–2007.
Chang L, Karin M. Mammalian MAP kinase signalling cascades. Nature. 2001;410(6824):37–40.
Downward J. Targeting RAS signalling pathways in cancer therapy. Nat Rev Cancer. 2003;3(1):11–22.
Hong SK, Wu PK, Karkhanis M, et al. ERK1/2 can feedback-regulate cellular MEK1/2 levels. Cell Signal. 2015;27(10):1939–48.
U.S. Food and Drug Administration. FDA-approved drugs: trametinib. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/204114s016lbl.pdf
U.S. Food and Drug Administration. FDA-approved drugs: selumetinib. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/213756s000lbl.pdf
U.S. Food and Drug Administration. FDA-approved drugs: cobimetinib. https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/206192s002lbl.pdf
U.S. Food and Drug Administration. FDA-approved drugs: binimetinib. https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/210498s001lbl.pdf
Yeh TC, Marsh V, Bernat BA, et al. Biological characterization of ARRY-142886 (AZD6244), a potent, highly selective mitogen-activated protein kinase kinase 1/2 inhibitor. Clin Cancer Res. 2007;13(5):1576–83.
Gilmartin AG, Bleam MR, Groy A, et al. GSK1120212 (JTP-74057) is an inhibitor of MEK activity and activation with favorable pharmacokinetic properties for sustained in vivo pathway inhibition. Clin Cancer Res. 2011;17(5):989–1000.
Lee PA, Wallace E, Marlow A, et al. Abstract 2515: preclinical development of ARRY-162, a potent and selective MEK 1/2 inhibitor. Cancer Res 2014.
Hoeflich KP, Merchant M, Orr C, et al. Intermittent administration of MEK inhibitor GDC-0973 plus PI3K inhibitor GDC-0941 triggers robust apoptosis and tumor growth inhibition. Cancer Res. 2012;72(1):210–9.
Cheng Y, Tian H. Current development status of MEK inhibitors. Molecules. 2017;22(10):1551.
Pheneger J, Wallace E, Marlow A, et al. Characterization of ARRY-438162, a potent MEK inhibitor in combination with methotrexate or ibuprofen in in vivo models of arthritis. In: Proceedings of the 2006 annual scientific meeting, Boston, MA, USA, 20–24 October 2006; p. 794.
Rice KD, Aay N, Anand NK, et al. Novel carboxamide-based allosteric MEK inhibitors: discovery and optimization efforts toward XL518 (GDC-0973). ACS Med Chem Lett. 2012;3(5):416–21.
Inaba K, Oda K, Ikeda Y, et al. Antitumor activity of a combination of dual PI3K/mTOR inhibitor SAR245409 and selective MEK1/2 inhibitor pimasertib in endometrial carcinomas. Gynecol Oncol. 2015;138(2):323–31.
Ciuffreda L, Del Bufalo D, Desideri M, et al. Growth-inhibitory and antiangiogenic activity of the MEK inhibitor PD0325901 in malignant melanoma with or without BRAF mutations. Neoplasia. 2009;11(8):720–31.
Iverson C, Larson G, Lai C, et al. RDEA119/BAY 869766: a potent, selective, allosteric inhibitor of MEK1/2 for the treatment of cancer. Cancer Res. 2009;69(17):6839–47.
Hatzivassiliou G, Haling JR, Chen H, et al. Mechanism of MEK inhibition determines efficacy in mutant KRAS- versus BRAF-driven cancers. Nature. 2013;501(7466):232–6.
Takahashi RH, Ma S, Robinson SJ, et al. Elucidating the mechanisms of formation for two unusual cytochrome P450-mediated fused ring metabolites of GDC-0623, a MAPK/ERK kinase inhibitor. Drug Metab Dispos. 2015;43(12):1929–33.
Martinez-Garcia M, Banerji U, Albanell J, et al. First-in-human, phase I dose-escalation study of the safety, pharmacokinetics, and pharmacodynamics of RO5126766, a first-in-class dual MEK/RAF inhibitor in patients with solid tumors. Clin Cancer Res. 2012;18(17):4806–19.
Hainsworth JD, Cebotaru CL, Kanarev V, et al. A phase II, open-label, randomized study to assess the efficacy and safety of AZD6244 (ARRY-142886) versus pemetrexed in patients with non-small cell lung cancer who have failed one or two prior chemotherapeutic regimens. J Thorac Oncol. 2010;5(10):1630–6.
Haura EB, Ricart AD, Larson TG, et al. A phase II study of PD-0325901, an oral MEK inhibitor, in previously treated patients with advanced non-small cell lung cancer. Clin Cancer Res. 2010;16(8):2450–7.
Blumenschein GR Jr, Smit EF, Planchard D, et al. A randomized phase II study of the MEK1/MEK2 inhibitor trametinib (GSK1120212) compared with docetaxel in KRAS-mutant advanced non-small-cell lung cancer (NSCLC)†. Ann Oncol. 2015;26(5):894–901.
Lopez-Chavez A, Thomas A, Rajan A, et al. Molecular profiling and targeted therapy for advanced thoracic malignancies: a biomarker-derived, multiarm, multihistology phase II basket trial. J Clin Oncol. 2015;33(9):1000–7.
Planchard D, Besse B, Groen HJM, et al. Dabrafenib plus trametinib in patients with previously treated BRAF(V600E)-mutant metastatic non-small cell lung cancer: an open-label, multicentre phase 2 trial. Lancet Oncol. 2016;17(7):984–93.
Planchard D, Smit EF, Groen HJM, et al. Dabrafenib plus trametinib in patients with previously untreated BRAF(V600E)-mutant metastatic non-small-cell lung cancer: an open-label, phase 2 trial. Lancet Oncol. 2017;18(10):1307–16.
Salama AKS, Li S, Macrae ER, et al. Dabrafenib and trametinib in patients with tumors with BRAF(V600E) mutations: results of the NCI-MATCH trial subprotocol H. J Clin Oncol. 2020;38(33):3895–904.
Jänne PA, Shaw AT, Pereira JR, et al. Selumetinib plus docetaxel for KRAS-mutant advanced non-small-cell lung cancer: a randomised, multicentre, placebo-controlled, phase 2 study. Lancet Oncol. 2013;14(1):38–47.
Melosky B, Bradbury P, Tu D, et al. Selumetinib in patients receiving standard pemetrexed and platinum-based chemotherapy for advanced or metastatic KRAS wildtype or unknown non-squamous non-small cell lung cancer: A randomized, multicenter, phase II study. Canadian Cancer Trials Group (CCTG) IND.219. Lung Cancer. 2019;133:48–55.
Jänne PA, Smith I, Mcwalter G, et al. Impact of KRAS codon subtypes from a randomised phase II trial of selumetinib plus docetaxel in KRAS mutant advanced non-small-cell lung cancer. Br J Cancer. 2015;113(2):199–203.
Gandara DR, Leighl N, Delord JP, et al. A phase 1/1b study evaluating trametinib plus docetaxel or pemetrexed in patients with advanced non-small cell lung cancer. J Thorac Oncol. 2017;12(3):556–66.
Jänne PA, Van Den Heuvel MM, Barlesi F, et al. Selumetinib plus docetaxel compared with docetaxel alone and progression-free survival in patients with KRAS-mutant advanced non-small cell lung cancer: the SELECT-1 randomized clinical trial. JAMA. 2017;317(18):1844–53.
Kaufman J, Stinchcombe TE. Treatment of KRAS-mutant non-small cell lung cancer: the end of the beginning for targeted therapies. JAMA. 2017;317(18):1835–7.
Soria JC, Fülöp A, Maciel C, et al. SELECT-2: a phase II, double-blind, randomized, placebo-controlled study to assess the efficacy of selumetinib plus docetaxel as a second-line treatment of patients with advanced or metastatic non-small-cell lung cancer. Ann Oncol. 2017;28(12):3028–36.
Greystoke A, Steele N, Arkenau HT, et al. SELECT-3: a phase I study of selumetinib in combination with platinum-doublet chemotherapy for advanced NSCLC in the first-line setting. Br J Cancer. 2017;117(7):938–46.
Seto T, Hirai F, Saka H, et al. Safety and tolerability of selumetinib as a monotherapy, or in combination with docetaxel as second-line therapy, in Japanese patients with advanced solid malignancies or non-small cell lung cancer. Jpn J Clin Oncol. 2018;48(1):31–42.
Rittmeyer A, Barlesi F, Waterkamp D, et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet. 2017;389(10066):255–65.
Herbst RS, Baas P, Kim DW, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet. 2016;387(10027):1540–50.
Choi H, Deng J, Li S, et al. Pulsatile MEK inhibition improves anti-tumor immunity and T cell function in murine Kras mutant lung cancer. Cell Rep. 2019;27(3):806-819.e805.
Poon E, Mullins S, Watkins A, et al. The MEK inhibitor selumetinib complements CTLA-4 blockade by reprogramming the tumor immune microenvironment. J Immunother Cancer. 2017;5(1):63.
Hellmann MD, Kim TW, Lee CB, et al. Phase Ib study of atezolizumab combined with cobimetinib in patients with solid tumors. Ann Oncol. 2019;30(7):1134–42.
Gaudreau PO, Lee JJ, Heymach JV, et al. Phase I/II trial of immunotherapy with durvalumab and tremelimumab with continuous or intermittent MEK inhibitor selumetinib in NSCLC: early trial report. Clin Lung Cancer. 2020;21(4):384–8.
Li S, Chen S, Jiang Y, et al. Synergistic interaction between MEK inhibitor and gefitinib in EGFR-TKI-resistant human lung cancer cells. Oncol Lett. 2015;10(4):2652–6.
Carter CA, Rajan A, Keen C, et al. Selumetinib with and without erlotinib in KRAS mutant and KRAS wild-type advanced nonsmall-cell lung cancer. Ann Oncol. 2016;27(4):693–9.
Oxnard GR, Yang JC, Yu H, et al. TATTON: a multi-arm, phase Ib trial of osimertinib combined with selumetinib, savolitinib, or durvalumab in EGFR-mutant lung cancer. Ann Oncol. 2020;31(4):507–16.
Asco. Phase I study of afatinib plus selumetinib in patients with KRAS mutation-positive colorectal, non-small cell lung and pancreatic cancer. 2020.
Esmo. Phase I study of lapatinib and trametinib in patients with KRAS mutant colorectal, non-small cell lung and pancreatic cancer. 2019.
Villanueva J, Infante JR, Krepler C, et al. Concurrent MEK2 mutation and BRAF amplification confer resistance to BRAF and MEK inhibitors in melanoma. Cell Rep. 2013;4(6):1090–9.
Emery CM, Vijayendran KG, Zipser MC, et al. MEK1 mutations confer resistance to MEK and B-RAF inhibition. Proc Natl Acad Sci U S A. 2009;106(48):20411–6.
Ciuffreda L, McCubrey JA, Milella M. Signaling intermediates (PI3K/PTEN/AKT/mTOR and RAF/MEK/ERK Pathways) as therapeutic targets for anti-cancer and anti-angiogenesis treatments. Curr Signal Transduct Ther. 2009;4(2):130–43.
Yao W, Yue P, Zhang G, et al. Enhancing therapeutic efficacy of the MEK inhibitor, MEK162, by blocking autophagy or inhibiting PI3K/Akt signaling in human lung cancer cells. Cancer Lett. 2015;364(1):70–8.
Meng J, Dai B, Fang B, et al. Combination treatment with MEK and AKT inhibitors is more effective than each drug alone in human non-small cell lung cancer in vitro and in vivo. PLoS ONE. 2010;5(11):e14124.
Engelman JA, Chen L, Tan X, et al. Effective use of PI3K and MEK inhibitors to treat mutant Kras G12D and PIK3CA H1047R murine lung cancers. Nat Med. 2008;14(12):1351–6.
Li H, Schmid-Bindert G, Wang D, et al. Blocking the PI3K/AKT and MEK/ERK signaling pathways can overcome gefitinib-resistance in non-small cell lung cancer cell lines. Adv Med Sci. 2011;56(2):275–84.
Falcone I, Conciatori F, Bazzichetto C, et al. Tumor microenvironment: implications in melanoma resistance to targeted therapy and immunotherapy. Cancers (Basel). 2020;12(10):2870.
Qu Y, Wu X, Yin Y, et al. Antitumor activity of selective MEK1/2 inhibitor AZD6244 in combination with PI3K/mTOR inhibitor BEZ235 in gefitinib-resistant NSCLC xenograft models. J Exp Clin Cancer Res. 2014;33:52.
Planchard D, Kim TM, Mazieres J, et al. Dabrafenib in patients with BRAF(V600E)-positive advanced non-small-cell lung cancer: a single-arm, multicentre, open-label, phase 2 trial. Lancet Oncol. 2016;17(5):642–50.
Tolcher AW, Kurzrock R, Valero V, et al. Phase I dose-escalation trial of the oral AKT inhibitor uprosertib in combination with the oral MEK1/MEK2 inhibitor trametinib in patients with solid tumors. Cancer Chemother Pharmacol. 2020;85(4):673–83.
Shapiro GI, Lorusso P, Cho DC, et al. A phase Ib open-label dose escalation study of the safety, pharmacokinetics, and pharmacodynamics of cobimetinib (GDC-0973) and ipatasertib (GDC-0068) in patients with locally advanced or metastatic solid tumors. Invest New Drugs 2020.
Bardia A, Gounder M, Rodon J, et al. Phase Ib study of combination therapy with MEK inhibitor binimetinib and phosphatidylinositol 3-kinase inhibitor buparlisib in patients with advanced solid tumors with RAS/RAF alterations. Oncologist. 2020;25(1):e160–9.
Shapiro GI, Lorusso P, Kwak E, et al. Phase Ib study of the MEK inhibitor cobimetinib (GDC-0973) in combination with the PI3K inhibitor pictilisib (GDC-0941) in patients with advanced solid tumors. Invest New Drugs. 2020;38(2):419–32.
Ramanathan RK, Von Hoff DD, Eskens F, et al. Phase Ib trial of the PI3K inhibitor copanlisib combined with the allosteric MEK inhibitor refametinib in patients with advanced cancer. Target Oncol. 2020;15(2):163–74.
Sato H, Yamamoto H, Sakaguchi M, et al. Combined inhibition of MEK and PI3K pathways overcomes acquired resistance to EGFR-TKIs in non-small cell lung cancer. Cancer Sci. 2018;109(10):3183–96.
Acknowledgements
Not applicable.
Funding
This work was supported by a grant from the Medical Science and Technology Foundation of Henan Province (No. 201601026) and in part by the National Natural Science Foundation of China (No. 81272600), Henan Provincial Training Abroad Foundation for Leaders of Medical Science (No. 201082), Henan Provincial Special Funds for Health and Technological Innovative Talents (No. 2011020155), Henan Provincial Research Program of Application Foundation and Advanced Technology (No. 112300410033), a project co-sponsored by the Henan Province and Ministry of Health of Medical Science and Technology Program (No. 201601026), the 51282 project Leading Talent of Henan Provincial Health Science and Technology Innovation Talents (No. [2016]32), and Wu Jieping Medical Foundation for Clinical Research (No. 320.6799.15018). It was also supported by the Program for Science and Technology Innovation Talents in Universities of Henan Province (No. 18HASTIT044). The funders had no role in the study design, data collection/analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
All of the authors participated in the discussion and development of consensus management approaches and contributed to correcting the draft manuscript and providing additional recommendations. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Written informed consent for publication was obtained from all participants.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Han, J., Liu, Y., Yang, S. et al. MEK inhibitors for the treatment of non-small cell lung cancer. J Hematol Oncol 14, 1 (2021). https://doi.org/10.1186/s13045-020-01025-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13045-020-01025-7