Abstract
Non-coding RNAs (ncRNAs) have been the focus of many studies over the last few decades, and their fundamental roles in human diseases have been well established. Transfer RNAs (tRNAs) are housekeeping ncRNAs that deliver amino acids to ribosomes during protein biosynthesis. tRNA fragments (tRFs) are a novel class of small ncRNAs produced through enzymatic cleavage of tRNAs and have been shown to play key regulatory roles similar to microRNAs. Development and application of high-throughput sequencing technologies has provided accumulating evidence of dysregulated tRFs in cancer. Aberrant expression of tRFs has been found to participate in cell proliferation, invasive metastasis, and progression in several human malignancies. These newly identified functional tRFs also have great potential as new biomarkers and therapeutic targets for cancer treatment. In this review, we focus on the major biological functions of tRFs including RNA silencing, translation regulation, and epigenetic regulation; summarize recent research on the roles of tRFs in different types of cancer; and discuss the potential of using tRFs as clinical biomarkers for cancer diagnosis and prognosis and as therapeutic targets for cancer treatment.
Similar content being viewed by others
Introduction
The intricate molecular mechanisms of tumorigenesis and development have always been a prime focus for cancer research. In addition to protein-coding messenger RNAs (mRNAs), research over the past few decades has elucidated roles for non-coding RNAs (ncRNAs), including long non-coding RNAs (lncRNAs) and small non-coding RNAs (sncRNAs), in various biological processes. ncRNAs’ involvement in complex mechanisms that play crucial roles in the development and progression of cancers [1,2,3,4] has been elucidated, thus challenging the previous views of these molecules as merely transcriptional “junk.”
In recent years, with the development of high-throughput sequencing technology and improvements in bioinformatics analysis, a new class of sncRNAs derived from tRNAs has been discovered. These tRNA-derived ncRNAs are called tRNA fragments (tRFs). Far from being random tRNA degradation products [5,6,7], the biogenesis of tRFs is actually controlled by a set of highly conservative and precise site-specific cutting mechanisms that produce transcripts that are 14–50 nucleotides in length [8,9,10,11].
There is increasing evidence that tRFs can regulate gene expression at transcriptional and post-transcriptional levels. tRFs participate in various molecular processes such as gene silencing, RNA processing, and protein translation and different physiological processes such as cell stress, cell growth, and cell differentiation [5, 12,13,14,15]. tRFs also play significant roles in various human diseases, including cancer [16, 17], neurodegenerative disease [18, 19], virus infection [20,21,22], metabolic disorder [23], and inflammation [24]. Here, we will review the biogenesis and discovery of tRFs and delineate some of the major biological functions of tRFs as well as recent reports on the roles of tRFs in different types of cancer. Finally, we will explore the potential of using tRFs as clinical biomarkers for cancer diagnosis and prognosis and therapeutic targets for cancer treatment.
Biogenesis and discovery of tRFs
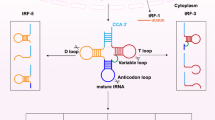
Generally, tRFs are defined and named according to the cleavage positions on pre- and mature tRNAs in various cell types and organisms. These tRFs can be roughly classified into four categories (Fig. 1). tRNAs undergo extensive processing and a series of chemical modifications in their life cycle. During tRNA maturation, the 3′-trailer sequences are removed from pre-tRNA by the endonuclease Z (RNase Z, ELAC2), which results in the production of 1-tRF [8, 25]. The other two classes of tRFs are generated from mature tRNAs: 5′-tRF as produced by cleavage of the 5′ end in the D-loop and 3′-tRF as produced through cleavage of the 3′ end in the T-loop. The final longer tRF type, 30–50 bases in length, are called tRNA-derived stress-induced RNAs (tiRNAs) or tRNA halves and are generated by specific cleavage in the anticodon loops of mature tRNAs by angiogenin (ANG) under stress conditions, such as hypoxia, starvation, virus infection, arsenite, heat shock, or heavy metal-induced cellular stress/toxicity [15, 26,27,28,29,30]. As a stress-activated and secreted ribonuclease, ANG can be transported to the cytoplasm under stress conditions. In the cytoplasm, it cleaves tRNAs to produce tiRNAs, which is highly related to tRNA modifications [30, 31].
Different types of tRNA-derived RNA fragments produced from either pre-tRNAs or mature tRNAs. The 1-tRF series is produced by RNase Z (or ELAC2) cleavage of the pre-tRNA during the tRNA processing. Mature tRNA can be cleaved in the anticodon loop by ANG to produce 5′-tiRNA and 3′-tiRNA series under stress conditions. The 5′-tRF series is derived from the 5′-end of mature tRNAs by endonucleolytic cleavage and exonuclease digestion in the D-loop. The cleavage in the T-loop results in the production of the 3′-tRF series
In recent years, the most commonly used techniques for identifying tRFs are deep sequencing [32, 33] and microarrays [34, 35]. Such large-scale discovery of tRFs has promoted the development of several tRF-related databases (Table 1). The first, tRFdb, was the first attempt to present the tRF sequences and read counts from eight species, including humans, and is available at http://genome.bioch.virginia.edu/trfdb/ [7]. Then came MINTbase, which is a repository tabulating tRF information that arises from the nucleic and mitochondrial tRNAs and is freely accessible at http://cm.jefferson.edu/MINTbase/. It contains information about sequence, expression abundance, parental tRNAs, and other genomic information [36]. Another study identified hundreds of distinct tRFs in the National Cancer Institute 60 (NCI-60) cell lines and TCGA tumor samples. The expression profile of these tRFs was compiled into a public database, tRFexplorer (https://trfexplorer.cloud/) [37]. Another web server, named tRF2Cancer (http://rna.sysu.edu.cn/tRFfinder/), can be used for identifying tRFs from small RNA sequencing datasets from various cancer types [10]. However, these databases only contain tRF expression across cancer types or focus on the identification of tRFs from small RNA sequencing data. We recently integrated large-scale small RNA sequencing, RNA sequencing, clinicopathologic datasets from TCGA, information from chemical modification sites on parental tRNAs, and from other validated literature manually curated from PubMed, and used these to construct a comprehensive database named OncotRF (http://bioinformatics.zju.edu.cn/OncotRF). OncotRF adopts a highly conserved filtering strategy in which only the tRFs with 10th quantile reads per million (RPM) > 1 are retained in the reported candidate list. This ensures robust results in downstream analysis. It provides a valuable tRF resource for users to identify diagnostic and prognostic biomarkers, develop cancer therapy, and study cancer pathogenesis [38].
Although standard deep sequencing methods are used to discover tRFs and quantify their abundance, it has been reported that tRNA (and tRF) has been heavily modified, and these chemical modifications may affect the detection and quantification of tRFs using standard sequencing methods [33, 39]. Several newly developed methods may overcome this obstacle such as AlkB-facilitated RNA methylation sequencing (ARM-seq) [40] and engineered demethylases-based tRNA sequencing (DM-tRNA-seq) [41]. Besides the classical northern blot assay, qRT-PCR was used to quantitatively analyze the abundance of individual tRFs after removing the tRNA modifications [23, 42]. We recently improved dumbbell-PCR (Db-PCR) to detect tRFs, which is a TaqMan qRT-PCR-based method and can distinctively quantify 5′ and 3′ end variants of RNA fragments at the single base resolution [43] (unpublished). In addition, most current studies usually ignore the important role of tRNA modifications when using synthetic RNAs to mimic endogenous tRFs. A recent study developed a simple method to isolate tiRNAs in vivo, which may facilitate functional studies of tiRNAs by addressing this modification problem [30].
Biological functions of tRFs
Though the biological functions of tRFs are complex and require further elucidation, our current knowledge of their function, as presented here, has been summarized into three categories: RNA silencing, translation regulation, and epigenetic regulation (Fig. 2). These three categories of tRF function have also been a key focus in cancer research.
Biological functions of tRFs. (A) RNA splicing. tRFs can affect RNA splicing by targeting the 3′-UTR regions of mRNAs or competitive binding of target mRNAs. (B) Translation regulation. YB-1 binding tRFs repress global translation by displacing translation eukaryotic initiation factor and induce assembly of SGs. tRFs can also regulate translation by interacting with ribosomes. (C) Epigenetic regulation. tRFs can inhibit LTR-retrotransposons or participate in non-coding RNA regulation
RNA silencing
Analysis of a human Photoactivatable-Ribonucleoside-Enhanced Crosslinking and Immunoprecipitation (PAR-CLIP) data has revealed that some 5′-tRFs and 3′-tRFs bind to Argonautes (AGO) in a manner similar to miRNAs, with the exception that they preferentially bind to AGO1, 3, and 4 rather than AGO2. Most 5′-tRFs and 3′-tRFs have been shown to interact with RNAs in the cells, suggesting that the majority of tRFs may play important roles in RNA interference (RNAi)-mediated silencing [9]. The observation that the regulation of tRF level has significant effects on the silencing activities of microRNAs (miRNAs) and small interfering RNAs (siRNAs), rather than on the abundance of miRNAs and siRNAs, also suggested tRF involvement in the global control of small RNA silencing [44]. In addition, 3′-tRFs are seen to post-transcriptionally repress genes in HEK293T cells. This 3′-tRF-mediated repression is Dicer-independent, but AGO-dependent, and targets are recognized by sequence complementarity [45]. All of these studies suggest that tRFs play a role in gene silencing by directly targeting mRNAs in a manner similar to miRNAs, or even competing with miRNAs to bind to their targets. Considering the difference between tRFs and miRNAs, a study has developed an unbiased approach that combines biochemical screening, analysis of gene expression data, and in silico prediction to identify tRF targets. It was found for the first time that the endogenous target of a 5′-tRF (derived from tRNA-Glu-CTC) can promote respiratory syncytial virus replication by targeting the 3′-UTR region of the APOER2 gene [20].
Another mechanism for gene silencing by tRFs is through competitive binding of target proteins with mRNAs. For example, unlike the above mechanism of tRF in viral infections, a chr10.tRNA2-Ser(TGA)-derived 1-tRF, tRF_U3_1, can bind directly to La/SSB protein and inhibit La/SSB-dependent viral gene expression [46]. This means that tRFs may have involvement in multiple different mechanisms within the same disease.
Translation regulation
Although tRNAs are essential components of translational machinery, the mode of translation regulation by tRFs is not simply the result of changes in the amount of mature tRNAs involved in the synthesis of proteins.
Stress-induced 5′-tiRNAs, but not 3′-tiRNAs, were found to inhibit protein synthesis [26] and trigger the phospho-elF2α-independent assembly of stress granules (SGs) [14]. Further investigations clarified that 5′-tiRNAs, such as 5′-tiRNAAla and 5′-tiRNACys, can repress global translation by displacing translation eukaryotic initiation factor eIF4G and eIF4A from mRNAs and displacing eIF4F from isolated m7G cap [47]. This 5′-tiRNA-associated translational silencer YB-1 contributes to the induction of SG assembly and stress-induced translational repression [47]. However, YB-1 was later noted to directly bind to tiRNAs by its cold shock domain, which is indispensable in packaging tiRNA-repressed mRNAs into SGs, but is not necessary for tiRNA-mediated translational inhibition [48].
A further important breakthrough came when Ivanov et al. showed in 2014 that translationally active 5′-tiRNAs assembled unique G-quadruplex structures that are essential for translational suppression [49]. Lyons’ group later added the observation that RNA G-quadruplex (RG4), a critical component of cellular stress response, is found to be required for functions of tRFs in the regulation of mRNA translation, and where the destruction of RG4 deprives tRFs of the ability to trigger the formation of SGs in vivo [50]. More recently still, Gkatza et al. showed that the loss of NSUN2, a cytosine-5 RNA methyltransferase, altered the biogenesis of tiRNAs in response to stress, leading to impaired regulation of protein synthesis [51].
In addition to tiRNAs, a universal conserved “GG” dinucleotide in 5′-tRFs is noted to also inhibit the process of protein translation [52]. tRFs can also regulate translation by interacting with ribosomes. One specific 3′-tRF derived from Leu-CAG tRNA (LeuCAG3’tsRNA) was seen to bind to a coding and non-coding 3′-UTR sequence in the ribosomal protein S28 (RPS28) mRNA to enhance its translation and ultimately the number of ribosomes. This may represent a conserved tRF-regulated translational mechanism among vertebrates [33, 53]. This ribosome-associated translation regulation mechanism is also observed in other invertebrate organisms, including the Trypanosoma brucei parasite [54] and the archaeon Haloferax volcanii [55].
Alternatively, epigenetic modifications can affect tRF’s role in translation control. For example, PUS7, the “writer” of pseudouridylation, can modify and activate a novel network of tRF targeting the translation initiation complex. Inactivation of PUS7 in embryonic stem cells weakens tRF-mediated translation regulation, leading to an increase of protein biosynthesis and the defect of germ layer specification [56].
Epigenetic regulation
The expression of biogenetic information is controlled by both DNA sequence and epigenetic information. Epigenetics regulate gene expression mainly through DNA methylation, histone modification, chromatin remodeling, and ncRNA regulation [57, 58]. Several studies have demonstrated that tRFs can regulate gene expression by affecting different epigenetic processes.
Transposable elements (TEs) and their repetitive sequences contribute to the formation and function of chromosomes, induce epigenetic regulation of specific genes, and drive transcription. However, TE mobility is driven by intact, active transposons and is highly mutagenic, necessitating tight control [59]. Transcription of TEs is generally repressed by epigenetic marks such as histone modification and DNA methylation. In the absence of epigenetic transcriptional suppression, 3′-tRFs can strongly inhibit long terminal repeat (LTR)-retrotransposon or endogenous retrovirus (ERV) activity in mice by targeting the highly conserved primer binding site of the LTR-retrotransposons [60].
tRFs can also participate in ncRNA regulation. tRNA methyltransferase Dnmt2 limits the extent of tRNA fragmentation in heat shock responses. The resulting tRFs can inhibit the activity of Dicer-2 on long double-stranded RNAs (dsRNAs). Consequently, heat-shocked Dnmt2 mutations lead to the accumulation of dsRNAs and the production of fewer siRNAs, resulting in dysregulation of siRNA pathway-dependent genes [61]. Recently, Boskovic et al. identified a specific 5′-tRF, tRF-GG, that plays a role in the production of a wide variety of small ncRNAs, the stability and activity of which were seen as dependent on Cajal bodies by directly binding to RNA-binding proteins hnRNPF/H. Importantly, the regulation of the U7 snRNA by tRF-GG regulated the heterochromatin-mediated transcriptional repression of endogenous retroelement MERVL elements by providing sufficient histone proteins [62].
Roles of tRFs in cancer
Growing evidence indicates that tRFs contribute to various biological processes associated with cancer development and progression. We will now proceed to describe recent advances in the study of biological functions and the underlying molecular mechanisms of dysregulated tRFs and the associated machinery in the pathogenesis of various types of cancers (Fig. 3 and Table 2).
Roles of tRFs in different types of cancer. tRFs are associated with many types of cancer including breast cancer, prostate cancer, leukemia, lung cancer, colorectal cancer, hepatocellular carcinoma, ovarian cancer, urinary bladder carcinoma, cervical carcinoma, uveal melanoma, and pancreatic cancer. These tRFs can play differing biological functions in different types of cancer
Breast cancer
A few tRFs have been noted as differentially expressed in breast cancers (BC) [35]. Many mRNAs are also differentially expressed between normal breast and triple-negative breast cancer (TNBC) in tandem with isomiR or tRF dysregulation [77]. All of these implicate tRFs as potential candidates for BC diagnostic and prognostic biomarkers and therapeutic targets. The role of tRFs in BC has received increasing attention over the past few years (Fig. 4).
Mechanisms of action of tRFs in breast cancer. Hypoxia-induced tRNAGlu, tRNAAsp, tRNAGly, and tRNATyr can interact with YBX1 and suppress breast cancer metastasis. Hypoxia-induced tDR-0009 and tDR-7336 can facilitate the doxorubicin resistance in triple-negative breast cancer cells. tRF3E can inhibit cell proliferation by binding with NCL. 5′-tiRNAVal inhibits breast cancer progression by directly targeting FZD3 3′-UTR sequence. RUNX1-regulated tRFs and sex hormone-dependent tiRNA (SHOT-RNAs) are associated with cell proliferation
Hypoxic stress induces the production of a new class of tRFs derived from tRNAGlu, tRNAAsp, tRNAGly, and tRNATyr. This class of tRFs can suppress the development of BC metastasis by binding to the oncogenic RNA-binding protein YBX1 and displacing multiple oncogenic transcripts such as EIF4EBP1 and AKT1. This results in the suppression of their stability [32]. tDR-0009 and tDR-7336, which were significantly upregulated under hypoxia conditions, have been found to facilitate the doxorubicin resistance in TNBC cells [63]. These data indicate that hypoxia-induced specific tRFs may act either as tumor suppressors or as a novel class of regulatory factors involved in hypoxia-induced chemoresistance.
Other tRFs that act as tumor suppressors have also been identified. tRF3E, a 3′-tRF derived from tRNA-GluTTC, is expressed in healthy mammary glands but not in BC. The level of serum tRF3E in patients with HER2-positive BC decreased with the increase of malignancy. As a tumor suppressor, tRF3E causes the release of p53 mRNA and further promotes p53 translation through competitive interactions with nucleolin (NCL), an RNA-binding protein overexpressed in BC, resulting in inhibition of cancer cell proliferation [64]. Another tRF, 5′-tiRNAVal, can inhibit the frizzled class receptor 3 (FZD3)-mediated Wnt/β-Catenin signaling pathway in BC cells by directly targeting the FZD3 3′-UTR sequence [65]. Its downregulation in serum was positively correlated with BC stage progression and lymph node metastasis.
Conversely, some tRFs have been found to act as oncogenes in BC. Sex hormone-dependent tRNA-derived RNAs (SHOT-RNAs) are specifically and abundantly expressed in ER-positive BC cell lines and patients. Similarly, 5′-SHOT-RNA can also enhance cell proliferation in BC cells [66]. Inhibition of ts-112, the expression of which is regulated by the tumor suppressor runt-related transcription factor 1 (RUNX1), can reduce the proliferative capacity of aggressive breast cancer cells while its overexpression promotes cell growth in normal-like mammary epithelial cells [67].
Prostate cancer
Small RNA sequencing of prostate cancer revealed a pronounced increase in tRFs in prostate cancers that occurred with metastatic lymph nodes compared with more organ-confined disease [78]. Analysis of a tRF profile in a large cohort of prostate adenocarcinoma (PRAD) patients from The Cancer Genome Atlas (TCGA) also found that tRFs have extensive correlations with mRNAs which were disrupted in PRAD. Interestingly, the profile of tRFs differed in patients of different races [79].
These findings provide clues for work on the elucidation of any putative roles of tRFs in PRAD carcinogenesis. Such a work has begun where, for example, one report details a specific 1-tRF called tRF-1001 which can act as an oncogene in PRAD. tRF-1001 is present in the cytoplasm and is produced by tRNA 3′-endonuclease ELAC2 (a prostate cancer susceptibility gene), and it is required for the proliferation of prostate cancer cells [68]. In addition, the expression of 5′-SHOT-RNA (as previously mentioned) is also seen as promoted by sex hormones and their receptors in androgen receptor (AR)-positive prostate cancer cell lines and has significant functional involvement in cell proliferation [66].
Leukemia
Studies have also begun to uncover tRF signatures in chronic lymphocytic leukemia (CLL). One such study demonstrated that ts-101, ts-53, ts-46, and ts-47 were all downregulated in CLL samples. ts-101 and ts-53 interact not only with Ago proteins but also with Piwil2, a protein involved in the silencing of transposons [35]. Similarly, another report revealed that ts-43 and ts-44, derived from distinct genes of pre-tRNAHis, as well as 5′-tRFs from mature tRNAHis, are all downregulated in CLL samples. Further investigations of the expression of tRFs in both aggressive CLL and indolent CLL revealed drastic dysregulation of the expression of mature tRFs in CLL [69]. These results suggest that tRFs are dysregulated and may have cancer-associated functions in CLL.
Several functional tRFs were also observed in other types of leukemia. For example, tRF-3019 perfectly matches the sequence of the primer binding site of human T cell leukemia virus type 1 (HTLV-1), the causative agent of adult T cell leukemia/lymphoma (ATLL). An in vitro reverse transcriptase assay further verified that tRF-3019 was capable of activating HTLV-1 reverse transcriptase and therefore may represent a new target to control HTLV-1 infection [70]. Another tRF, CU1276, was found abundant in normal germinal center B cells but absent in germinal center-derived lymphomas. Furthermore, the expression of CU1276 in a lymphoma cell line could inhibit cell proliferation and regulate the molecular response to DNA damage due to inhibition of endogenous replication protein A1 (RPA1), which is involved in many important aspects of DNA dynamics [71].
Lung cancer
At present, research on the role of tRFs in lung cancer is still lacking, especially related to their molecular mechanisms. However, the four tRFs of the tRF signatures in CLL mentioned above (ts-101, ts-53, ts-46, ts-47) are also seen to be downregulated in lung cancer. Overexpression of ts-46 and ts-47 in two lung cancer cell lines strongly inhibited the colony formation of cells, confirming that tRFs can affect lung cancer cell growth and survival [35].
The level of tRF-Leu-CAG was higher in human non-small cell lung cancer (NSCLC) tumor tissues than in normal tissues. Inhibition of tRF-Leu-CAG in H1299 cells suppressed cell proliferation and impeded the cell cycle, possibly due to the repression of aurora kinase A (AURKA). AURKA can be also directly targeted by miR-137 and miR-32 to affect the progression of NSCLC [72]. However, whether AURKA is a direct target of tRF-Leu-CAG and whether tRF-Leu-CAG can interact with miR-137 or miR-32 still require investigation.
Colorectal cancer
tRF-1001, as mentioned above, was also strongly associated with colon cancer cell proliferation. In the HCT-116 cell line, knockdown of tRF-1001 increased the proportion of cells in the G2 phase of the cell cycle and led to a significant decrease in cell viability [68]. A comprehensive small RNA sequencing study then identified 16 differentially expressed tRFs between colon cancer and paired paracancerous tissue. Meanwhile, 55 differentially expressed mRNAs were identified as potential targets of these tRFs and were noted as primarily enriched in vitamin metabolic pathways and the cyclic guanine monophosphate-protein kinase G signaling pathway [80].
Relating to the metastasis of colorectal cancer (CRC), one study demonstrated that tRF/miR-1280, derived from both tRNA-Leu and pre-miRNA, suppresses Notch/Gata and miR-200b signaling through a direct interaction with the 3′-UTR of Notch ligand JAG2 and plays important roles in CRC growth and metastasis [73]. Another study revealed that increased ANG to CRC growth and metastasis was due to the production of ANG-cleaved tiRNAs. Among them, a 5′-tiRNA from tRNA-Val was highly expressed in CRC patients and was closely related to tumor metastasis [74]. These results provide valuable cues for further insights into the roles of tRFs in colon cancer.
Other cancers
In addition to the abovementioned cancers, functional tRFs have been associated with several other types of cancer, but the amount and depth of these studies are rather limited and further research is needed.
In hepatocellular carcinomas (HCC), tRF_U3_1 is more abundant in the HCC cell line Huh7 and cancerous liver tissues compared to primary hepatocytes and normal liver tissues, which can inhibit viral gene expression and its precursors [46]. Whether the inhibition of viral gene expression by tRF_U3_1 has an impact on HCC still requires verification.
For ovarian cancer, it has been suggested that tRF-03357 promotes cell proliferation, migration, and invasion of high-grade serous ovarian cancer partly by downregulating HMBOX1 [75]. This also requires confirmation.
For urinary bladder carcinomas, one study isolated a tRF from the conditioned medium of human urinary bladder carcinoma cells, which can inhibit the growth of endothelial cells, demonstrating a new in vitro role for tRFs as a selective endothelial cell inhibitor [76].
A direct sequencing method was also used to characterize miRNA profiles and other small RNA species from six human cervical carcinoma cell lines and five normal cervical samples. A significant expression variation in the number of tRFs was observed among these sequencing samples [81]. Furthermore, by comprehensively characterizing the small RNA profiles in 80 primary uveal melanoma tumor samples, researchers showed that the abundance profiles of isomiRs and tRFs were correlated with various molecular phenotypes, metastatic disease, and patient survival [82]. Coincidentally, another tRF sequencing study screened out a total of 48 tRFs in clinical pancreatic cancer samples [83]. All of these tRF profiling studies suggest that further investigation and characterization of these differentially expressed tRFs will improve our understanding of the mechanisms underlying these cancers.
Potential clinical applications of tRFs
Although tRF researches are still in their infancy, tRFs have gradually become clinical biomarkers for cancer diagnosis and prognosis and therapeutic targets for cancer treatment. In this section, we will discuss the potential of tRFs in clinical application.
Clinical biomarkers for cancer diagnosis and prognosis
Early detection, diagnosis, and treatment are always considered a key towards an improved prognosis for most cancers. At present, clinical and pathological methods for predicting postoperative clinical results remain limited. Therefore, it is essential to find accurate biomarkers for the early diagnosis and accurate prognosis of cancer. Recently, extracellular RNAs are gaining more and more clinical attention as non-invasive biofluid-based markers for diseases, especially cancer. In particular, tRFs are emerging as such biomarkers with high potentials.
As early as 1979, scientists discovered that tRNA breakdown products could be used as cancer biomarkers. They found 7 tRNA breakdown products in the urine of 26 patients with 13 different malignancies. The level of the excretion of these factors varied with the stage of the disease [84]. Nevertheless, it was not until recent years, with the development of high-throughput sequencing technology, that the potential of tRFs as biomarkers has been further explored. Several studies have demonstrated that tRFs can be present in a rich complex in serum [85], or are highly abundant in human saliva [86]. In addition, tRFs can also be selectively exported into extracellular vesicles (EVs) [87]. Some 5′-tRFs and tiRNAs have been found in exosomes and are upregulated during stress responses and cancer [88], suggesting that tRFs can circulate in the blood in a stable form and can act as novel forms of signaling molecules. For example, one study identified the high abundance of tRFs in EVs derived uniquely from the MCF7 breast cancer cell line [89].
Increasing numbers of examples of abnormal tRF expression are being discovered in various cancers. They are easy to detect in bodily fluids, and some are clear indicators of important biological functions in cancers. For example, as mentioned above, the expression of tRF3E [64] and 5′-tiRNAVal [65] in the blood is related to the degree of malignancy of BC and may serve as diagnostic biomarkers. In particular, 5′-tiRNAVal can be used to distinguish differentiated BC from healthy controls, with a sensitivity of 90.0% and a specificity of 62.7% [65]. Meanwhile, the detection of the functional tRF-Leu-CAG in the serum of NSCLC is related to progression stage, suggesting that it may be a new diagnostic marker and potential therapeutic target for NSCLC [72]. Circulating tRF-Lys and another two miRNAs in EVs can discriminate early-stage BCs at stage 0 from controls with an AUC (area under the curve, a criterion for judging the pros and cons of a binary classification prediction model) of 0.92 [90]. All these studies indicated that tRFs have great potential as clinical biomarkers for cancer diagnosis and prognosis.
Therapeutic targets
As small molecules, certain tRF mimetics or antisense molecules against tRFs can be used as small molecule drugs [91], which makes certain tRFs also have great potential as therapeutic targets. For example, transfection of mimetics of four tRFs that bind YBX1 can significantly inhibit lung metastasis in vivo [32], indicating that mimetics of certain tRFs can be used to treat tumor progression and metastasis.
Furthermore, drug resistance has always been one of the main causes of cancer treatment failure. The causes of drug resistance are usually multifactorial, and there are still many challenges in improving the outcome of patients [92, 93]. In addition to the abovementioned tDR-0009 and tDR-7336 related to doxorubicin resistance [63], tRF-30-JZOYJE22RR33 and tRF-27-ZDXPHO53KSN were associated with trastuzumab resistance in HER-2-positive BC patients [42]. Compared with sensitive individuals, these two tRFs were upregulated in trastuzumab-resistant patients. The higher the expression level of tRF-30-JZOYJE22RR33 and tRF-27-ZDXPHO53KSN in HER-2-positive BC patients, the shorter the progression-free survival of the patient.
These functional tRFs with easy-to-detect properties may be used as potential biomarkers and intervention targets in clinical treatment. However, as a specific biomarker, it is still necessary to clarify the specificity and applicability of these tRFs among different types of cancer. As an efficient intervention target, the role and mechanism of these tRFs in cancer still needs to be fully explored.
Conclusion and future perspectives
Although the recognition of the existence of tRNA breakdown products as cancer markers has been noted since the 1970s [84], the exact function of tRFs in cancer has only recently been the subject of direct study. The application of high-throughput sequencing technology has led to the discovery of the aberrant expression of many tRFs in a variety of cancer types. Some of these tRFs have been collated and deposited in several databases, which in turn has facilitated further discovery and functional research relating to tRFs. tRFs are constitutively generated in human cells, in both normal and stress conditions, and their composition and abundances are shaped by many factors, including gender, population origin, tissue, and disease subtype [36]. Increasing evidence suggests that tRFs are involved in many aspects of cancer and can be used as potential non-invasive biomarkers for the diagnosis and prognosis of cancer.
According to our review, most of these functional tRFs can regulate gene expression and/or translation and affect cellular signaling cascades in order to regulate the proliferation, metastasis, and drug resistance of tumor cells. However, the mechanisms of how tRFs regulate carcinogenesis and progression are largely unknown. Meanwhile, the depth and breadth of the biological function of tRFs seem significantly different in different types of cancer. Considering the abnormal expression of many tRFs in most cancer types, we believe that these tRFs may also play important functions in these cancer types. Further functional research will help to identify tRFs as diagnostic and prognostic biomarkers and therapeutic targets for clinical application.
We also realize that the expression of tRFs varied greatly with different types of cancer, but the underlying mechanism for the differential expression of tRFs is still poorly understood. The discovery of SHOT-RNAs in breast and prostate cancer led to the suggestion that the status of the hormone receptors may impact the production of tRFs in hormone-dependent tumors [66]. In addition, hypoxia-induced tRFs play important roles in BC [32, 63] and other stress-induced tiRNAs are also found to be important to breast and colon cancer [65, 94]. Interestingly, a recent study showed that in several instances, the abundance of tRFs is regulated without changes in mature tRNA levels. This suggests that tRNA genes can be selectively expressed to regulate noncanonical tRNA functions performed by tRFs without compromising the mature tRNA pool [39]. The modification status of tRNA nucleotides can affect stress-induced endonuclease activities and have an important impact on the tRNA fragmentation process [95]. For example, the m1A demethylated tRNA was found to be more sensitive to ANG cleavage that generates tiRNAs [96]. Another recent study has demonstrated that the 2′-O-methylation at position 34 of human elongator tRNAMet(CAT) prohibits site-specific cleavage of tRNAMet (CAT) into tRFs by the stress-responsive endoribonuclease ANG [97]. All of these results reveal the complexity and challenge in the determination of exactly what conditions can cause tRF production, and whether this production is related to epigenetic modifications of tRNAs and/or to certain characteristics of cancer progression, and consequently how these tRNA/tRF modifications contribute to tumorigenesis and progression.
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Abbreviations
- tRNAs:
-
Transfer RNAs
- tRFs:
-
tRNA fragments
- mRNAs:
-
Messenger RNAs
- ncRNAs:
-
Non-coding RNAs
- lncRNAs:
-
Long non-coding RNAs
- sncRNAs:
-
Small non-coding RNAs
- tiRNAs:
-
tRNA-derived stress-induced RNAs
- ANG:
-
Angiogenin
- PAR-CLIP:
-
Photoactivatable-Ribonucleoside-Enhanced Crosslinking and Immunoprecipitation
- AGO:
-
Argonautes
- RNAi:
-
RNA interference
- miRNAs:
-
MicroRNAs
- siRNAs:
-
Small interfering RNAs
- SG:
-
Stress granules
- RG4:
-
RNA G-quadruplex
- RPS28:
-
Ribosomal protein S28
- TEs:
-
Transposable elements
- LTR:
-
Long terminal repeat
- ERV:
-
Endogenous retrovirus
- dsRNAs:
-
Double-stranded RNAs
- BC:
-
Breast cancers
- TNBC:
-
Triple-negative breast cancer
- NCL:
-
Nucleolin
- FZD3:
-
Frizzled class receptor 3
- SHOT-RNAs:
-
Sex hormone-dependent tRNA-derived RNAs
- RUNX1:
-
Runt-related transcription factor 1
- PRAD:
-
Prostate adenocarcinoma
- TCGA:
-
The Cancer Genome Atlas
- AR:
-
Androgen receptor
- CLL:
-
Chronic lymphocytic leukemia
- HTLV-1:
-
Human T cell leukemia virus type 1
- ATLL:
-
Adult T cell leukemia/lymphoma
- RPA1:
-
Replication protein A1
- NSCLC:
-
Non-small cell lung cancer
- AURKA:
-
Aurora kinase A
- CRC:
-
Colorectal cancer
- HCC:
-
Hepatocellular carcinomas
- EVs:
-
Extracellular vesicles
- AUC:
-
Area under the curve
- RPM:
-
Reads per million
- ROC:
-
Receiver operating characteristic
References
Anastasiadou E, Jacob LS, Slack FJ. Non-coding RNA networks in cancer. Nat Rev Cancer. 2018;18:5–18..
Iyer MK, Niknafs YS, Malik R, Singhal U, Sahu A, Hosono Y, et al. The landscape of long noncoding RNAs in the human transcriptome. Nat Genet. 2015;47:199–208.
Yang X, Liu M, Li M, Zhang S, Hiju H, Sun J, et al. Epigenetic modulations of noncoding RNA: a novel dimension of cancer biology. Mol Cancer. 2020;19:64.
Wolin SL, Maquat LE. Cellular RNA surveillance in health and disease. Science. 2019;366:822–7.
Guzzi N, Bellodi C. Novel insights into the emerging roles of tRNA-derived fragments in mammalian development. RNA Biol. 2020. https://doi.org/10.1080/15476286.2020.1732694:1-9.
Megel C, Hummel G, Lalande S, Ubrig E, Cognat V, Morelle G, et al. Plant RNases T2, but not Dicer-like proteins, are major players of tRNA-derived fragments biogenesis. Nucleic Acids Res. 2019;47:941–52.
Kumar P, Mudunuri SB, Anaya J, Dutta A. tRFdb: a database for transfer RNA fragments. Nucleic Acids Res. 2015;43:D141–5.
Gebetsberger J, Polacek N. Slicing tRNAs to boost functional ncRNA diversity. RNA Biol. 2013;10:1798–806.
Kumar P, Anaya J, Mudunuri SB, Dutta A. Meta-analysis of tRNA derived RNA fragments reveals that they are evolutionarily conserved and associate with AGO proteins to recognize specific RNA targets. BMC Biol. 2014;12:78.
Zheng LL, Xu WL, Liu S, Sun WJ, Li JH, Wu J, et al. tRF2Cancer: a web server to detect tRNA-derived small RNA fragments (tRFs) and their expression in multiple cancers. Nucleic Acids Res. 2016;44:W185–93.
Shen Y, Yu X, Zhu L, Li T, Yan Z, Guo J. Transfer RNA-derived fragments and tRNA halves: biogenesis, biological functions and their roles in diseases. J Cell Mol Med. 2018;96:1167–76.
Kumar P, Kuscu C, Dutta A. Biogenesis and function of transfer RNA-related fragments (tRFs). Trends Biochem Sci. 2016;41:679–89.
Zhu L, Liu X, Pu W, Peng Y. tRNA-derived small non-coding RNAs in human disease. Cancer Lett. 2018;419:1–7.
Emara MM, Ivanov P, Hickman T, Dawra N, Tisdale S, Kedersha N, et al. Angiogenin-induced tRNA-derived stress-induced RNAs promote stress-induced stress granule assembly. J Biol Chem. 2010;285:10959–68.
Goncalves KA, Silberstein L, Li S, Severe N, Hu MG, Yang H, et al. Angiogenin promotes hematopoietic regeneration by dichotomously regulating quiescence of stem and progenitor cells. Cell. 2016;166:894–906.
Sun C, Fu Z, Wang S, Li J, Li Y, Zhang Y, et al. Roles of tRNA-derived fragments in human cancers. Cancer Lett. 2018;414:16–25.
Zhu L, Ge J, Li T, Shen Y, Guo J. tRNA-derived fragments and tRNA halves: the new players in cancers. Cancer Lett. 2019;452:31–7.
Schaffer Ashleigh E, Eggens Veerle RC, Caglayan Ahmet O, Reuter Miriam S, Scott E, Coufal Nicole G, et al. CLP1 founder mutation links tRNA splicing and maturation to cerebellar development and neurodegeneration. Cell. 2014;157:651–63.
Blanco S, Dietmann S, Flores JV, Hussain S, Kutter C, Humphreys P, et al. Aberrant methylation of tRNAs links cellular stress to neuro-developmental disorders. The EMBO J. 2014;33:2020–39.
Deng J, Ptashkin RN, Chen Y, Cheng Z, Liu G, Phan T, et al. Respiratory syncytial virus utilizes a tRNA fragment to suppress antiviral responses through a novel targeting mechanism. Mol Ther. 2015;23:1622–9.
Li Z, Ender C, Meister G, Moore PS, Chang Y, John B. Extensive terminal and asymmetric processing of small RNAs from rRNAs, snoRNAs, snRNAs, and tRNAs. Nucleic Acids Res. 2012;40:6787–99.
Wang Q, Lee I, Ren J, Ajay SS, Lee YS, Bao X. Identification and functional characterization of tRNA-derived RNA fragments (tRFs) in respiratory syncytial virus infection. Mol Ther. 2013;21:368–79.
Cosentino C, Toivonen S, Diaz Villamil E, Atta M, Ravanat J-L, Demine S, et al. Pancreatic β-cell tRNA hypomethylation and fragmentation link TRMT10A deficiency with diabetes. Nucleic Acids Res. 2018;46:10302–18.
Zhong F, Hu Z, Jiang K, Lei B, Wu Z, Yuan G, et al. Complement C3 activation regulates the production of tRNA-derived fragments Gly-tRFs and promotes alcohol-induced liver injury and steatosis. Cell Res. 2019;29:548–61.
Phizicky EM, Hopper AK. tRNA biology charges to the front. Genes Dev. 2010;24:1832–60.
Yamasaki S, Ivanov P, Hu GF, Anderson P. Angiogenin cleaves tRNA and promotes stress-induced translational repression. J Cell Biol. 2009;185:35–42.
Liu S, Chen Y, Ren Y, Zhou J, Ren J, Lee I, et al. A tRNA-derived RNA fragment plays an important role in the mechanism of arsenite-induced cellular responses. Sci Rep. 2018;8:16838.
Saikia M, Krokowski D, Guan BJ, Ivanov P, Parisien M, Hu GF, et al. Genome-wide identification and quantitative analysis of cleaved tRNA fragments induced by cellular stress. J Biol Chem. 2012;287:42708–25.
Saikia M, Hatzoglou M. The many virtues of tRNA-derived stress-induced RNAs (tiRNAs): discovering novel mechanisms of stress response and effect on human health. J Biol Chem. 2015;290:29761–8.
Akiyama Y, Kharel P, Abe T, Anderson P, Ivanov P. Isolation and initial structure-functional characterization of endogenous tRNA-derived stress-induced RNAs. RNA Biol. 2020;17:1116–24.
Prehn JHM, Jirstrom E. Angiogenin and tRNA fragments in Parkinson’s disease and neurodegeneration. Acta Pharmacol Sin. 2020;41:442–6.
Goodarzi H, Liu X, Nguyen HC, Zhang S, Fish L, Tavazoie SF. Endogenous tRNA-derived fragments suppress breast cancer progression via YBX1 displacement. Cell. 2015;161:790–802.
Kim HK, Fuchs G, Wang S, Wei W, Zhang Y, Park H, et al. A transfer-RNA-derived small RNA regulates ribosome biogenesis. Nature. 2017;552:57–62.
Pekarsky Y, Balatti V, Palamarchuk A, Rizzotto L, Veneziano D, Nigita G, et al. Dysregulation of a family of short noncoding RNAs, tsRNAs, in human cancer. Proc Natl Acad Sci U S A. 2016;113:5071–6.
Balatti V, Nigita G, Veneziano D, Drusco A, Stein GS, Messier TL, et al. tsRNA signatures in cancer. Proc Natl Acad Sci U S A. 2017;114:8071–6.
Pliatsika V, Loher P, Magee R, Telonis AG, Londin E, Shigematsu M, et al. MINTbase v2.0: a comprehensive database for tRNA-derived fragments that includes nuclear and mitochondrial fragments from all The Cancer Genome Atlas projects. Nucleic Acids Res. 2018;46:D152-D159.
La Ferlita A, Alaimo S, Veneziano D, Nigita G, Balatti V, Croce CM, et al. Identification of tRNA-derived ncRNAs in TCGA and NCI-60 panel cell lines and development of the public database tRFexplorer. Database (Oxford). 2019. https://doi.org/10.1093/database/baz115.
Yao D, Sun X, Zhou L, Amanullah M, Pan X, Liu Y, et al. OncotRF: an online resource for exploration of tRNA-derived fragments in human cancers. RNA Biol. 2020;17:1081–91.
Torres AG, Reina O, Stephan-Otto Attolini C, Ribas de Pouplana L. Differential expression of human tRNA genes drives the abundance of tRNA-derived fragments. Proc Natl Acad Sci U S A. 2019;116:8451–6.
Cozen AE, Quartley E, Holmes AD, Hrabeta-Robinson E, Phizicky EM, Lowe TM. ARM-seq: AlkB-facilitated RNA methylation sequencing reveals a complex landscape of modified tRNA fragments. Nat Methods. 2015;12:879–84.
Zheng G, Qin Y, Clark WC, Dai Q, Yi C, He C, et al. Efficient and quantitative high-throughput tRNA sequencing. Nat Methods. 2015;12:835–7.
Sun C, Yang F, Zhang Y, Chu J, Wang J, Wang Y, et al. tRNA-derived fragments as novel predictive biomarkers for trastuzumab-resistant breast cancer. Cell Physiol Biochem. 2018;49:419–31.
Honda S, Kirino Y. Dumbbell-PCR: a method to quantify specific small RNA variants with a single nucleotide resolution at terminal sequences. Nucleic Acids Res. 2015;43:e77.
Haussecker D, Huang Y, Lau A, Parameswaran P, Fire AZ, Kay MA. Human tRNA-derived small RNAs in the global regulation of RNA silencing. RNA. 2010;16:673–95.
Kuscu C, Kumar P, Kiran M, Su Z, Malik A, Dutta A. tRNA fragments (tRFs) guide Ago to regulate gene expression post-transcriptionally in a Dicer-independent manner. RNA. 2018;24:1093–105.
Cho H, Lee W, Kim GW, Lee SH, Moon JS, Kim M, et al. Regulation of La/SSB-dependent viral gene expression by pre-tRNA 3' trailer-derived tRNA fragments. Nucleic Acids Res. 2019;47:9888–901.
Ivanov P, Emara Mohamed M, Villen J, Gygi Steven P, Anderson P. Angiogenin-induced tRNA fragments inhibit translation initiation. Mol Cell. 2011;43:613–23.
Lyons SM, Achorn C, Kedersha NL, Anderson PJ, Ivanov P. YB-1 regulates tiRNA-induced stress granule formation but not translational repression. Nucleic Acids Res. 2016;44:6949–60.
Ivanov P, O’Day E, Emara MM, Wagner G, Lieberman J, Anderson P. G-quadruplex structures contribute to the neuroprotective effects of angiogenin-induced tRNA fragments. Proc Natl Acad Sci U S A. 2014;111:18201–6.
Lyons SM, Gudanis D, Coyne SM, Gdaniec Z, Ivanov P. Identification of functional tetramolecular RNA G-quadruplexes derived from transfer RNAs. Nat Commun. 2017;8:1127.
Gkatza NA, Castro C, Harvey RF, Heiss M, Popis MC, Blanco S, et al. Cytosine-5 RNA methylation links protein synthesis to cell metabolism. PLoS Biol. 2019;17:e3000297.
Sobala A, Hutvagner G. Small RNAs derived from the 5′ end of tRNA can inhibit protein translation in human cells. RNA Biol. 2013;10:553–63.
Kim HK, Xu J, Chu K, Park H, Jang H, Li P, et al. A tRNA-derived small RNA regulates ribosomal protein S28 protein levels after translation initiation in humans and mice. Cell Rep. 2019; 29:3816-3824 e4.
Fricker R, Brogli R, Luidalepp H, Wyss L, Fasnacht M, Joss O, et al. A tRNA half modulates translation as stress response in Trypanosoma brucei. Nat Commun. 2019;10:118.
Gebetsberger J, Wyss L, Mleczko AM, Reuther J, Polacek N. A tRNA-derived fragment competes with mRNA for ribosome binding and regulates translation during stress. RNA Biol. 2017;14:1364–73.
Guzzi N, Cieśla M, Ngoc PCT, Lang S, Arora S, Dimitriou M, et al. Pseudouridylation of tRNA-derived fragments steers translational control in stem cells. Cell. 2018;173:1204–1216.e26.
Henikoff S, Greally JM. Epigenetics, cellular memory and gene regulation. Current Biol. 2016;26:R644–8.
Dawson MA, Kouzarides T. Cancer epigenetics: from mechanism to therapy. Cell. 2012;150:12–27.
Slotkin RK, Martienssen R. Transposable elements and the epigenetic regulation of the genome. Nat Rev Genet. 2007;8:272–85.
Schorn AJ, Gutbrod MJ, LeBlanc C, Martienssen R. LTR-retrotransposon control by tRNA-derived small RNAs. Cell. 2017;170:61–71 e11.
Durdevic Z, Mobin Mehrpouya B, Hanna K, Lyko F, Schaefer M. The RNA methyltransferase Dnmt2 is required for efficient Dicer-2-dependent siRNA pathway activity in Drosophila. Cell Rep. 2013;4:931–7.
Boskovic A, Bing XY, Kaymak E, Rando OJ. Control of noncoding RNA production and histone levels by a 5′ tRNA fragment. Genes Dev. 2020;34:118–31.
Cui Y, Huang Y, Wu X, Zheng M, Xia Y, Fu Z, et al. Hypoxia-induced tRNA-derived fragments, novel regulatory factor for doxorubicin resistance in triple-negative breast cancer. J Cell Physiol. 2018;234:8740–51.
Falconi M, Giangrossi M, Zabaleta ME, Wang J, Gambini V, Tilio M, et al. A novel 3'-tRNA(Glu)-derived fragment acts as a tumor suppressor in breast cancer by targeting nucleolin. FASEB J. 2019;33:13228–40.
Mo D, Jiang P, Yang Y, Mao X, Tan X, Tang X, et al. A tRNA fragment, 5'-tiRNA(Val), suppresses the Wnt/beta-catenin signaling pathway by targeting FZD3 in breast cancer. Cancer Lett. 2019;457:60–73.
Honda S, Loher P, Shigematsu M, Palazzo JP, Suzuki R, Imoto I, et al. Sex hormone-dependent tRNA halves enhance cell proliferation in breast and prostate cancers. Proc Natl Acad Sci U S A. 2015;112:E3816–25.
Farina NH, Scalia S, Adams CE, Hong D, Fritz AJ, Messier TL, et al. Identification of tRNA-derived small RNA (tsRNA) responsive to the tumor suppressor, RUNX1, in breast cancer. J Cell Physiol. 2020;235:5318–27.
Lee YS, Shibata Y, Malhotra A, Dutta A. A novel class of small RNAs: tRNA-derived RNA fragments (tRFs). Genes Dev. 2009;23:2639–49.
Veneziano D, Tomasello L, Balatti V, Palamarchuk A, Rassenti LZ, Kipps TJ, et al. Dysregulation of different classes of tRNA fragments in chronic lymphocytic leukemia. Proc Natl Acad Sci U S A. 2019;116:24252–8.
Ruggero K, Guffanti A, Corradin A, Sharma VK, De Bellis G, Corti G, et al. Small noncoding RNAs in cells transformed by human T-cell leukemia virus type 1: a role for a tRNA fragment as a primer for reverse transcriptase. J Virol. 2014;88:3612–22.
Maute RL, Schneider C, Sumazin P, Holmes A, Califano A, Basso K, et al. tRNA-derived microRNA modulates proliferation and the DNA damage response and is down-regulated in B cell lymphoma. Proc Natl Acad Sci U S A. 2013;110:1404–9.
Shao Y, Sun Q, Liu X, Wang P, Wu R, Ma Z. tRF-Leu-CAG promotes cell proliferation and cell cycle in non-small cell lung cancer. Chem Biol & Drug Des. 2017;90:730–8.
Huang B, Yang H, Cheng X, Wang D, Fu S, Shen W, et al. tRF/miR-1280 Suppresses stem cell–like cells and metastasis in colorectal cancer. Cancer Res. 2017;77:3194–206.
Li S, Shi X, Chen M, Xu N, Sun D, Bai R, et al. Angiogenin promotes colorectal cancer metastasis via tiRNA production. Int J Cancer. 2019. https://doi.org/10.1002/ijc.32245.
Zhang M, Li F, Wang J, He W, Li Y, Li H, et al. tRNA-derived fragment tRF-03357 promotes cell proliferation, migration and invasion in high-grade serous ovarian cancer. Onco Targets Ther. 2019;12:6371–83.
Zhao H, Bojanowski K, Ingber DE, Panigrahy D, Pepper MS, Montesano R, et al. New role for tRNA and its fragment purified from human urinary bladder carcinoma conditioned medium: inhibition of endothelial cell growth. J Cell Biochem. 1999;76:109–17.
Telonis AG, Rigoutsos I. Race disparities in the contribution of miRNA isoforms and tRNA-derived fragments to triple-negative breast cancer. Cancer Res. 2018;78:1140–54.
Martens-Uzunova ES, Jalava SE, Dits NF, van Leenders GJ, Moller S, Trapman J, et al. Diagnostic and prognostic signatures from the small non-coding RNA transcriptome in prostate cancer. Oncogene. 2012;31:978–91.
Magee RG, Telonis AG, Loher P, Londin E, Rigoutsos I. Profiles of miRNA isoforms and tRNA fragments in prostate cancer. Sci Rep. 2018;8:5314.
Xiong W, Wang X, Cai X, Xiong W, Liu Y, Li C, et al. Identification of tRNA-derived fragments in colon cancer by comprehensive small RNA sequencing. Oncol Rep. 2019;42:735–44.
Lui WO, Pourmand N, Patterson BK, Fire A. Patterns of known and novel small RNAs in human cervical cancer. Cancer Res. 2007;67:6031–43.
Londin E, Magee R, Shields CL, Lally SE, Sato T, Rigoutsos I. IsomiRs and tRNA-derived fragments are associated with metastasis and patient survival in uveal melanoma. Pigm Cell Melanoma Res. 2019;33:52–62.
Jin L, Zhu C, Qin X. Expression profile of tRNA-derived fragments in pancreatic cancer. Oncol Lett. 2019;18:3104–14.
Speer J, Gehrke CW, Kuo KC, Waalkes TP, Borek E. tRNA breakdown products as markers for cancer. Cancer. 1979;44:2120–3.
Dhahbi JM, Spindler SR, Atamna H, Yamakawa A, Boffelli D, Mote P, et al. 5' tRNA halves are present as abundant complexes in serum, concentrated in blood cells, and modulated by aging and calorie restriction. BMC Genomics. 2013;14:298.
Li F, Kaczor-Urbanowicz KE, Sun J, Majem B, Lo HC, Kim Y, et al. Characterization of human salivary extracellular RNA by next-generation sequencing. Clin Chem. 2018;64:1085–95.
Chiou NT, Kageyama R, Ansel KM. Selective export into extracellular vesicles and function of tRNA fragments during T cell activation. Cell Rep. 2018; 25:3356-3370 e4.
Schorn AJ, Martienssen R. Tie-Break: host and retrotransposons play tRNA. Trends Cell Biol. 2018;28:793–806.
Guzman N, Agarwal K, Asthagiri D, Yu L, Saji M, Ringel MD, et al. Breast cancer-specific miR signature unique to extracellular vesicles includes “microRNA-like” tRNA fragments. Mol Cancer Res. 2015;13:891–901.
Koi Y, Tsutani Y, Nishiyama Y, Ueda D, Ibuki Y, Sasada S, et al. Predicting the presence of breast cancer using circulating small RNAs, including those in the extracellular vesicles. Cancer Sci. 2020. https://doi.org/10.1111/cas.14393.
Warner KD, Hajdin CE, Weeks KM. Principles for targeting RNA with drug-like small molecules. Nat Rev Drug Discov. 2018;17:547–58.
Konieczkowski DJ, Johannessen CM, Garraway LA. A convergence-based framework for cancer drug resistance. Cancer Cell. 2018;33:801–15.
Zugazagoitia J, Guedes C, Ponce S, Ferrer I, Molina-Pinelo S, Paz-Ares L. Current challenges in cancer treatment. Clin Ther. 2016;38:1551–66.
Li S, Shi X, Chen M, Xu N, Sun D, Bai R, et al. Angiogenin promotes colorectal cancer metastasis via tiRNA production. Int J Cancer. 2019;145:1395–407.
Durdevic Z, Schaefer M. tRNA modifications: necessary for correct tRNA-derived fragments during the recovery from stress? Bioessays. 2013;35:323–7.
Chen Z, Qi M, Shen B, Luo G, Wu Y, Li J, et al. Transfer RNA demethylase ALKBH3 promotes cancer progression via induction of tRNA-derived small RNAs. Nucleic Acids Res. 2019;47:2533–45.
Vitali P, Kiss T. Cooperative 2'-O-methylation of the wobble cytidine of human elongator tRNA(Met)(CAT) by a nucleolar and a Cajal body-specific box C/D RNP. Genes Dev. 2019;33:741–6.
Acknowledgements
We thank anonymous reviewers, Anna Williams, and Christopher R. Wood for reading and commenting on the manuscript.
Funding
This work has been supported in part by the National Natural Science Foundation of China (81871864 and 81772766), Key Program of Zhejiang Provincial Natural Science Foundation of China (LZ20H160001), Medical Health Science and Technology Key Project of Zhejiang Provincial Health Commission (WKJ-ZJ-2007 and 2017211914), National Key Research and Development Program of China (2019YFC1315700 and 2016YFA0501800), and Zhejiang Province Key Research and Development Program of China (2019C03010, 2020C04003 and 2020C03034).
Author information
Authors and Affiliations
Contributions
YL and PL designed the study. MY drafted the manuscript. YL, PL, JZ, JD, and BL revised the manuscript. All of the authors have read and approved the paper.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
All authors have agreed to publish this manuscript.
Competing interests
No potential conflicts of interest were disclosed.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Yu, M., Lu, B., Zhang, J. et al. tRNA-derived RNA fragments in cancer: current status and future perspectives. J Hematol Oncol 13, 121 (2020). https://doi.org/10.1186/s13045-020-00955-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13045-020-00955-6