Abstract
Background
Limited data is available to guide the choice of the conditioning regimen for patients with acute myeloid leukemia (AML) undergoing transplant with persistent disease.
Methods
We retrospectively compared outcome of fludarabine-treosulfan (FT), thiotepa-busulfan-fludarabine (TBF), and sequential fludarabine, intermediate dose Ara-C, amsacrine, total body irradiation/busulfan, cyclophosphamide (FLAMSA) conditioning in patients with refractory or relapsed AML.
Results
Complete remission rates at day 100 were 92%, 80%, and 88% for FT, TBF, and FLAMSA, respectively (p = 0.13). Non-relapse mortality, incidence of relapse, acute (a) and chronic (c) graft-versus-host disease (GVHD) rates did not differ between the three groups. Overall survival at 2 years was 37% for FT, 24% for TBF, and 34% for FLAMSA (p = 0.10). Independent prognostic factors for survival were Karnofsky performance score and patient CMV serology (p = 0.01; p = 0.02), while survival was not affected by age at transplant. The use of anti-thymocyte globulin (ATG) was associated with reduced risk of grade III–IV aGVHD (p = 0.02) and cGVHD (p = 0.006), with no influence on relapse.
Conclusions
In conclusion, FT, TBF, and FLAMSA regimens provided similar outcome in patients undergoing transplant with active AML. Survival was determined by patient characteristics as Karnofsky performance score and CMV serology, however was not affected by age at transplant. ATG appears able to reduce the incidence of acute and chronic GVHD without influencing relapse risk.
Similar content being viewed by others
Background
Allogeneic hematopoietic stem cell transplant is the only potentially curative option for patients with acute myeloid leukemia (AML) in primary induction failure or refractory relapse. This population represents a big challenge for transplant physicians; nevertheless, according to recent evidence [1, 2], long-term survival can be achieved in about one third of patients undergoing transplant with active leukemia, and recent recommendations support prompt transplant in this setting, avoiding further chemotherapy [3]. The choice of the conditioning regimen is of vast importance in these fragile patients, as the need for powerful cytoreduction should not negate an acceptable toxicity profile of the protocol [4]. Historically, regimens employed in this setting included mainly standard myeloablative protocols based on alkylators or total-body irradiation (TBI) [5,6,7]. More recently, alternative strategies have been developed. The sequential fludarabine, intermediate dose Ara-C, amsacrine, total body irradiation/busulfan, cyclophosphamide (FLAMSA) regimen, designed by Kolb and colleagues in the early 2000s [8, 9], has shown promising outcome and currently represents one of the most widely employed protocols in this setting. On the other hand, the relentless effort of transplant physicians to temper conditioning toxicity while retaining a significant myeloablative power recently prompted the design of novel regimens, taking advantage in time of rather old drugs like thiotepa or treosulfan or combining two alkylators at reduced doses. The combination of thiotepa, busulfan, and fludarabine (TBF) was initially proposed as a preparative regimen for cord blood transplant [10]; subsequently, it has demonstrated excellent anti-leukemic activity in haploidentical [11, 12], matched sibling donor (MSD) and unrelated donor (UD) transplant [13, 14]. An additional option is represented by the association of fludarabine and treosulfan (FT), which has been intensely investigated in the last decade [15,16,17,18]. Preliminary results of a prospective randomized trial demonstrated promising outcome following FT conditioning in patients with AML and MDS [19]. Furthermore, in a recent retrospective study comparing treosulfan with busulfan-based regimens in patients with active leukemia at the time of transplant, FT protocol resulted in improved outcome [20]. Given the lack of available reports analyzing and comparing the alternative conditioning protocols in patients with refractory or relapsed AML, we designed the current study to compare outcome of FT, TBF, and FLAMSA regimens in this particularly challenging setting.
Methods
Study design and data collection
This is a registry-based retrospective study. Data were provided and the study design was approved by the Acute Leukemia Working Party (ALWP) of the European Society for Blood and Marrow Transplantation (EBMT), in accordance with the EBMT guidelines for retrospective studies. EBMT is a voluntary working group of more than 600 transplant centers which are required to report all consecutive stem cell transplantations and follow-up once a year. Audits are routinely performed to determine the accuracy of the data. Since 1990, patients have been able to provide informed consent that authorizes the use of their transplant information for research purposes. The ALWP of the EBMT granted ethical approval for this study.
We included in the analysis AML patients older than 18 years, who had received FT, TBF, or FLAMSA as conditioning regimen for transplant from matched sibling donor (MSD) or unrelated donor (UD) as first transplant in active disease status (defined as > 5% bone marrow blasts or detectable blasts in peripheral blood at the time of transplant). Patients with primary refractory AML, first or second relapse were included in the analysis. Stem cell transplants were performed between January 2005 and December 2016, and all data were reported to the ALWP of the EBMT. All unrelated donors were HLA-matched (10/10) or mismatched at one HLA locus (9/10). Patients who received conditioning regimens including oral busulfan or T-depleted grafts were excluded.
End-point definitions and statistical analysis
Non-relapse mortality (NRM) was defined as death from any cause in the absence of prior disease recurrence. Disease relapse was defined according to standard hematologic criteria. Leukemia-free survival (LFS) was defined as survival without relapse. Overall survival (OS) was calculated from the day of transplant until death from any cause or last follow-up. GVHD-free relapse-free survival (GRFS) was defined by the first of the following events: acute GVHD grades III to IV, extensive chronic GVHD, relapse, or death [21]. Patients with no event were censored at last contact. The cause of death was categorized according to standard criteria. The cause of death of patients who experienced relapse at any time before death was considered relapse related. Acute and chronic GVHD were graded according to standard criteria. All outcomes were measured from the time of stem cell infusion. Follow-up was estimated using the reverse Kaplan-Meier method. LFS, OS, and GRFS were estimated using the Kaplan-Meier method [22], whereas NRM, relapse, and GVHD were estimated using cumulative incidence analysis considering competing risks [23]. Univariate comparisons were performed using the log-rank test for LFS, OS, and GRFS and Gray’s test for GVHD, relapse incidence, and NRM. For all univariate analyses, continuous variables were categorized and the median used as a cut-off point. Multivariate analyses were performed using the Cox proportional hazards model. All factors differing significantly in distribution between the three groups or associated with one outcome were included in the Cox model. The FLAMSA group was used as the reference group in all comparisons. Results are expressed as hazard ratio (HR) with 95% confidence interval (CI). All p values were two-sided, and p < 0.05 were considered statistically significant. Statistical analyses were performed with SPSS 22.0 (IBM Corp., Armonk, NY) and R3.2.3 software packages(R Development Core Team, Vienna, Austria).
Results
Patient, disease, and transplant characteristics
Eight hundred and fifty-six patients fulfilled the inclusion criteria for the present analysis. Among them, 113 patients received FT, 112 TBF, and 631 received the FLAMSA regimen. Three hundred and sixty-two patients (42%) were transplanted from a MSD, 347 (41%) from a 10/10 UD, and 147 (17%) from a 9/10 UD. The FLAMSA protocol was busulfan- or TBI-based in 32% and 68% of the patients, respectively. Busulfan total dose was 6.4 mg/kg in 210 patients (157 FLAMSA, 53 TBF) and 9.6 mg/kg in 61 patients (8 FLAMSA, 53 TBF), while it was 12.8 mg/kg in 43 patients (37 FLAMSA, 6 TBF). In the group receiving TBI as part of the FLAMSA regimen, the dose was 4 Gy for all patients. Treosulfan dose was 30 mg/m2, 36 mg/m2, or 42 mg/m2 in 9, 21, and 83 patients, respectively. Anti-thymocyte globulin (ATG) administration was more frequent in FLAMSA as compared to TBF and FT cohorts (88%, 58%, and 39%, respectively, p < 10−3). The median year of transplant was 2011, 2015, and 2010 for FT, TBF, and FLAMSA, respectively (p < 10−3). The FT group included significantly older patients compared to the TBF and FLAMSA cohorts (median age 58, 52, and 52 years, respectively, p < 10−3). Cytogenetic data were available in 56% of the patients; among them, 6% of patients were with favorable, 63% with intermediate, and 31% with adverse cytogenetics, with no significant difference between the three groups. Cytomegalovirus (CMV) serology of donor and patient differed between the three cohorts (p < 10−3). Disease status (primary refractory or relapsed AML), Karnofsky performance score (KPS), type of donor, and donor/patient gender match did not differ between the groups. Donor lymphocyte infusions were administered to 101 (16%) patients in the FLAMSA group, 10 (9%) TBF recipients, and 14 (13%) patients within the FT cohort. Fifty-seven patients in the FLAMSA, 8 patients in the TBF, and 6 patients in the FT group received a second allogeneic transplant. Patient, disease, and transplant characteristics are summarized in Table 1. Detailed information on drug doses and post transplant cell therapy is provided in the supplementary material (Additional file 1).
Engraftment, disease response, and graft-vs-host disease
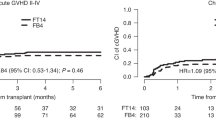
Engraftment rate was 98%, 91%, and 95% with median time to neutrophil engraftment of 16, 15, and 14 days in the FT, TBF, and FLAMSA cohorts, respectively (p = 0.1; p = 0.02). Median time to platelet engraftment was 15 days in the TBF group and 14 days in FT and FLAMSA groups. Graft failure was observed in four patients in the FLAMSA group and in one patient in the TBF and FT groups each. Secondary graft rejection was observed in six patients in the FLAMSA group while in none of the others. Cumulative incidence of complete remission for patients that reached day 100 was 92%, 80%, and 88% for the FT, TBF, and FLAMSA groups, respectively (p = 0.13). Global incidence of grade II–IV and III–IV acute GVHD (aGVHD) was 28% and 11%, respectively. The incidence of grade II–IV aGVHD was similar between the three groups, being 24%, 29%, and 28% in FT, TBF, and FLAMSA, respectively (p = 0.7). Similarly, the incidence of grade III–IV aGVHD did not differ between the three cohorts, 10% for FT, 12% for TBF, and 11% for FLAMSA, respectively (p = 0.9). Frequencies of chronic GVHD (cGVHD) and severe cGVHD in the global population were 27% and 12%, respectively. By univariate analysis, the cumulative incidence of cGVHD and severe cGVHD was similar in the three groups, being 33%, 26%, and 26% (p = 0.4) and 13%, 19%, and 11% (p = 0.5) for FT, TBF, and FLAMSA, respectively (Additional file 1). In multivariate analysis, the only factors associated with increased risk of developing aGVHD were transplant from mismatched unrelated donor and female/male donor/patient gender match. The use of ATG was independently associated with reduced risk of grade III–IV aGVHD and cGVHD (Table 2).
NRM, relapse, and survival
Global NRM rate was 7% at 100 days and 22% at 2 years. Six (5%) patients following FT, 14 (13%) following TBF and 40 (6%) following FLAMSA regimen died within 100 days. By univariate analysis, non-relapse mortality at 2 years was similar between the three groups: 26%, 24%, and 20% in FT, TBF, and FLAMSA, respectively (p = 0.24) (Fig. 1). In multivariate analysis, factors associated with increased NRM risk were older age and transplant from mismatched UD (Table 2). Leading causes of NRM were GVHD and infectious complications; the complete list of causes of death and their relative incidence are detailed in Table 3.
Cumulative incidence of relapse in the entire population was 52% at 2 years. By univariate analysis, 2-year relapse incidence was not statistically different between the three groups; 46%, 54%, and 53% for FT, TBF, and FLAMSA, respectively (p = 0.33). Multivariate analysis confirmed those results. Factors independently associated with higher risk of relapse were age at transplant, relapsed vs primary refractory AML, and patient CMV positive serology. Of note, the use of ATG did not influence relapse risk.
Leukemia-free survival, overall survival, and GRFS in the global population were 27%, 34%, and 20%, respectively. Leukemia-free survival at 2 years was similar among the three groups: 29%, 22%, and 27% for FT, TBF, and FLAMSA, respectively (p = 0.28). Overall survival did not significantly differ as well, being 37% for FT, 24% for TBF, and 34% for FLAMSA (p = 0.10). In multivariate analysis, patient CMV positive serology was associated with inferior LFS. The factors predicting inferior OS were KPS lower than 80% and patient CMV positive serology. The composite endpoint GRFS at 2 years was 23%, 13%, and 20% for FT, TBF, and FLAMSA, respectively (p = 0.15) (Fig. 2). In multivariate analysis, KPS lower than 80% and patient CMV positive serology were independently associated with inferior GRFS.
Discussion
Limited data is available to guide the choice of the conditioning regimen for patients with primary refractory or relapsed AML. We thus analyzed and compared the outcome of three commonly used conditioning regimens for active AML namely fludarabine-treosulfan, thiotepa-busulfan-fludarabine, and FLAMSA sequential regimen. Our results indicate global survival of 34% at 2 years; the type of conditioning protocol did not significantly affect survival, which was mostly determined by patient characteristics.
A major obstacle in transplanting patients with active leukemia is the high risk of non-relapse mortality; in fact, historical trials employing standard busulfan- or TBI-based regimens report a NRM rate of approximately 30–40% at day 100 after transplant [24,25,26]. In our study including patients up to 76 years of age, NRM at day 100 was around 5% following FT and FLAMSA and 13% after TBF, this difference being not statistically significant. Similarly, NRM at 2 years did not differ among the three regimens. It is important to highlight that the FT cohort included significantly older patients as compared to TBF and FLAMSA groups; in fact, 70% of FT patients were older than 50 years (median age of FT group, 58 years). Different strategies have been followed by researchers aiming to reduce mortality and improve the outcome of patients undergoing transplant with persistent leukemia. The design of the sequential FLAMSA regimen by the German group has represented a major breakthrough in this setting, combining promising anti-leukemic activity with acceptable NRM (about 22% at 2 years) [9]. On the other hand, some recent evidence suggests that redesigning standard myeloablative regimens could be an alternative strategy [1]. In fact, the Gruppo Italiano Trapianto di Midollo Osseo (GITMO) selected the TBF protocol as conditioning regimen for the GANDALF prospective trial, whose results have been recently presented, reporting a NRM rate of 35% at 2 years [27]. An alternative regimen is represented by the combination of fludarabine and treosulfan, which was shown to provide an interesting safety profile and promising outcome in patients undergoing transplant in remission [17] or with persistent leukemia [18, 20].
Importantly, the tolerability of the conditioning regimen should not compromise powerful antitumor activity in patients undergoing transplant with active AML. In the current study, we observed a complete remission rate at day 100 of about 90% following FT and FLAMSA, while CR rate was 80% after TBF, this difference being not statistically significant. Similarly, survival did not significantly differ among the three groups. Two-year survival rates were around 35% following FT and FLAMSA protocols; the latter was in accordance with the original report by the Munich group [8]. Conversely, TBF regimen was associated with a survival rate of 24% at 2 years, consistently with recent evidence from the GITMO trial employing the same protocol (OS at 2 years, 18%) [27]. Incidence of acute and chronic GVHD did not differ between the three regimens. In previous reports including patients with AML in remission, FT protocol has been associated with lower rates of GVHD as compared to busulfan-based regimens; we could not confirm this finding in our population of patients undergoing transplant with persistent leukemia [18, 28]. In fact, previous literature indicates a higher incidence of GVHD in patients transplanted with active disease in comparison to patients undergoing transplant in remission [29]. Nevertheless, we observed a tendency towards better GRFS following the FT protocol.
The rather large cohort included in the present study has allowed us to perform a multivariate analysis on factors predicting transplant outcome in the setting of active AML. Karnofsky performance score below 80% and patient positive CMV serology were strongly associated with poor survival. Further, patients with relapsed AML showed significantly higher risk of disease recurrence after transplant as compared to primary refractory AML. These findings are in accordance with a great body of previous evidence [30,31,32]. Transplant from mismatched UD predicted higher risk of grade III–IV aGVHD and non-relapse mortality, with no significant impact on survival. Interestingly, the use of ATG was associated with significantly lower incidence of both acute and chronic GVHD and a strong tendency towards better GRFS, with no influence on relapse rates. The benefit of ATG in terms of reduced incidence of GVHD is well established [33, 34]. However, as graft-versus-leukemia correlates with GVHD, there is a theoretic concern that the use of ATG could result in increased relapse rates, especially in patients undergoing transplant with active leukemia. Interestingly, in the historical ATG trial by GITMO, which included a high proportion of patients with active AML receiving transplant from unrelated donors, the use of ATG was associated with lower incidence of acute and chronic GVHD with no impact on relapse [35]. The findings of the present study are in line with these data. Furthermore, a recent report on patients with high risk AML undergoing transplant following a reduced intensity conditioning regimen including ATG confirmed no increased relapse risk [36]. Finally, it is of interest that in our cohort of patients aged up to 76 years, age at transplant did not influence survival, indicating that older age should not be taken as a criterion to withhold transplant in patients with active AML.
The limitations of the present study are mostly related to its retrospective design; in fact, limited information is available on the reason why a specific patient was allocated to a certain regimen. Further, scarce data on minimal residual disease status after transplant was available in the database; similarly, information on treatment administered after transplant other than cellular therapies (i.e., chemotherapy, target therapies, hypometilating drugs) was incomplete. Nevertheless, since a prospective randomized study comparing the three different conditioning protocols in patients with active AML has not been conducted yet and it is unlikely to be performed in the near future, we believe the results of the present analysis might serve to guide physicians practice in this very high-risk patients.
In conclusion, allogeneic transplant should be strongly considered in primary refractory and relapsed AML, as it is able to provide long-term survival in about one third of these patients. FT, TBF, and FLAMSA represent three possible alternative conditioning options in this setting, providing similar efficacy, toxicity, and survival. In fact, outcome was strongly affected by patient characteristics including Karnofsky performance score and CMV serology, while age should not be taken per se as a criterion to select patients for transplant. The use of ATG was associated with reduced incidence of severe acute and chronic GVHD without influencing relapse risk. Relapse remains the major cause of transplant failure; novel post-transplant strategies are thus in need to prevent disease recurrence in this extremely high-risk population.
Abbreviations
- ALWP:
-
Acute Leukemia Working Party
- AML:
-
Acute myeloid leukemia
- BM:
-
Bone marrow
- EBMT:
-
European Society for Blood and Marrow Transplantation
- FLAMSA:
-
Fludarabine, intermediate dose Ara-C, amsacrine, total body irradiation/busulfan, cyclophosphamide sequential regimen
- FT:
-
Fludarabine-treosulfan
- GVHD:
-
Graft-versus-host disease
- LFS:
-
Leukemia-free survival
- MAC:
-
Myeloablative
- MSD:
-
Matched sibling donor
- NRM:
-
Non-relapse mortality
- OS:
-
Overall survival
- PBSCs:
-
Peripheral blood stem cells
- RI:
-
Relapse incidence
- TBF:
-
Thiotepa-busulfan-fludarabine
- TBI:
-
Total-body irradiation
- UD:
-
Unrelated donor
References
Decroocq J, Itzykson R, Vigouroux S, Michallet M, Yakoub-Agha I, Huynh A, et al. Similar outcome of allogeneic stem cell transplantation after myeloablative and sequential conditioning regimen in patients with refractory or relapsed acute myeloid leukemia: a study from the Société Francophone de Greffe de Moelle et de Thérapie Cellulaire. Am J Hematol. 2018;93(3):416–23.
Brissot E, Labopin M, Ehninger G, Stelljes M, Brecht A, Ganser A, et al. Haploidentical versus unrelated allogeneic stem cell transplantation for relapsed/refractory acute myeloid leukemia: a report of 1578 patients from the Acute Leukemia Working Party of EBMT. Haematologica. 2018. https://doi.org/10.3324/haematol.2017.187450 [Epub ahead of print].
Thol F, Schlenk RF, Heuser M, Ganser A. How I treat refractory and early relapsed acute myeloid leukemia. Blood. 2015;126(3):319–27.
Jethava YS, Sica S, Savani B, Socola F, Jagasia M, Mohty M, et al. Conditioning regimens for allogeneic hematopoietic stem cell transplants in acute myeloid leukemia. Bone Marrow Transplant. 2017;52(11):1504–11.
Oyekunle AA, Kroger N, Zabelina T, Ayuk F, Schieder H, Renges H, et al. Allogeneic stem-cell transplantation in patients with refractory acute leukemia: a long-term follow-up. Bone Marrow Transplant. 2006;37(1):45–50.
Craddock C, Labopin M, Pillai S, Finke J, Bunjes D, Greinix H, et al. Factors predicting outcome after unrelated donor stem cell transplantation in primary refractory acute myeloid leukaemia. Leukemia. 2011;25:808–13.
Nagler A, Savani BN, Labopin M, Polge E, Passweg J, Finke J, et al. Outcomes after use of two standard ablative regimens in patients with refractory acute myeloid leukaemia: a retrospective, multicentre, registry analysis. Lancet Haematol. 2015;2:e384–92.
Schmid C, Schleuning M, Ledderose G, Tischer J, Kolb HJ. Sequential regimen of chemotherapy, reduced-intensity conditioning for allogeneic stem-cell transplantation, and prophylactic donor lymphocyte transfusion in high-risk acute myeloid leukemia and myelodysplastic syndrome. J Clin Oncol. 2005;23(24):5675–87.
Schmid C, Schleuning M, Schwerdtfeger R, Hertenstein B, Mischak-Weissinger E, Bunjes D, et al. Long-term survival in refractory acute myeloid leukemia after sequential treatment with chemotherapy and reduced-intensity conditioning for allogeneic stem cell transplantation. Blood. 2006;108(3):1092–9.
Sanz J, Sanz MA, Saavedra S, Lorenzo I, Montesinos P, Senent L, et al. Cord blood transplantation from unrelated donors in adults with high-risk acute myeloid leukemia. Biol Blood Marrow Transplant. 2010;16(1):86–94.
Raiola AM, Dominietto A, Ghiso A, Di Grazia C, Lamparelli T, Gualandi F, et al. Unmanipulated haploidentical bone marrow transplantation and posttransplantation cyclophosphamide for hematologic malignancies after myeloablative conditioning. Biol Blood Marrow Transplant. 2013;19(1):117–22.
Di Bartolomeo P, Santarone S, De Angelis G, Picardi A, Cudillo L, Cerretti R, et al. Haploidentical, unmanipulated, G-CSF-primed bone marrow transplantation for patients with high-risk hematologic malignancies. Blood. 2013;121(5):849–57.
Saraceni F, Labopin M, Hamladji RM, Mufti G, Socié G, Shimoni A, et al. Thiotepa-busulfan-fludarabine compared to busulfan-fludarabine for sibling and unrelated donor transplant in acute myeloid leukemia in first remission. Oncotarget. 2017;9(3):3379–93.
Saraceni F, Beohou E, Labopin M, Arcese W, Bonifazi F, Stepensky P, et al. Thiotepa, busulfan and fludarabine compared to busulfan and cyclophosphamide as conditioning regimen for allogeneic stem cell transplant from matched siblings and unrelated donors for acute myeloid leukemia. Am J Hematol. 2018;93(10):1211–9.
Casper J, Knauf W, Kiefer T, Wolff D, Steiner B, Hammer U, et al. Treosulfan and fludarabine: a new toxicity-reduced conditioning regimen for allogeneic hematopoietic stem cell transplantation. Blood. 2004;103:725–31.
Kroger N, Shimoni A, Zabelina T, Schieder H, Panse J, Ayuk F, et al. Reduced-toxicity conditioning with treosulfan, fludarabine and ATG as preparative regimen for allogeneic stem cell transplantation (alloSCT) in elderly patients with secondary acutemyeloid leukemia (sAML) or myelodysplastic syndrome (MDS). Bone Marrow Transplant. 2006;37:339–44.
Shimoni A, Shem-Tov N, Volchek Y, Danylesko I, Yerushalmi R, Nagler A. Allo-SCT for AML and MDS with treosulfan compared with BU-based regimens: reduced toxicity vs reduced intensity. Bone Marrow Transplant. 2012;47:1274–82.
Nagler A, Labopin M, Beelen D, Ciceri F, Volin L, Shimoni A, et al. Long-term outcome after a treosulfan-based conditioning regimen for patients with acute myeloid leukemia: a report from the Acute Leukemia Working Party of the European Society for Blood and Marrow Transplantation. Cancer. 2017;123:2671–9.
Beelen D. W. et al. Results of a randomized phase III trial comparing treosulfan/fludarabine to reduced-intensity conditioning with busulfan/fludarabine before allogeneic hematopoietic stem cell transplantation in acute myeloid leukemia or myelodysplastic syndrome. Oral abstract #OS8-2. 2018 European Society for Blood and Marrow Transplantation (EBMT) annual meeting, Lisbon, PT.
Shimoni A, Labopin M, Savani B, Hamladji RM, Beelen 5, Mufti G et al. Intravenous busulfan compared with treosulfan-based conditioning for allogeneic stem cell transplantation in acute myeloid leukemia: a study on behalf of the Acute Leukemia Working Party of European Society for Blood and Marrow Transplantation. Biol Blood Marrow Transplant. 2018;24(4):751–757.
Ruggeri A, Labopin M, Ciceri F, Mohty M, Nagler A. Definition of GvHD-free, relapse-free survival for registry-based studies: an ALWP-EBMT analysis on patients with AML in remission. Bone Marrow Transplant. 2016;51:610–1.
Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–81.
Gooley TA, Leisenring W, Crowley J, Storer BE. Estimation of failure probabilities in the presence of competing risks: new representations of old estimators. Stat Med. 1999;18:695–706.
Biggs JC, Horowitz MM, Gale RP, Ash RC, Atkinson K, Helbig W, et al. Bone marrow transplants may cure patients with acute leukemia never achieving remission with chemotherapy. Blood. 1992;80:1090–3.
Singhal S, Powles R, Henslee-Downey PJ, Chiang KY, Treleaven J, Godder K, et al. Allogeneic transplantation from HLA-matched sibling or partially HLA-mismatched related donors for primary refractory acute leukemia. Bone Marrow Transplant. 2002;29(4):291–5.
Todisco E, Ciceri F, Oldani E, Boschini C, Micò C, Vanlint MT, et al. The CIBMTR score predicts survival of AML patients undergoing allogeneic transplantation with active disease after a myeloablative or reduced intensity conditioning: a retrospective analysis of the Gruppo Italiano Trapianto Di Midollo Osseo. Leukemia. 2013;27(10):2086–91.
Ciceri F, Bernasconi P, Picardi A, et al. Alternative donor transplantation in patients with active acute leukemia at transplant (GANDALF): final analysis of a prospective study from Gruppo Italiano Trapianto Midollo Osseo (GITMO). EBMT 2018 oral O024.
Danylesko I, Shimoni A, Nagler A. Treosulfan-based conditioning before hematopoietic SCT: more than a BU look-alike. Bone Marrow Transplant. 2011;47:5–14.
Duval M, Klein JP, He W, Cahn JY, Cairo M, Camitta BM, et al. Hematopoietic stem-cell transplantation for acute leukemia in relapse or primary induction failure. J Clin Oncol. 2010;28(23):3730–8.
Sorror M, Storer B, Sandmaier BM, Maloney DG, Chauncey TR, Langston A, et al. Hematopoietic cell transplantation comorbidity index and Karnofsky performance status are independent predictors of morbidity and mortality after allogeneic nonmyeloablative hematopoietic cell transplantation. Cancer. 2008;112(9):1992–2001.
Brissot E, Labopin M, Stelljes M, Ehninger G5, Schwerdtfeger R6, Finke J, et al. Comparison of matched sibling donors versus unrelated donors in allogeneic stem cell transplantation for primary refractory acute myeloid leukemia: a study on behalf of the Acute Leukemia Working Party of the EBMT. J Hematol Oncol. 2017.
Clift RA, Buckner CD, Appelbaum FR, Schoch G, Petersen FB, Bensinger WI, et al. Allogeneic marrow transplantation during untreated first relapse of acute myeloid leukemia. J Clin Oncol. 1992;10(11):1723–9.
Finke J, Bethge WA, Schmoor C, Ottinger HD, Stelljes M, Zander AR, et al. Standard graft-versus-host disease prophylaxis with or without anti-T-cell globulin in haematopoietic cell transplantation from matched unrelated donors: a randomised, open-label, multicentre phase 3 trial. Lancet Oncol. 2009;10(9):855–64.
Kröger N, Solano C, Wolschke C, Bandini G, Patriarca F, Pini M, et al. Antilymphocyte globulin for prevention of chronic graft-versus-host disease. N Engl J Med. 2016;374(1):43–53.
Bacigalupo A, Lamparelli T, Bruzzi P, Guidi S, Alessandrino PE, di Bartolomeo P, et al. Antithymocyte globulin for graft-versus-host disease prophylaxis in transplants from unrelated donors: 2 randomized studies from Gruppo Italiano Trapianti Midollo Osseo (GITMO). Blood. 2001;98(10):2942–7.
Ofran Y, Beohou E, Labopin M, Blaise D, Cornelissen JJ, de Groot MR, et al. Anti-thymocyte globulin for graft-versus-host disease prophylaxis in patients with intermediate- or high-risk acute myeloid leukaemia undergoing reduced-intensity conditioning allogeneic stem cell transplantation in first complete remission - a survey on behalf of the Acute Leukaemia Working Party of the European Society for Blood and Marrow Transplantation. Br J Haematol. 2018. https://doi.org/10.1111/bjh.15131.
Acknowledgments
The authors would like to thank all EBMT centers for contributing patients to the study and data managers for their great work. A complete list of the EBMT members appears in the supplement.
Funding
Not applicable.
Availability of data and materials
The study relies on the EBMT dataset.
Author information
Authors and Affiliations
Contributions
FS, AN, and BS designed the study, the synopsis of which was approved by the Acute Leukemia Working Party of the EBMT. ML performed all the statistical analysis. FS wrote the first draft of the manuscript. AN and BS reviewed the manuscript. All co-authors contributed data to the EBMT registry, read the manuscript, and approved the final version.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Since 1990, patients provide informed consent authorizing the use of their personal information for research purposes.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Additional file
Additional file 1:
Table S1. Post-transplant cell therapy. Table S2. Drug doses in the three conditioning regimens (TBF, FT, FLAMSA). Table S3. Univariate analysis of ATG yes vs no stratified by conditioning regimen. (DOC 110 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Saraceni, F., Labopin, M., Brecht, A. et al. Fludarabine-treosulfan compared to thiotepa-busulfan-fludarabine or FLAMSA as conditioning regimen for patients with primary refractory or relapsed acute myeloid leukemia: a study from the Acute Leukemia Working Party of the European Society for Blood and Marrow Transplantation (EBMT). J Hematol Oncol 12, 44 (2019). https://doi.org/10.1186/s13045-019-0727-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13045-019-0727-4