Abstract
Background
The ovarian function and therefore the ovarian reserve may be compromised by the pathogenesis of autoimmune diseases of which, Hashimoto’s thyroiditis (HT) is the most common in women of reproductive age. Furthermore, a prolonged reduction in thyroid hormone concentration results in a broad spectrum of reproductive alteration. Previous reports in the literature have been controversial regarding the impact of hypothyroidism and alterations in the ovarian reserve. Thus, this prospective and comparative study aimed to evaluate the association of hypothyroidism with low ovarian reserve.
Materials and Methods
A subset of 27 patients with primary autoimmune hypothyroidism were compared to healthy women. The ovarian reserve was assessed through the anti-Mullerian hormone (AMH) and the antral follicle count (AFC).
Results
Overall, the two groups did not display significant differences in length of their menstrual cycles neither in the AMH serum levels nor the AFC.
Conclusions
No significant alteration was found in the ovarian reserve of women with HT.
Similar content being viewed by others
Background
Autoimmunity is considered to be involved in the pathogenesis of 4–30 % of premature ovarian failure (POF) cases [1]. The alteration of the immune system may cause disruption of the ovarian function or even deletion of follicles causing a reduced ovarian reserve [2,3,4]. The most common autoimmune disease in women of reproductive age is the thyroid dysfunction with a prevalence of 5–20 % [5,6,7]. An altered thyroid function affects a wide variety of functions including growth, development and metabolism [8]. Hypothyroidism is originated either by thyroid autoimmune disease (TAID) or by an insufficient iodine intake [9]. It is well known that a prolonged reduction in thyroid hormone concentration results in a broad spectrum of reproductive alteration, including abnormal folliculogenesis, alterations in the ovulation and fertilization rate, and ovarian failure [9,10,11,12]. There have been reports of alterations in the menstrual cycle of patients with Hashimoto’s thyroiditis [11] and also, women with thyroid-related diseases have a higher frequency of infertility compared to healthy individuals [9].
The presence of thyroid hormone receptors in the ovary suggests that thyroid hormones are important for ovarian functions [13]. Currently, the physiopathology behind the hypothyroidism on follicular development and the ovarian follicular reserve is not clear and studies using animal models are contradictory. For instance, some reports have claimed that low levels of thyroid hormones produce a significant decrease in basal luteinizing hormone (LH) release resulting in ovarian atrophy [14] or prolonged periods of vaginal diestrus [15, 16]. Other reports showed small changes in LH and follicle-stimulating hormone (FSH) release and the presence of mature follicles and corpus luteum [17]. In humans, some studies support the relationship between hypothyroidism and a diminished ovarian reserve (DOR) [18, 19]. The DOR is defined by a reduced response to ovarian stimulation in women of reproductive age with regular menstrual cycles when compared to women of equivalent age.
The ovarian reserve (OR) can be accurately assessed by the measurement of the anti-Müllerian hormone (AMH), which is secreted by the granulosa cells of ovarian follicles [20, 21] and which levels are stable throughout the cycle. There is preliminary evidence that thyroid disorders are associated with low OR [22] and also of a possible association between TAID and a DOR [23]. Additionally, the prevalence of DOR in patients with low levels of thyroid hormones is not fully established. The aim of this prospective and comparative study was to evaluate if women with TAID but being euthyroid on replacement therapy have lower ovarian reserve.
Materials and Methods
Subjects and samples
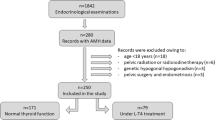
This study was approved by the Bioethics Committee of the Hospital Universitario “Dr. José Eleuterio González” (#GI07-020) and complied with the Declaration of Helsinki principles. Patients were provided with written and verbal information about the study before consenting to participate in the study. We performed a comparative and prospective study that included women of reproductive age (20–35 years old) with primary autoimmune hypothyroidism.
These patients displayed positive anti-TPO antibodies, high levels of thyroid-stimulating hormone (TSH) and low levels of free thyroxine (FT4). The reference values were 0.27–4.2 mIU/l and 9.3–17.0 ng/l (12–23.3 pmol/l) for TSH and FT4, respectively. At the time of the study, the patients were euthyroid due to the administration of thyroid hormones. The average age of patients when hypothyroidism was diagnosed was 25.6 years. The length of replacement therapy with L-thyroxine was 27.04 ± 8.04 months. Positive thyroid antibodies were found at least 1 year before the initiation of this study. A group of patients with normal levels of thyroid hormones and negative for anti-TPO antibodies was included as a control. Patients with history of endometriosis, Poly Cystic Ovarian Syndrome (PCOS), hysterectomy, oophorectomy or any other ovarian surgery, or with the usage of hormonal replacement for menopausal symptoms at the initiation of the study or during follow-up; or taking hormonal contraception for ≥ 3 months before the study onset was not included in the study. The body mass index (BMI) was calculated using the formula BMI = Weight (Kg)/Height (m2). According to the BMI, individuals with values between 18.5 and 24.5 were classified as normal weight whilst a BMI of 25 and 29 were considered overweight and above 30 as obese.
Transvaginal ultrasonography
Transvaginal ultrasonography with the Voluson Expert 730 with a 7.5 vaginal transducer (GE Healthcare) was used for the determination of the AFC and the ovarian volume on the third day of the menstrual cycle. Briefly, once the individual was with an empty bladder and in a lithotomy position, the transducer was advanced about 6 to 8 cm into the vagina angling laterally until the ovary was visualized. The length and Antero-Posterior (AP) measurements were obtained in the longitudinal plane, while in the transverse plane, the antral follicles measuring 2–10 mm in diameter in both ovaries were counted for the AFC.
Measurement of anti‐mullerian hormone
After overnight fast, blood samples were taken from the participants. Briefly, blood was drawn into plain serum tubes, centrifugation at 3500 rpm for 5 minutes was performed within 1 h of blood collection and the serum was separated and stored at -20°C until analysis. Serum anti-Mullerian hormone (AMH) levels were assessed by the Mullerian Inhibiting Substance/Anti-Mullerian (MIS/AMH) enzyme linked immunosorbent assay (ELISA) test (Diagnostic Systems, Workingham, UK) following the manufacturer recommendations. The assay demonstrated stable intra- and inter-assay coefficients of variation of 5 % and a functional sensitivity of 0.35 ng/ml.
Calculations and statistical analysis
Data are expressed as mean ± standard deviation (SD). Statistical comparisons were performed by Student t-test. Statistical analysis was performed with GraphPad Prism version 4.0 for Windows (GraphPad Software, San Diego, CA, USA). A probability (p) value p < 0.05 was considered statistically significant.
Results
A total of 52 patients were enrolled in this study, of which, 27 were diagnosed with TAID and 25 displayed normal levels of thyroid hormones and therefore used as a control group. The general features of the patients are shown in Table 1. The age of both groups was not significantly different. In contrast, the TAID group showed higher values of BMI (p = 0.02) compared to the control group.
The length of the menstrual cycle was longer in patients with hypothyroidism (4.59 ± 1.15) than in the control group (3.92 ± 0.702). However, no differences were observed in the minimum and maximum cycle length between both groups (Table 1).
We used two approaches to estimate the OR, the AMH and the AFC. The basal FSH levels were not different between the TAID and control group with 4.78 ± 2.59 and 4.37 ± 1.96, respectively (Table 2). Similarly, there was no difference in the AMH levels between both groups. Accordingly, no significant differences were found for the AFC and the ovary volume when the groups were compared.
Discussion
The importance of thyroid hormones for ovarian function has been extensively revised recently and the authors concluded that abnormal levels of thyroid hormones, especially during puberty and fertile age, might result in ovarian dysfunction throughout the entire life [19]. In this prospective study, we observed that there is no significant alteration in the ovarian reserve of women with TAID. Patients with a diminished ovarian reserve presented higher levels of TSH suggesting that thyroid disorders are associated with the ovarian reserve [24]. Other studies have shown the presence of anti-thyroid antibodies in ovarian follicular fluid from women with TAID which were positive correlated with their levels on serum [25]. However, evidence on the correlation between TAID with a low ovarian reserve is controversial [23, 26,27,28]. Osuka and coworkers [29] reported that thyroid autoantibodies are not likely to influence ovarian reserve in euthyroid women whose TSH levels are in the normal range. In a similar study, Ke et al. [30] analyzed the association between TAID and DOR and found that the presence of antibodies had no impact on ovarian reserve in euthyroid women. They also observed that these autoantibodies do not impact of the ART outcome, similar to what others observed in a subset of patients undergoing ART [9].
The analysis of our results, however, requires an important consideration that need to be taken into account. It was reported that after LT4 supplementation, the AMH level in patients with thyroglobulin antibody-positive and thyroid peroxidase antibody-negative significantly increased [31]. This possibility may introduce a bias in our observations, However, the limited number of patients that were included in Kuroda et al. [31] required that this possibility is explored in larger subsets of patients. Another important consideration is related to the association that has been found between ovarian reserve markers and body mass index (BMI) [32]. In this regard, we have not found a significant association between both parameters in the control group or in the patients (Pearson correlation coefficient of 0.22 and − 0.14, respectively).
The pathophysiological mechanism of the association of the TAID with the ovarian reserve is not completely elucidated. It is proposed that TPO antibodies pass through the blood follicle barrier during follicular development which damages the growing follicles and oocytes. Hypothyroidism patients can have alterations in the menstrual cycle where the most common alteration is oligomenorrhea [9]. In our study, the duration of the menstrual cycles was different among groups despite that all the patients were euthyroid through medication at the time of evaluation.
A study by Chen et al. demonstrated that low ovarian reserve with lower serum concentration of AMH was associated with more frequent positive TPO Ab rather than thyroid function or Tg Ab positivity [27]. The thyroglobulin antibodies were not assessed in this study and the TAID was determined based on the serum TPO Ab measurement.
Several approaches are used to evaluate the ovarian reserve, including the FSH, E2, inhibin B, AMH, the AFC and the ovarian volume (by ultrasound), the clomiphene test, and the exogenous FSH test. Even though we did not assess the inhibin B, the AMH has been indicated to be a reliable biomarker for the ovarian reserve since its levels are constant through the cycle [33].
Finally, although our results did not show significant differences between TAID patients and the control group, it is important to consider the existence of preantral follicles that are not visualized by ultrasound that can produce AMH. Thus, it would be important to assess the ovarian reserve (AMH and AFC) in individuals with primary hypothyroidism before the initiation of the treatment. Our findings also indicate a lack of fertility affectation at the time of the OR assessment. However, the monitoring of the OR through the disease progression is important to provide fertility advice to these patients in a timely manner.
Availability of data and materials
Available.
References
Ebrahimi M, Asbagh FA. The role of autoimmunity in premature ovarian failure. Iran J Reprod Med. 2015;13(8):461–72.
Anasti JN. Premature ovarian failure: an update. Fertil Steril. 1998;70(1):1–15. https://doi.org/10.1016/s0015-0282(98)00099-5.
Thöne J, Kollar S, Nousome D, Ellrichmann G, Kleiter I, Gold R, et al. Serum anti-Müllerian hormone levels in reproductive-age women with relapsing-remitting multiple sclerosis. Mult Scler. 2015;21(1):41–7. https://doi.org/10.1177/1352458514540843.
Rasool S, Shah D. Fertility with early reduction of ovarian reserve: the last straw that breaks the Camel’s back. Fertil Res Pract. 2017;3:15. https://doi.org/10.1186/s40738-017-0041-1.
Vanderpump MP, Tunbridge WM, French JM, Appleton D, Bates D, Clark F, et al. The incidence of thyroid disorders in the community: a twenty-year follow-up of the Whickham Survey. Clin Endocrinol (Oxf). 1995;43(1):55–68. https://doi.org/10.1111/j.1365-2265.1995.tb01894.x ;[cited 2019 Oct 23].
Canaris GJ, Manowitz NR, Mayor G, Ridgway EC. The Colorado thyroid disease prevalence study. Arch Intern Med. 2000;160(4):526–34.https://doi.org/10.1001/archinte.160.4.526http://archinte.jamanetwork.com/article.aspx? [cited 2019 Oct 23].
Aoki Y, Belin RM, Clickner R, Jeffries R, Phillips L, Mahaffey KR. Serum TSH and total T4 in the United States population and their association with participant characteristics: National Health and Nutrition Examination Survey (NHANES 1999–2002). Thyroid. 2007;17(12):1211–23. https://doi.org/10.1089/thy.2006.0235https://www.liebertpub.com/ [cited 2019 Oct 23].
McAninch EA, Bianco AC. Thyroid hormone signaling in energy homeostasis and energy metabolism. Ann N Y Acad Sci. 2014;1311(1):77–87 http://www.ncbi.nlm.nih.gov/pubmed/24697152 .[cited 2019 Oct 23].
Krassas GE, Poppe K, Glinoer D. Thyroid function and human reproductive health. Endocr Rev. 2010;31(5):702–55 https://academic.oup.com/edrv/article/31/5/702/2354820. [cited 2019 Oct 23].
Abalovich M, Mitelberg L, Allami C, Gutierrez S, Alcaraz G, Otero P, et al. Subclinical hypothyroidism and thyroid autoimmunity in women with infertility. Gynecol Endocrinol. 2007;23(5):279–83. https://doi.org/10.1080/09513590701259542http://www.tandfonline.com/doi/full/. [cited 2019 Oct 23].
Joshi JV, Bhandarkar SD, Chadha M, Balaiah D, Shah R. Menstrual irregularities and lactation failure may precede thyroid dysfunction or goitre. J Postgrad Med. 39(3):137–41. http://www.ncbi.nlm.nih.gov/pubmed/8051643. [cited 2019 Oct 23].
Vissenberg R, Manders VD, Mastenbroek S, Fliers E, Afink GB, Ris-Stalpers C, et al. Pathophysiological aspects of thyroid hormone disorders/thyroid peroxidase autoantibodies and reproduction. Hum Reprod Update. 2015;21(3):378–87 http://academic.oup.com/humupd/article/21/3/378/676494/Pathophysiological-aspects-of-thyroid-hormone. [cited 2019 Oct 23].
Aghajanova L, Lindeberg M, Carlsson IB, Stavreus-Evers A, Zhang P, Scott JE, et al. Receptors for thyroid-stimulating hormone and thyroid hormones in human ovarian tissue. Reprod Biomed Online. 2009;18(3):337–47 http://www.ncbi.nlm.nih.gov/pubmed/19298732. [cited 2021 Jan 25].
Ortega E, Rodriguez E, Ruiz E, Osorio C. Activity of the hypothalamo-pituitary ovarian axis in hypothyroid rats with or without triiodothyronine replacement. Life Sci. 1990;46(6):391–5 https://linkinghub.elsevier.com/retrieve/pii/0024320590900812. [cited 2019 Oct 23].
Hapon MB, Simoncini M, Via G, Jahn GA. Effect of hypothyroidism on hormone profiles in virgin, pregnant and lactating rats, and on lactation. Reproduction. 2003;126(3):371–82 http://www.ncbi.nlm.nih.gov/pubmed/12968945. [cited 2019 Oct 23].
Mattheij JA, Swarts JJ, Lokerse P, van Kampen JT, Van der Heide D. Effect of hypothyroidism on the pituitary-gonadal axis in the adult female rat. J Endocrinol. 1995;146(1):87–94 https://joe.bioscientifica.com/view/journals/joe/146/1/joe_146_1_012.xml. [cited 2019 Oct 23].
Armada-Dias L, Carvalho JJ, Breitenbach MM, Franci CR, Moura EG. Is the infertility in hypothyroidism mainly due to ovarian or pituitary functional changes? Brazilian J Med Biol Res Rev=Bras Pesqui medicas e Biol. 2001;34(9):1209–15 http://www.ncbi.nlm.nih.gov/pubmed/11514846. [cited 2019 Oct 23].
Goswami R, Marwaha RK, Goswami D, Gupta N, Ray D, Tomar N, et al. Prevalence of thyroid autoimmunity in sporadic idiopathic hypoparathyroidism in comparison to type 1 diabetes and premature ovarian failure. J Clin Endocrinol Metab. 2006;91(11):4256–9 http://www.ncbi.nlm.nih.gov/pubmed/16895958. [cited 2019 Oct 23].
Colella M, Cuomo D, Giacco A, Mallardo M, De Felice M, Ambrosino C. Thyroid Hormones and Functional Ovarian Reserve: Systemic vs. Peripheral Dysfunctions. J Clin Med. 2020;9(6). http://www.ncbi.nlm.nih.gov/pubmed/32492950. [cited 2021 Jan 25]
Nardo LG, Gelbaya TA, Wilkinson H, Roberts SA, Yates A, Pemberton P, et al. Circulating basal anti-Müllerian hormone levels as predictor of ovarian response in women undergoing ovarian stimulation for in vitro fertilization. Fertil Steril. 2009;92(5):1586–93 http://www.ncbi.nlm.nih.gov/pubmed/18930213; [cited 2019 Oct 23].
La Marca A, Sighinolfi G, Radi D, Argento C, Baraldi E, Artenisio AC, et al. Anti-Mullerian hormone (AMH) as a predictive marker in assisted reproductive technology (ART). Hum Reprod Update. 2010;16(2):113–30 http://www.ncbi.nlm.nih.gov/pubmed/19793843. [cited 2019 Oct 23].
Bahri S, Tehrani FR, Amouzgar A, Rahmati M, Tohidi M, Vasheghani M, et al. Overtime trend of thyroid hormones and thyroid autoimmunity and ovarian reserve: a longitudinal population study with a 12-year follow up. BMC Endocr Disord. 2019;19(1):47 https://bmcendocrdisord.biomedcentral.com/articles/10.1186/s12902-019-0370-7. [cited 2019 Oct 23].
Polyzos NP, Sakkas E, Vaiarelli A, Poppe K, Camus M, Tournaye H. Thyroid autoimmunity, hypothyroidism and ovarian reserve: a cross-sectional study of 5000 women based on age-specific AMH values. Hum Reprod. 2015;30(7):1690–6 https://academic.oup.com/humrep/article-lookup/doi/https://doi.org/10.1093/humrep/dev089. [cited 2019 Oct 23].
Michalakis KG, Mesen TB, Brayboy LM, Yu B, Richter KS, Levy M, et al. Subclinical elevations of thyroid-stimulating hormone and assisted reproductive technology outcomes. Fertil Steril. 2011;95(8):2634–7 https://linkinghub.elsevier.com/retrieve/pii/S0015028211003694. [cited 2019 Oct 23].
Monteleone P, Parrini D, Faviana P, Carletti E, Casarosa E, Uccelli A, et al. Female infertility related to thyroid autoimmunity: the ovarian follicle hypothesis. Am J Reprod Immunol. 2011;66(2):108–14 https://doi.org/http://doi.wiley.com/10.1111/j.1600-0897.2010.00961.x. [cited 2019 Oct 23].
Meng L, Rijntjes E, Swarts HJM, Keijer J, Teerds KJ. Prolonged hypothyroidism severely reduces ovarian follicular reserve in adult rats. J Ovarian Res. 2017;10(1):19 http://ovarianresearch.biomedcentral.com/articles/https://doi.org/10.1186/s13048-017-0314-7. [cited 2019 Oct 23].
Chen C-W, Huang Y-L, Tzeng C-R, Huang R-L, Chen C-H. Idiopathic low ovarian reserve is associated with more frequent positive thyroid peroxidase antibodies. Thyroid. 2017;27(9):1194–200 https://www.liebertpub.com/doi/https://doi.org/10.1089/thy.2017.0139. [cited 2019 Oct 23].
Saglam F, Onal ED, Ersoy R, Koca C, Ergin M, Erel O, et al. Anti-Müllerian hormone as a marker of premature ovarian aging in autoimmune thyroid disease. Gynecol Endocrinol. 2015;31(2):165–8 http://www.tandfonline.com/doi/full/https://doi.org/10.3109/09513590.2014.973391. [cited 2019 Oct 23].
Osuka S, Iwase A, Goto M, Takikawa S, Nakamura T, Murase T, et al. Thyroid autoantibodies do not impair the ovarian reserve in Euthyroid infertile women: A cross-sectional study. Horm Metab Res. 2018;50(7):537–42 http://www.ncbi.nlm.nih.gov/pubmed/29991084. [cited 2021 Jan 25].
Ke H, Hu J, Zhao L, Ding L, Jiao X, Qin Y. Impact of thyroid autoimmunity on ovarian reserve, pregnancy outcomes, and offspring health in euthyroid women following in vitro fertilization/intracytoplasmic sperm injection. Thyroid. 2020;30(4):588–97 http://www.ncbi.nlm.nih.gov/pubmed/31928166. [cited 2021 Jan 25].
Kuroda M, Kuroda K, Segawa T, Noh JY, Yoshihara A, Ito K, et al. Levothyroxine supplementation improves serum anti-Müllerian hormone levels in infertile patients with Hashimoto’s thyroiditis. J Obstet Gynaecol Res. 2018;44(4):739–46 http://www.ncbi.nlm.nih.gov/pubmed/29297967. [cited 2021 Jan 25].
Moslehi N, Shab-Bidar S, Ramezani Tehrani F, Mirmiran P, Azizi F. Is ovarian reserve associated with body mass index and obesity in reproductive aged women? A meta-analysis Menopause. 2018;25(9):1046–55 http://www.ncbi.nlm.nih.gov/pubmed/29738413. [cited 2021 Jan 25].
Fanchin R, Taieb J, Lozano DHM, Ducot B, Frydman R, Bouyer J. High reproducibility of serum anti-Mullerian hormone measurements suggests a multi-staged follicular secretion and strengthens its role in the assessment of ovarian follicular status. Hum Reprod. 2005;20(4):923–7 http://academic.oup.com/humrep/article/20/4/923/701092/High-reproducibility-of-serum-antiMüllerian. [cited 2019 Oct 23].
Acknowledgements
We would like to thank Dr Selene Garcia Luna for her technical assistance.
Funding
There was no funding to declare.
Author information
Authors and Affiliations
Contributions
Conceptualization of the study: FAM and LHS. Patient Selection and Conduct of study: MMR, OHV, OVG, and JGG. Data Analysis, manuscript preparation, and manuscript editing: FAM, LHS, and SGL. The author(s) read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All research protocols were approved by the Institutional Ethics Committee of Hospital Universitario “Dr. José Eleuterio González” and were congruent with the declaration of Helsinki. Informed consent was obtained from all subjects.
Consent for publication
All authors give consent for publication.
Competing interests
The authors declare no conflicts of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Morales-Martínez, F.A., Sordia-Hernández, L.H., Ruiz, M.M. et al. Association between thyroid autoimmunity and ovarian reserve in women with hypothyroidism. Thyroid Res 14, 6 (2021). https://doi.org/10.1186/s13044-021-00095-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13044-021-00095-0