Abstract
Many neurodegenerative disorders, including Parkinson’s, Alzheimer’s, and amyotrophic lateral sclerosis, are well known to involve the accumulation of disease-specific proteins. Less well known are the accumulations of another set of proteins, neuronal intermediate filaments (NFs), which have been observed in these diseases for decades. NFs belong to the family of cytoskeletal intermediate filament proteins (IFs) that give cells their shape; they determine axonal caliber, which controls signal conduction; and they regulate the transport of synaptic vesicles and modulate synaptic plasticity by binding to neurotransmitter receptors. In the last two decades, a number of rare disorders caused by mutations in genes that encode NFs or regulate their metabolism have been discovered. These less prevalent disorders are providing novel insights into the role of NF aggregation in the more common neurological disorders.
Similar content being viewed by others
Background
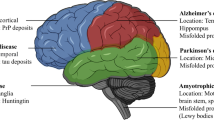
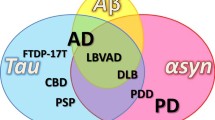
The majority of neurodegenerative disorders are proteinopathies, i.e., they are diseases of protein homeostasis with proteins misfolding and accumulating in aggregates [1,2,3]. Advances in molecular medicine have begun to reveal specific proteins that accumulate in specific syndromes—for instance, α-synuclein in Parkinson’s disease (PD); Aβ and tau in Alzheimer’s disease (AD); polyglutamine proteins in various CAG trinucleotide repeat disorders; superoxide dismutase 1 (SOD1), TAR DNA-binding protein 43 (TDP43), FUS, optineurin (OPTN), ubiquilin 2 (UBQLN2), and dipeptide repeat protein (DRP) in amyotrophic lateral sclerosis (ALS) [4,5,6,7].
It is worth noting, however, that protein accumulation in neurons was already a well-recognized phenomenon in the pre-genetic era. Silver stains developed by Camillo Golgi in 1873, which depend on the so-called “black reaction” and which were improved upon by David Bodian 60 years later, demonstrated the presence of protein tangles and accumulations in the brains of patients with dementia at autopsy [8, 9]. These aggregates were later found to contain specific proteins that form cytoskeletal polymers called neurofilaments (NFs) (Table 1) [22,23,24]. Within a few years, NFs were found to overlap with tau neurofibrillary tangles in brains affected by AD [10] and were discovered within Lewy bodies in PD dopaminergic neurons [11] and in skeins and aggregates in the dystrophic neurites of ALS motor neurons [12]. Hirano bodies, a term used to describe the crystalloid structures found in in the soma of neurons in a variety of degenerative conditions including ALS and AD, also stained strongly for NFs [25].
We now know that NFs belong to the larger family of intermediate filaments (IFs), so called because their approximately 10 nm diameter falls between those of the two other cytoskeletal polymers, microtubules (25 nm) and actin filaments (6 nm) [26]. Based on primary amino acid sequence and tissue of distribution, IFs have been classified into six major types (I-VI) [27]. Adult neurons in the central nervous system (CNS) express the pan-neuronal type IV IFs (NF triplet proteins: light, middle and heavy; henceforth called NF-L, NF-M, NF-H; and α-internexin, INA) [28], while neurons in the peripheral nervous system (PNS) express the NF triplet proteins along with the type III IF peripherin [29]. The immature nervous system expresses the class III IF vimentin and the class VI IFs nestin and synemin. These IF proteins are thought to be more dynamic at a time when developmental processes such as neurite extension and synapse formation warrant a more changeable cytoskeleton [30].
NF structure and functions
At a molecular level, IF proteins share a common tripartite structure. They consist of a conserved central α-helical rod domain flanked by two variable head and tail domains located at the C- and N-terminus, respectively [31]. Our knowledge of how they polymerize has come from studying IF assembly. Taking advantage of IF’s ability to dissolve in chaotropic reagents (e.g., urea), IF assembly can be studied in vitro under controlled conditions by dialysis against defined ionic strength buffers. The assembled intermediates can then be assessed by a combination of analytical centrifugation, chemical cross-linking, and electron microscopy (EM) [32]. IF monomers form an in-parallel coil-coiled dimer (2 nm in diameter) from tight hydrophobic interactions of the rod domains; the dimers interact in an anti-parallel fashion to form tetramers (3.6 nm in diameter). Eight tetramers associate to form unit-length filaments (ULFs; ~ 18 nm diameter) that in turn undergo radial compaction and join end-to-end to form mature, 10 nm-long polymers [33, 34]. NFs have a greater subunit complexity: NF-M and NF-H copolymerize with NF-L to form two heterotetramers, NF-L/NF-M and NF-L/NF-H. These heterotramers also in distinct neuronal populations incorporate INA or peripherin, although many of the details of this incorporation appear less clear [35]. The stoichiometry of assembled NFs, nevertheless, appears to be regulated: for instance in the CNS (optic nerve and spinal cord) the ratio of NF polymers is 4:2:2:1 (NF-L:ΙΝΑ:NF-M:NF-H) [28], while in the PNS (the sciatic nerve) the molar ratio of the NF quadruplets is 4:2:1:1 (NF-L:NF-M:peripherin:NF-H) [29].
Because IF polymers are higly stable in vitro they were initially thought to be static and relatively inert [36, 37]. However, in living cells they are dynamic—they undergo cycles of severing and end-to-end annealing, and also show subunit exchange along their length [38, 39]. Indeed, besides their mechanical role, IFs organize the cellular environment, position the nucleus, and dock organelles such as mitochondria and endoplasmic reticulum; they also participate in intracellular signaling and transcription [40]. In the nervous system, NFs regulate neurite outgrowth and axonal caliber; the latter controls the cable properties of the neuron [41, 42]. Some of the neuronal functions of NFs are driven by specific subunits. NF-L interacts with the molecular motor myosin Va to help transport synaptic vesicles [43]; NF-L also directly interacts with the N-methyl-D-aspartate (NMDA) receptor subunit NR1, anchoring NMDARs on the neuronal membrane at the level of dendrites and growth cone [44]. NF-M binds the D1 dopamine receptor and regulates its surface expression [45]. NF-H directly binds the C-terminal domain of tubulin in a phosphorylation-dependent manner, modulating microtubule polymerization [46, 47]. Not all NF functions are dependent on their polymeric nature; for instance, shorter particles and even soluble oligomers bind NMDA and other neurotransmitter receptors to regulate synaptic function [48].
The behavior of NFs is modulated by post-translational modifications (PTMs) such as phosphorylation, O-linked glycosylation, ubiquitination, oxidation and nitration [49, 50]. Phosphorylation is the best studied and is thought to play a major role in driving NF assembly and disassembly. Phosphorylation of the head domain regulates NF polymerization and is mediated by protein kinase A (PKA), protein kinase C (PKC) and calcium/calmodulin dependent protein kinase II (CAMKII) [51,52,53,54]. The tail domains of NF-M and NF-H, which mediate spacing between NF polymers, are also phosphorylated at specific Lys-Ser-Pro (KSP) motifs by CDC2-like kinase (CLK), cyclin-dependent kinase 5 (CDK5), and mitogen-activated protein kinases (MAPKs) [55,56,57]. This was initially thought to modulate the lateral growth of the NF lattice and by extension the radial growth of axons [58], but NF-M mutants in which all serines of KSP repeats have been replaced with phosphorylation-incompetent alanines fail to show major alterations in the caliber of their axons [59]. The phosphorylation of the head and tail domains is thought to occur in different regions of the neuron, with the head domain being phosphorylated in the cell body, while that of the tail domain occurs after entering the axon. In fact, C-terminal phosphorylation inhibits phosphorylation of the tail-domain, suggesting that cross-talk between signaling events regulates subunit assembly and possibly transport down the axon [60].
Much less is known about the other PTMs, although the proximity of O-linked glycosylation sites to the phosphorylation sites on both head and tail domains of NF-M and NF-H subunits suggests that this PTM competes with phosphorylation to regulate NF dynamics [61].
NF aggregation and its role in neurodegeneration
The mechanism by which NFs aggregate is still unknown, but hyper-phosphorylation is considered one of the main triggers for NF aggregation [62]. This model is similar to what has been proposed for tau, which also tends to aggregate when hyper-phosphorylated. Indeed, for many years it was thought that NFs did not really aggregate in AD and related tauopathies, and that their presence was due to antibody cross-reaction with phosopho-tau epitopes [63,64,65]. NF aggregation, however, has since then been convincingly demonstrated by proteomic findings, which do not rely on antibody detection at all [24].
There are several ways that phosphorylation could cause aggregation. First, it could alter ionic interactions among the subunits to create aberrant intermediates that are prone to aggregation or drive assembly over disassembly [66, 67]. Second, hyper-phosphorylation could alter the association of NF subunits with molecular motors and disrupt their transport, leading to their aggregation; NF mutants that mimic permanent phosphorylation states display lower rates of transport, and premature phosphorylation sequesters NF subunits within the cell soma [68, 69]. Third, phosphorylation could protect NFs from proteolytic cleavage, which could enhance their biochemical stability and trigger aggregation through the imbalance in the tight stoichiometry among the different subunits that is required for correct filament formation [70, 71]. There is evidence to support this stoichiometric model too (Table 2): transgenic mice overexpressing wild type NF subtypes can mimic strategic mutant versions that impair NF assembly in their ability to develop abnormal neurofilamentous axonal swellings and progressive neuropathy that are highly reminiscent of those found in ALS [72, 76, 84]. Moreover, these data supported a causal role for NF aggregates in causing neurodegeneration [90]. In the absence of disease-causing mutations, however, these experiments did not prompt inquiry into possible roles of NFs in the pathophysiology of bona fide neurodegenerative diseases.
The pathogenic role of NF dysmetabolism began to be studied more closely only after the discovery of rare neurological disorders that involve NF accumulation and are caused by mutations in NF genes (Table 3). These NF Mendelian disorders fall under the rubric of Charcot-Marie-Tooth (CMT) diseases, which typically cause sensory and motor peripheral neuropathy. The first neurofilament-related CMT to be discovered was CMT2E, an autosomal dominant disease that can be caused by any of more than 20 different mutations distributed through the head, rod and tail domains of the NF-L encoding NEFL gene [100]. When expressed in cell lines, some of these NF-L mutants display altered phosphorylation patterns that suppress the filament assembly process, which confirms the importance of phosphorylation for NF aggregation [101].
The second NF-related CMT, called CMT2CC, is caused by frameshift variants in NEFH, which encodes the NF-H, leading to stop loss and translation of a cryptic amyloidogenic element (CAE) in the 3’UTR with a propensity toward aggregation [93]. It is worth noting that indels in NEFH and missense mutations in peripherin-encoding PRPH have been also linked to susceptibility to ALS, another disease that involves NF accumulation [102, 103].
What is the connection between NF aggregation and neurodegeneration? One possibility is that NF aggregates hinder axonal transport. This could in turn impair the sub-cellular distribution of vesicles and key organelles such as mitochondria. In support of this possibility are two lines of evidence. First, ultrastructural analyses of CMT sural biopsies have demonstrated that NF inclusions often cause the misplacement and accumulation of mitochondria, lysosomes and other membranous bodies [91]. Second, in rat primary neurons and neuronal cell lines overexpressing mutant NF proteins, mitochondria accumulate within the cell body and almost completely disappear from the distal segments of axons and dendrites [104, 105]. Another study in cell lines overexpressing mutant NF-L found fragmentation of the Golgi apparatus and endoplasmic reticulum, which could underlie dysfunctions of the vacuolar compartment in addition to mitochondrial mislocalization [106].
Another possibility is that NF accumulation occurs downstream of other events caused by the non-structural roles of NF proteins. Indeed, studies in primary neurons from Nefl knockout mice have shown that NF-L ablation alters mitochondrial shape, fusion and motility [107]. Furthermore, abnormalities in mitochondrial morphology and dynamics in CMT2E cellular models have been described prior to the disruption of the NF network and the appearance of visible NF deposits [108]. There is also at least one autosomal recessive neuropathic disease, CMT1F, where nonsense mutations in NEFL produce truncated forms of NF-L that are unstable and unable to assemble with NF-M and NF-H subunits into NFs. In this disease the neuropathy is thought to result from a reduction in NFs rather than accumulation [92, 109, 110]. Due to the absence of a functional NF lattice, CMT1F axons fail to develop their proper diameter during development, and the diminished axonal caliber leads to defects in myelination and lower conduction velocities.
Molecular mechanisms of NF-mediated neurotoxicity
To truly understand the role of NFs in disease it would be important to find tools that modulate NF levels or, better yet, disassemble aggregated NF proteins. Hitherto, this has been difficult to do since NFs are amongst the most stable cytoskeletal polymers, with a half-life of more than 2.5 months [111].
Here another rare disease, giant axonal neuropathy (GAN), has provided insights. In GAN, the NF accumulation is so severe that the axons become distended. Clinically, GAN overlaps with CMTs in producing sensory and motor neuropathies, but it is a much more devastating disease because it affects the CNS as well: patients develop ataxia, dysarthria, nystagmus, ptosis, facial paralysis and ophthalmoplegia, and typically die in the second or third decade of life [112]. Another difference is that GAN is caused not by mutations of NF genes, but rather by mutations in the gene that encodes gigaxonin, a protein that targets NFs for degradation. Gigaxonin belongs to the broad-complex, tramtrack, and bric-à-brac (BTB)/Kelch family of adaptors for the Cullin3-E3 ubiquitin ligase complex [113,114,115,116]. We have studied gigaxonin’s role in NF clearance using dorsal root ganglia (DRG) from Gan-null mice, in which even large accumulations can be readily cleared by overexpressing wild-type gigaxonin. These neurons are beginning to shed light on pathogenic pathways likely downstream from NF aggregation. For instance, we have found that NF accumulations closely correlate with mitochondrial dysmotility and bioenergetic defects [117, 118]. Gan-null neurons experience indeed greater metabolic demands and are more prone to oxidative stress [117].
Overexpressing wild type gigaxonin rapidly clears NF aggregates and rescues mitochondrial motility and metabolic defects. Since E3 ligase adaptors have multiple substrates, which also appears to be the case with gigaxonin [119, 120], it is still not entirely clear the extent to which NF aggregates contribute to pathology. Some aspects of the disease could well stem from derangements in other cellular processes. This would explain why GAN pathology is more severe and affects more neuronal subtypes that those affected in the CMT disorders. Even with this shortcoming in our knowledge, gigaxonin promises to become a tool to study NF degradation and clearance. The therapeutic potential of gigaxonin is also being tested in clinical trials where viral vectors are being used to deliver gigaxonin to the nervous system of GAN patients [121].
Conclusions
For decades, the role of NF accumulation in many neurological disorders has been neglected. But with the discovery of Mendelian diseases affecting NF proteins or those involved in their metabolism, we are beginning to gain novel insights into the role of NFs in disease. But GAN is not the only disease caused by mutations in factors directly involved in NF metabolism. There are a few other recently discovered disorders that feature NF aggregation and promise to shed light on NF quality control mechanisms (Table 3). These include diseases such as giant axonal neuropathy 2 (GAN2), a disease also characterized by enlarged neurons, but in which the pathology is due to loss of function mutations in another E3 ligase adaptor named DDB1 and CUL4 associated factor 8 (DCAF8), which interacts with Cullin4 (instead of Cullin3) [95]. Others are due to pathological mutations in molecular chaperones that help nascent NFs acquire a correct tertiary structure: this is the case with CMT2F and CMT2L, two CMT subtypes due to dominant mutations in the heat shock protein (HSP)-encoding genes HSPB1 and HSPB8, respectively [97, 98]. There is also myofibrillar myopathy 6 (MFM6), a severe neuromuscular disorder caused by mutations in BCL2 associated athanogene 3 (BAG3), a gene encoding a factor that regulates the HSP70 protein family [99].
The available data support a model in which multiple triggers are able to cause NF aggregation by reducing their physiological turnover and promoting their pathological buildup. Mutations in NF-coding or chaperone-coding genes can directly increase the resistance of NFs towards degradation by affecting their phosphorylation patterns or their folding. On the other hand, mutations in elements of the ubiquitin-proteasome system indirectly cause NF aggregation by impairing NF degradation pathways (Fig. 1). In the future, it would be important to assess whether any of the cellular pathways identified in these rare disorders are also dysregulated in the more common neurodegenerative diseases characterized by NF inclusions. There could also be pathology driven by signaling processes gone awry. For instance, abnormal NF phosphorylation in AD has been connected to an imbalance in the concerted activity between protein phosphatase 1 (PP1) and 2A (PP2A), and the kinases CDK5 and MAPKs [122,123,124]. Investigations into these possibilities is likely to provide further insights into NF aggregation mechanisms that, while historically the oldest neuropathological phenomena, still resist full explanation.
Molecular mechanisms of NF aggregation. The scheme shows the principal pathways triggering neurofilament (NF) aggregation in the neurodegenerative diseases listed in Tables 1 and 3. NF subunits can undergo hyper-phosphorylation and accumulation due to pathological mutations in NF-coding genes (inner circle). Alternatively, NF accumulation can be caused by damaging mutations in genes directly involved in NF metabolism such as factors regulating NF turnover and degradation; gigaxonin is shown as an example (intermediate circle). Lastly, NF aggregation can be the result of the dysregulation in cellular signaling pathways converging on NF metabolism such as specific protein kinase cascades (outer circle). While the first two mechanisms are at the root of rare neurological disorders like giant axon neuropathy (GAN) and Charcot-Marie-Tooth (CMT) syndromes, the latter is likely to explain NF aggregation in the more common neurodegenerative diseases
Abbreviations
- AD:
-
Alzheimer’s disease
- ALS:
-
Amyotrophic lateral sclerosis
- BAG3:
-
BCL2 associated athanogene 3
- BTB:
-
Broad-complex, tramtrack, and bric-à-brac
- CDK5:
-
Cyclin-dependent kinase 5
- CLK:
-
CDC2-like kinase
- CMT:
-
Charcot-Marie-Tooth
- CNS:
-
Central nervous system
- DCAF8:
-
DDB1 and CUL4 associated factor 8
- DRG:
-
Dorsal root ganglia
- DRP:
-
Dipeptide repeat protein
- ET:
-
Essential tremor
- FTD:
-
Frontotemporal dementia
- FXTAS:
-
Fragile X tremor/ataxia syndrome
- GAN:
-
Giant axonal neuropathy
- HSP:
-
Heat shock protein
- IFs:
-
Intermediate filaments
- INA:
-
α-internexin
- MAPKs:
-
Mitogen-activated protein kinases
- MFM6:
-
Myofibrillar myopathy 6
- MSA-C:
-
Multiple system atrophy-cerebellar
- NEDMAGA:
-
Neurodevelopmental disorder with movement abnormalities, abnormal gait, and autistic features
- NFs:
-
Neurofilaments
- NIID:
-
Neuronal intranuclear inclusion disease
- NMDA:
-
N-methyl-D-aspartate
- PD:
-
Parkinson’s disease
- PEHO syndrome:
-
Progressive encephalopathy syndrome with edema, hypsarrhythmia and optic atrophy
- PKA:
-
Protein kinase A
- PKC:
-
Protein kinase C
- PP1:
-
Protein phosphatase 1
- PP2A:
-
Protein phosphatase 2A
- PTMs:
-
Post-translational modifications
- SCA1:
-
Spinocerebellar ataxia type 1
- SMA:
-
Spinal muscular atrophy
- SOD1:
-
Superoxide dismutase 1
- TDP43:
-
TAR DNA-binding protein 43
- ULFs:
-
Unit-length filaments
References
Douglas PM, Dillin A. Protein homeostasis and aging in neurodegeneration. J Cell Biol. 2010;190:719–29.
Sweeney P, Park H, Baumann M, Dunlop J, Frydman J, Kopito R, McCampbell A, Leblanc G, Venkateswaran A, Nurmi A, Hodgson R. Protein misfolding in neurodegenerative diseases: implications and strategies. Transl Neurodegener. 2017;6:6.
Soto C, Pritzkow S. Protein misfolding, aggregation, and conformational strains in neurodegenerative diseases. Nat Neurosci. 2018;21:1332–40.
Breydo L, Wu JW, Uversky VN. Alpha-synuclein misfolding and Parkinson's disease. Biochim Biophys Acta. 2012;1822:261–85.
Stoyas CA, La Spada AR. The CAG-polyglutamine repeat diseases: a clinical, molecular, genetic, and pathophysiologic nosology. Handb Clin Neurol. 2018;147:143–70.
Irvine GB, El-Agnaf OM, Shankar GM, Walsh DM. Protein aggregation in the brain: the molecular basis for Alzheimer's and Parkinson's diseases. Mol Med. 2008;14:451–64.
Blokhuis AM, Groen EJ, Koppers M, van den Berg LH, Pasterkamp RJ. Protein aggregation in amyotrophic lateral sclerosis. Acta Neuropathol. 2013;125:777–94.
Pannese E. The black reaction. Brain Res Bull. 1996;41:343–9.
Bodian, David: A new method for staining nerve fibers and nerve endings in mounted paraffin sections. The Anatomical Record. 1936;65:89–97.
Ishii T, Haga S, Tokutake S. Presence of neurofilament protein in Alzheimer's neurofibrillary tangles (ANT). An immunofluorescent study. Acta Neuropathol. 1979;48:105–12.
Goldman JE, Yen SH, Chiu FC, Peress NS. Lewy bodies of Parkinson's disease contain neurofilament antigens. Science. 1983;221:1082–4.
Delisle MB, Carpenter S. Neurofibrillary axonal swellings and amyotrophic lateral sclerosis. J Neurol Sci. 1984;63:241–50.
Perry G, Stewart D, Friedman R, Manetto V, Autilio-Gambetti L, Gambetti P. Filaments of Pick's bodies contain altered cytoskeletal elements. Am J Pathol. 1987;127:559–68.
Iwahashi CK, Yasui DH, An HJ, Greco CM, Tassone F, Nannen K, Babineau B, Lebrilla CB, Hagerman RJ, Hagerman PJ. Protein composition of the intranuclear inclusions of FXTAS. Brain. 2006;129:256–71.
Cifuentes-Diaz C, Nicole S, Velasco ME, Borra-Cebrian C, Panozzo C, Frugier T, Millet G, Roblot N, Joshi V, Melki J. Neurofilament accumulation at the motor endplate and lack of axonal sprouting in a spinal muscular atrophy mouse model. Hum Mol Genet. 2002;11:1439–47.
Louis ED, Yi H, Erickson-Davis C, Vonsattel JP, Faust PL. Structural study of Purkinje cell axonal torpedoes in essential tremor. Neurosci Lett. 2009;450:287–91.
Denora PS, Smets K, Zolfanelli F, Ceuterick-de Groote C, Casali C, Deconinck T, Sieben A, Gonzales M, Zuchner S, Darios F, et al. Motor neuron degeneration in spastic paraplegia 11 mimics amyotrophic lateral sclerosis lesions. Brain. 2016;139:1723–34.
Palmer EE, Kumar R, Gordon CT, Shaw M, Hubert L, Carroll R, Rio M, Murray L, Leffler M, Dudding-Byth T, et al. A recurrent De novo nonsense variant in ZSWIM6 results in severe intellectual disability without frontonasal or limb malformations. Am J Hum Genet. 2017;101:995–1005.
Palo J, Haltia M, Carpenter S, Karpati G, Mushynski W. Neurofilament subunit--related proteins in neuronal intranuclear inclusions. Ann Neurol. 1984;15:322–8.
Schmidt RE, Beaudet LN, Plurad SB, Dorsey DA. Axonal cytoskeletal pathology in aged and diabetic human sympathetic autonomic ganglia. Brain Res. 1997;769:375–83.
Haltia M, Somer M. Infantile cerebello-optic atrophy. Neuropathology of the progressive encephalopathy syndrome with edema, hypsarrhythmia and optic atrophy (the PEHO syndrome). Acta Neuropathol. 1993;85:241–7.
Gambetti P, Autilio Gambetti L, Papasozomenos SC. Bodian's silver method stains neurofilament polypeptides. Science. 1981;213:1521–2.
Balin BJ, Clark EA, Trojanowski JQ, Lee VM. Neurofilament reassembly in vitro: biochemical, morphological and immuno-electron microscopic studies employing monoclonal antibodies to defined epitopes. Brain Res. 1991;556:181–95.
Rudrabhatla P, Jaffe H, Pant HC. Direct evidence of phosphorylated neuronal intermediate filament proteins in neurofibrillary tangles (NFTs): phosphoproteomics of Alzheimer's NFTs. FASEB J. 2011;25:3896–905.
Hirano A. Hirano bodies and related neuronal inclusions. Neuropathol Appl Neurobiol. 1994;20:3–11.
Franke WW, Schmid E, Osborn M, Weber K. Different intermediate-sized filaments distinguished by immunofluorescence microscopy. Proc Natl Acad Sci U S A. 1978;75:5034–8.
Leduc C, Etienne-Manneville S. Intermediate filaments in cell migration and invasion: the unusual suspects. Curr Opin Cell Biol. 2015;32:102–12.
Yuan A, Rao MV, Sasaki T, Chen Y, Kumar A, Veeranna LRK, Eyer J, Peterson AC, Julien JP, Nixon RA. Alpha-internexin is structurally and functionally associated with the neurofilament triplet proteins in the mature CNS. J Neurosci. 2006;26:10006–19.
Yuan A, Sasaki T, Kumar A, Peterhoff CM, Rao MV, Liem RK, Julien JP, Nixon RA. Peripherin is a subunit of peripheral nerve neurofilaments: implications for differential vulnerability of CNS and peripheral nervous system axons. J Neurosci. 2012;32:8501–8.
Nixon RA, Shea TB. Dynamics of neuronal intermediate filaments: a developmental perspective. Cell Motil Cytoskeleton. 1992;22:81–91.
Herrmann H, Aebi U. Intermediate filaments: structure and assembly. Cold Spring Harb Perspect Biol. 2016;8:a018242.
Renner W, Franke WW, Schmid E, Geisler N, Weber K, Mandelkow E. Reconstitution of intermediate-sized filaments from denatured monomeric vimentin. J Mol Biol. 1981;149:285–306.
Herrmann H, Bar H, Kreplak L, Strelkov SV, Aebi U. Intermediate filaments: from cell architecture to nanomechanics. Nat Rev Mol Cell Biol. 2007;8:562–73.
Qin Z, Kreplak L, Buehler MJ. Hierarchical structure controls nanomechanical properties of vimentin intermediate filaments. PLoS One. 2009;4:e7294.
Gentil BJ, Tibshirani M, Durham HD. Neurofilament dynamics and involvement in neurological disorders. Cell Tissue Res. 2015;360:609–20.
Starger JM, Brown WE, Goldman AE, Goldman RD. Biochemical and immunological analysis of rapidly purified 10-nm filaments from baby hamster kidney (BHK-21) cells. J Cell Biol. 1978;78:93–109.
Aebi U, Cohn J, Buhle L, Gerace L. The nuclear lamina is a meshwork of intermediate-type filaments. Nature. 1986;323:560–4.
Colakoglu G, Brown A. Intermediate filaments exchange subunits along their length and elongate by end-to-end annealing. J Cell Biol. 2009;185:769–77.
Uchida A, Colakoglu G, Wang L, Monsma PC, Brown A. Severing and end-to-end annealing of neurofilaments in neurons. Proc Natl Acad Sci U S A. 2013;110:E2696–705.
Eriksson JE, Dechat T, Grin B, Helfand B, Mendez M, Pallari HM, Goldman RD. Introducing intermediate filaments: from discovery to disease. J Clin Invest. 2009;119:1763–71.
Liem RK, Messing A. Dysfunctions of neuronal and glial intermediate filaments in disease. J Clin Invest. 2009;119:1814–24.
Julien JP. Neurofilament functions in health and disease. Curr Opin Neurobiol. 1999;9:554–60.
Rao MV, Mohan PS, Kumar A, Yuan A, Montagna L, Campbell J, Veeranna EEM, Julien JP, Nixon RA. The myosin Va head domain binds to the neurofilament-L rod and modulates endoplasmic reticulum (ER) content and distribution within axons. PLoS One. 2011;6:e17087.
Ehlers MD, Fung ET, O'Brien RJ, Huganir RL. Splice variant-specific interaction of the NMDA receptor subunit NR1 with neuronal intermediate filaments. J Neurosci. 1998;18:720–30.
Kim OJ, Ariano MA, Lazzarini RA, Levine MS, Sibley DR. Neurofilament-M interacts with the D1 dopamine receptor to regulate cell surface expression and desensitization. J Neurosci. 2002;22:5920–30.
Miyasaka H, Okabe S, Ishiguro K, Uchida T, Hirokawa N. Interaction of the tail domain of high molecular weight subunits of neurofilaments with the COOH-terminal region of tubulin and its regulation by tau protein kinase II. J Biol Chem. 1993;268:22695–702.
Bocquet A, Berges R, Frank R, Robert P, Peterson AC, Eyer J. Neurofilaments bind tubulin and modulate its polymerization. J Neurosci. 2009;29:11043–54.
Yuan A, Nixon RA. Specialized roles of neurofilament proteins in synapses: relevance to neuropsychiatric disorders. Brain Res Bull. 2016;126:334–46.
Yuan A, Rao MV, Veeranna NRA. Neurofilaments at a glance. J Cell Sci. 2012;125:3257–63.
Snider NT, Omary MB. Post-translational modifications of intermediate filament proteins: mechanisms and functions. Nat Rev Mol Cell Biol. 2014;15:163–77.
Sihag RK, Nixon RA. Identification of Ser-55 as a major protein kinase a phosphorylation site on the 70-kDa subunit of neurofilaments. Early turnover during axonal transport. J Biol Chem. 1991;266:18861–7.
Sihag RK, Jaffe H, Nixon RA, Rong X. Serine-23 is a major protein kinase a phosphorylation site on the amino-terminal head domain of the middle molecular mass subunit of neurofilament proteins. J Neurochem. 1999;72:491–9.
Yates DM, Manser C, De Vos KJ, Shaw CE, McLoughlin DM, Miller CC. Neurofilament subunit (NFL) head domain phosphorylation regulates axonal transport of neurofilaments. Eur J Cell Biol. 2009;88:193–202.
Yuan A, Rao MV, Veeranna NRA. Neurofilaments and Neurofilament proteins in health and disease. Cold Spring Harb Perspect Biol. 2017;9:a018309.
Shetty KT, Link WT, Pant HC. cdc2-like kinase from rat spinal cord specifically phosphorylates KSPXK motifs in neurofilament proteins: isolation and characterization. Proc Natl Acad Sci U S A. 1993;90:6844–8.
Kesavapany S, Li BS, Pant HC. Cyclin-dependent kinase 5 in neurofilament function and regulation. Neurosignals. 2003;12:252–64.
Veeranna AND, Ahn NG, Jaffe H, Winters CA, Grant P, Pant HC. Mitogen-activated protein kinases (Erk1,2) phosphorylate Lys-Ser-pro (KSP) repeats in neurofilament proteins NF-H and NF-M. J Neurosci. 1998;18:4008–21.
Xu ZS, Liu WS, Willard MB. Identification of six phosphorylation sites in the COOH-terminal tail region of the rat neurofilament protein M. J Biol Chem. 1992;267:4467–71.
Garcia ML, Rao MV, Fujimoto J, Garcia VB, Shah SB, Crum J, Gotow T, Uchiyama Y, Ellisman M, Calcutt NA, Cleveland DW. Phosphorylation of highly conserved neurofilament medium KSP repeats is not required for myelin-dependent radial axonal growth. J Neurosci. 2009;29:1277–84.
Zheng YL, Li BS, Veeranna PHC. Phosphorylation of the head domain of neurofilament protein (NF-M): a factor regulating topographic phosphorylation of NF-M tail domain KSP sites in neurons. J Biol Chem. 2003;278:24026–32.
Dong DL, Xu ZS, Hart GW, Cleveland DW. Cytoplasmic O-GlcNAc modification of the head domain and the KSP repeat motif of the neurofilament protein neurofilament-H. J Biol Chem. 1996;271:20845–52.
Sihag RK, Inagaki M, Yamaguchi T, Shea TB, Pant HC. Role of phosphorylation on the structural dynamics and function of types III and IV intermediate filaments. Exp Cell Res. 2007;313:2098–109.
Nukina N, Kosik KS, Selkoe DJ. Recognition of Alzheimer paired helical filaments by monoclonal neurofilament antibodies is due to crossreaction with tau protein. Proc Natl Acad Sci U S A. 1987;84:3415–9.
Ksiezak-Reding H, Dickson DW, Davies P, Yen SH. Recognition of tau epitopes by anti-neurofilament antibodies that bind to Alzheimer neurofibrillary tangles. Proc Natl Acad Sci U S A. 1987;84:3410–4.
Lichtenberg-Kraag B, Mandelkow EM, Biernat J, Steiner B, Schroter C, Gustke N, Meyer HE, Mandelkow E. Phosphorylation-dependent epitopes of neurofilament antibodies on tau protein and relationship with Alzheimer tau. Proc Natl Acad Sci U S A. 1992;89:5384–8.
Sibille N, Huvent I, Fauquant C, Verdegem D, Amniai L, Leroy A, Wieruszeski JM, Lippens G, Landrieu I. Structural characterization by nuclear magnetic resonance of the impact of phosphorylation in the proline-rich region of the disordered tau protein. Proteins. 2012;80:454–62.
Lyons AJ, Gandhi NS, Mancera RL. Molecular dynamics simulation of the phosphorylation-induced conformational changes of a tau peptide fragment. Proteins. 2014;82:1907–23.
Ackerley S, Thornhill P, Grierson AJ, Brownlees J, Anderton BH, Leigh PN, Shaw CE, Miller CC. Neurofilament heavy chain side arm phosphorylation regulates axonal transport of neurofilaments. J Cell Biol. 2003;161:489–95.
Shea TB, Yabe JT, Ortiz D, Pimenta A, Loomis P, Goldman RD, Amin N, Pant HC. Cdk5 regulates axonal transport and phosphorylation of neurofilaments in cultured neurons. J Cell Sci. 2004;117:933–41.
Goldstein ME, Sternberger NH, Sternberger LA. Phosphorylation protects neurofilaments against proteolysis. J Neuroimmunol. 1987;14:149–60.
Pant HC. Dephosphorylation of neurofilament proteins enhances their susceptibility to degradation by calpain. Biochem J. 1988;256:665–8.
Cote F, Collard JF, Julien JP. Progressive neuronopathy in transgenic mice expressing the human neurofilament heavy gene: a mouse model of amyotrophic lateral sclerosis. Cell. 1993;73:35–46.
Marszalek JR, Williamson TL, Lee MK, Xu Z, Hoffman PN, Becher MW, Crawford TO, Cleveland DW. Neurofilament subunit NF-H modulates axonal diameter by selectively slowing neurofilament transport. J Cell Biol. 1996;135:711–24.
Eyer J, Peterson A. Neurofilament-deficient axons and perikaryal aggregates in viable transgenic mice expressing a neurofilament-beta-galactosidase fusion protein. Neuron. 1994;12:389–405.
Rao MV, Garcia ML, Miyazaki Y, Gotow T, Yuan A, Mattina S, Ward CM, Calcutt NA, Uchiyama Y, Nixon RA, Cleveland DW. Gene replacement in mice reveals that the heavily phosphorylated tail of neurofilament heavy subunit does not affect axonal caliber or the transit of cargoes in slow axonal transport. J Cell Biol. 2002;158:681–93.
Gama Sosa MA, Friedrich VL Jr, DeGasperi R, Kelley K, Wen PH, Senturk E, Lazzarini RA, Elder GA. Human midsized neurofilament subunit induces motor neuron disease in transgenic mice. Exp Neurol. 2003;184:408–19.
Lee VM, Elder GA, Chen LC, Liang Z, Snyder SE, Friedrich VL Jr, Lazzarini RA. Expression of human mid-sized neurofilament subunit in transgenic mice. Brain Res Mol Brain Res. 1992;15:76–84.
Vickers JC, Morrison JH, Friedrich VL Jr, Elder GA, Perl DP, Katz RN, Lazzarini RA. Age-associated and cell-type-specific neurofibrillary pathology in transgenic mice expressing the human midsized neurofilament subunit. J Neurosci. 1994;14:5603–12.
Elder GA, Friedrich VL Jr, Liang Z, Li X, Lazzarini RA. Enhancer trapping by a human mid-sized neurofilament transgene reveals unexpected patterns of neuronal enhancer activity. Brain Res Mol Brain Res. 1994;26:177–88.
Tu PH, Elder G, Lazzarini RA, Nelson D, Trojanowski JQ, Lee VM. Overexpression of the human NFM subunit in transgenic mice modifies the level of endogenous NFL and the phosphorylation state of NFH subunits. J Cell Biol. 1995;129:1629–40.
Wong PC, Marszalek J, Crawford TO, Xu Z, Hsieh ST, Griffin JW, Cleveland DW. Increasing neurofilament subunit NF-M expression reduces axonal NF-H, inhibits radial growth, and results in neurofilamentous accumulation in motor neurons. J Cell Biol. 1995;130:1413–22.
Rao MV, Campbell J, Yuan A, Kumar A, Gotow T, Uchiyama Y, Nixon RA. The neurofilament middle molecular mass subunit carboxyl-terminal tail domains is essential for the radial growth and cytoskeletal architecture of axons but not for regulating neurofilament transport rate. J Cell Biol. 2003;163:1021–31.
Monteiro MJ, Hoffman PN, Gearhart JD, Cleveland DW. Expression of NF-L in both neuronal and nonneuronal cells of transgenic mice: increased neurofilament density in axons without affecting caliber. J Cell Biol. 1990;111:1543–57.
Xu Z, Cork LC, Griffin JW, Cleveland DW. Increased expression of neurofilament subunit NF-L produces morphological alterations that resemble the pathology of human motor neuron disease. Cell. 1993;73:23–33.
Lee MK, Marszalek JR, Cleveland DW. A mutant neurofilament subunit causes massive, selective motor neuron death: implications for the pathogenesis of human motor neuron disease. Neuron. 1994;13:975–88.
Dequen F, Filali M, Lariviere RC, Perrot R, Hisanaga S, Julien JP. Reversal of neuropathy phenotypes in conditional mouse model of Charcot-Marie-tooth disease type 2E. Hum Mol Genet. 2010;19:2616–29.
Adebola AA, Di Castri T, He CZ, Salvatierra LA, Zhao J, Brown K, Lin CS, Worman HJ, Liem RK. Neurofilament light polypeptide gene N98S mutation in mice leads to neurofilament network abnormalities and a Charcot-Marie-tooth type 2E phenotype. Hum Mol Genet. 2015;24:2163–74.
Beaulieu JM, Nguyen MD, Julien JP. Late onset of motor neurons in mice overexpressing wild-type peripherin. J Cell Biol. 1999;147:531–44.
Ching GY, Chien CL, Flores R, Liem RK. Overexpression of alpha-internexin causes abnormal neurofilamentous accumulations and motor coordination deficits in transgenic mice. J Neurosci. 1999;19:2974–86.
Goldman JE, Yen SH. Cytoskeletal protein abnormalities in neurodegenerative diseases. Ann Neurol. 1986;19:209–23.
Fabrizi GM, Cavallaro T, Angiari C, Cabrini I, Taioli F, Malerba G, Bertolasi L, Rizzuto N. Charcot-Marie-tooth disease type 2E, a disorder of the cytoskeleton. Brain. 2007;130:394–403.
Yum SW, Zhang J, Mo K, Li J, Scherer SS. A novel recessive Nefl mutation causes a severe, early-onset axonal neuropathy. Ann Neurol. 2009;66:759–70.
Rebelo AP, Abrams AJ, Cottenie E, Horga A, Gonzalez M, Bis DM, Sanchez-Mejias A, Pinto M, Buglo E, Markel K, et al. Cryptic Amyloidogenic elements in the 3′ UTRs of Neurofilament genes trigger axonal neuropathy. Am J Hum Genet. 2016;98:597–614.
Koch T, Schultz P, Williams R, Lampert P. Giant axonal neuropathy: a childhood disorder of microfilaments. Ann Neurol. 1977;1:438–51.
Klein CJ, Wu Y, Vogel P, Goebel HH, Bonnemann C, Zukosky K, Botuyan MV, Duan X, Middha S, Atkinson EJ, et al. Ubiquitin ligase defect by DCAF8 mutation causes HMSN2 with giant axons. Neurology. 2014;82:873–8.
Zhai J, Lin H, Julien JP, Schlaepfer WW. Disruption of neurofilament network with aggregation of light neurofilament protein: a common pathway leading to motor neuron degeneration due to Charcot-Marie-tooth disease-linked mutations in NFL and HSPB1. Hum Mol Genet. 2007;16:3103–16.
Tang BS, Zhao GH, Luo W, Xia K, Cai F, Pan Q, Zhang RX, Zhang FF, Liu XM, Chen B, et al. Small heat-shock protein 22 mutated in autosomal dominant Charcot-Marie-tooth disease type 2L. Hum Genet. 2005;116:222–4.
Ylikallio E, Poyhonen R, Zimon M, De Vriendt E, Hilander T, Paetau A, Jordanova A, Lonnqvist T, Tyynismaa H. Deficiency of the E3 ubiquitin ligase TRIM2 in early-onset axonal neuropathy. Hum Mol Genet. 2013;22:2975–83.
Jaffer F, Murphy SM, Scoto M, Healy E, Rossor AM, Brandner S, Phadke R, Selcen D, Jungbluth H, Muntoni F, Reilly MM. BAG3 mutations: another cause of giant axonal neuropathy. J Peripher Nerv Syst. 2012;17:210–6.
Szeverenyi I, Cassidy AJ, Chung CW, Lee BT, Common JE, Ogg SC, Chen H, Sim SY, Goh WL, Ng KW, et al. The human intermediate filament database: comprehensive information on a gene family involved in many human diseases. Hum Mutat. 2008;29:351–60.
Sasaki T, Gotow T, Shiozaki M, Sakaue F, Saito T, Julien JP, Uchiyama Y, Hisanaga S. Aggregate formation and phosphorylation of neurofilament-L Pro22 Charcot-Marie-tooth disease mutants. Hum Mol Genet. 2006;15:943–52.
Al-Chalabi A, Andersen PM, Nilsson P, Chioza B, Andersson JL, Russ C, Shaw CE, Powell JF, Leigh PN. Deletions of the heavy neurofilament subunit tail in amyotrophic lateral sclerosis. Hum Mol Genet. 1999;8:157–64.
Corrado L, Carlomagno Y, Falasco L, Mellone S, Godi M, Cova E, Cereda C, Testa L, Mazzini L, D'Alfonso S. A novel peripherin gene (PRPH) mutation identified in one sporadic amyotrophic lateral sclerosis patient. Neurobiol Aging. 2011;32:552 e551–6.
Brownlees J, Ackerley S, Grierson AJ, Jacobsen NJ, Shea K, Anderton BH, Leigh PN, Shaw CE, Miller CC. Charcot-Marie-tooth disease neurofilament mutations disrupt neurofilament assembly and axonal transport. Hum Mol Genet. 2002;11:2837–44.
Perez-Olle R, Jones ST, Liem RK. Phenotypic analysis of neurofilament light gene mutations linked to Charcot-Marie-tooth disease in cell culture models. Hum Mol Genet. 2004;13:2207–20.
Perez-Olle R, Lopez-Toledano MA, Goryunov D, Cabrera-Poch N, Stefanis L, Brown K, Liem RK. Mutations in the neurofilament light gene linked to Charcot-Marie-tooth disease cause defects in transport. J Neurochem. 2005;93:861–74.
Gentil BJ, Minotti S, Beange M, Baloh RH, Julien JP, Durham HD. Normal role of the low-molecular-weight neurofilament protein in mitochondrial dynamics and disruption in Charcot-Marie-tooth disease. FASEB J. 2012;26:1194–203.
Tradewell ML, Durham HD, Mushynski WE, Gentil BJ. Mitochondrial and axonal abnormalities precede disruption of the neurofilament network in a model of charcot-marie-tooth disease type 2E and are prevented by heat shock proteins in a mutant-specific fashion. J Neuropathol Exp Neurol. 2009;68:642–52.
Abe A, Numakura C, Saito K, Koide H, Oka N, Honma A, Kishikawa Y, Hayasaka K. Neurofilament light chain polypeptide gene mutations in Charcot-Marie-tooth disease: nonsense mutation probably causes a recessive phenotype. J Hum Genet. 2009;54:94–7.
Fu J, Yuan Y. A novel homozygous nonsense mutation in NEFL causes autosomal recessive Charcot-Marie-tooth disease. Neuromuscul Disord. 2018;28:44–7.
Yuan A, Sasaki T, Rao MV, Kumar A, Kanumuri V, Dunlop DS, Liem RK, Nixon RA. Neurofilaments form a highly stable stationary cytoskeleton after reaching a critical level in axons. J Neurosci. 2009;29:11316–29.
Ben Hamida M, Hentati F, Ben Hamida C. Giant axonal neuropathy with inherited multisystem degeneration in a Tunisian kindred. Neurology. 1990;40:245–50.
Bomont P, Cavalier L, Blondeau F, Ben Hamida C, Belal S, Tazir M, Demir E, Topaloglu H, Korinthenberg R, Tuysuz B, et al. The gene encoding gigaxonin, a new member of the cytoskeletal BTB/kelch repeat family, is mutated in giant axonal neuropathy. Nat Genet. 2000;26:370–4.
Mahammad S, Murthy SN, Didonna A, Grin B, Israeli E, Perrot R, Bomont P, Julien JP, Kuczmarski E, Opal P, Goldman RD. Giant axonal neuropathy-associated gigaxonin mutations impair intermediate filament protein degradation. J Clin Invest. 2013;123:1964–75.
Lin NH, Huang YS, Opal P, Goldman RD, Messing A, Perng MD. The role of gigaxonin in the degradation of the glial-specific intermediate filament protein GFAP. Mol Biol Cell. 2016;27:3980–90.
Opal P, Goldman RD. Explaining intermediate filament accumulation in giant axonal neuropathy. Rare Dis. 2013;1:e25378.
Israeli E, Dryanovski DI, Schumacker PT, Chandel NS, Singer JD, Julien JP, Goldman RD, Opal P. Intermediate filament aggregates cause mitochondrial dysmotility and increase energy demands in giant axonal neuropathy. Hum Mol Genet. 2016;25:2143–57.
Lowery J, Jain N, Kuczmarski ER, Mahammad S, Goldman A, Gelfand VI, Opal P, Goldman RD. Abnormal intermediate filament organization alters mitochondrial motility in giant axonal neuropathy fibroblasts. Mol Biol Cell. 2016;27:608–16.
Veena MS, Wilken R, Zheng JY, Gholkar A, Venkatesan N, Vira D, Ahmed S, Basak SK, Dalgard CL, Ravichandran S, et al. p16 protein and gigaxonin are associated with the ubiquitination of NFkappaB in cisplatin-induced senescence of cancer cells. J Biol Chem. 2014;289:34921–37.
Scrivo A, Codogno P, Bomont P. Gigaxonin E3 ligase governs ATG16L1 turnover to control autophagosome production. Nat Commun. 2019;10:780.
Bailey RM, Armao D, Nagabhushan Kalburgi S, Gray SJ. Development of intrathecal AAV9 gene therapy for Giant axonal neuropathy. Mol Ther Methods Clin Dev. 2018;9:160–71.
Gong CX, Singh TJ, Grundke-Iqbal I, Iqbal K. Phosphoprotein phosphatase activities in Alzheimer disease brain. J Neurochem. 1993;61:921–7.
Gong CX, Shaikh S, Wang JZ, Zaidi T, Grundke-Iqbal I, Iqbal K. Phosphatase activity toward abnormally phosphorylated tau: decrease in Alzheimer disease brain. J Neurochem. 1995;65:732–8.
Veeranna KT, Boland B, Odrljin T, Mohan P, Basavarajappa BS, Peterhoff C, Cataldo A, Rudnicki A, Amin N, et al. Calpain mediates calcium-induced activation of the erk1,2 MAPK pathway and cytoskeletal phosphorylation in neurons: relevance to Alzheimer's disease. Am J Pathol. 2004;165:795–805.
Acknowledgements
We thank members of the Opal lab and Vicky Brandt for suggestions and thoughtful comments on the manuscript. We also thank Aayushi Garg and Saurav Chopra for their help with literature searches.
Funding
This study was supported by NIH grants 1R01NS062051, 1R01NS082351 and R56NS108639 to PO. AD holds a Marilyn Hilton Award for Innovation in MS Research from the Conrad N. Hilton Foundation (#17323).
Availability of data and materials
Not applicable.
Author information
Authors and Affiliations
Contributions
PO conceived and supervised the project. AD and PO reviewed the literature, drafted the manuscript, and prepared the figures. Both authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Authors’ information
Not applicable.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
Dr. Opal received compensation for medico-legal and ad hoc consulting and royalty payments from UpToDate.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Didonna, A., Opal, P. The role of neurofilament aggregation in neurodegeneration: lessons from rare inherited neurological disorders. Mol Neurodegeneration 14, 19 (2019). https://doi.org/10.1186/s13024-019-0318-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13024-019-0318-4