Abstract
Background
The economic burden of rare diseases on health systems is still not widely measured, with the generation of accurate information about the costs with medical care for subjects with rare diseases being crucial when defining health policies. Duchenne Muscular Dystrophy (DMD) is the most common form of muscular dystrophy, with new technologies recently being studied for its management. Information about the costs related to the disease in Latin America is scarce, and the objective of this study is to evaluate the annual hospital, home care and transportation costs per patient with DMD treatment in Brazil.
Results
Data from 27 patients were included, the median annual cost per patient was R$ 17,121 (IQR R$ 6,786; 25,621). Home care expenditures accounted for 92% of the total costs, followed by hospital costs (6%) and transportation costs (2%). Medications and loss of family, and patient’s productivity are among the most representative consumption items. When disease worsening due to loss of the ability to walk was incorporated to the analysis, it was shown that wheelchair users account for an incremental cost of 23% compared with non-wheelchair users.
Conclusions
This is an original study in Latin America to measure DMD costs using the micro-costing technique. Generating accurate information about costs is crucial to provide health managers with information that could help establish more sustainable policies when deciding upon rare diseases in emerging countries.
Similar content being viewed by others
Background
Duchenne Muscular Hystrophy (DMD) is a X-linked inherited genetic disease caused by mutations in the DMD gene. Variants leading to loss of dystrophin function, a product of this gene, modify the structure of the muscle cell that is progressively replaced by fibrosis and fatty tissue, resulting in a clinical setting of progressive muscle weakness. Most boys with DMD inherit the condition from their mother; however, any de novo mutations are reported in approximately 1/3 of the cases. Although there are variations in the clinical evolution, the condition leads to early loss of the ability to walk, followed by cardiac and respiratory complications throughout life [1,2,3]. In a recent meta-analysis, the global overall mean prevalence of the disease was 19.8 cases per 100,000 male live births (95% CI: 16.6–23.6) [4]. Data from a single-center study in the state of Ceará indicate a prevalence of 0.44 cases per 100,000 (95% CI: 0.31–0.6) [5]; however, the overall prevalence of DMD in Brazil is unknown. A recent study from Brazil suggested that the molecular epidemiology of DMD cases in Brazil is similar to that from other regions worldwide, with major deletions and duplications identified in approximately 70% of cases, followed by nonsense variants in 13% of cases [6].
Difficulty to walk and need for wheelchair during adolescence, or before that, make patients with DMD highly dependent on their relatives. Life expectancy decreases with age, with a median life expectancy of 28 years for patients born after 1990 [7]. Advances in the care related to cardiac and respiratory problems have increased survival and, consequently, costs associated with disease care [3, 8, 9]. Treatment for DMD usually includes the continuous use of corticosteroids associated with other outpatient follow-up interventions from multiple professionals at reference sites, which delays the disease progression and allows an improved quality of life [10, 11].
Ataluren is a new drug recommended for patients with nonsense mutations in DMD gene, which is present in 10-15% of patients. It is not currently available in the Brazilian Unified Health System [Sistema Único de Saúde (SUS)], and it has been approved by the Brazilian National Health Surveillance Agency [Agência Nacional de Vigilância Sanitária (Anvisa)] in 2019 for male children who are able to walk, aged ≥ 5 years old, with DMD due to this genetic variant. Other medications have been recently approved in the United States for patients with specific mutations: eteplirsen (in 2016), golodirsen and viltolarsen (in 2020), and casimersen (in 2021). However, these medications are not approved by Anvisa for use in Brazil [12].
The lack of accurate information about the costs related to rare diseases, specifically for DMD, limits the ability to better manage the resources available for treating such diseases [13]. Generating costs and detailed information is crucial to target efficient use of resources to manage patients with this rare condition in the health systems [14].
The objective of this study is to evaluate the annual hospital, home care and transportation costs per patient for the treatment of DMD in Brazil.
Methods
The study is characterized as a cohort analysis, with retrospective collection of the patients’ data, and the cost analyses using microcosting techniques are under the perspective of the public health system and of the patients (family).
Patients diagnosed with DMD treated throughout 2019 at Hospital de Clínicas de Porto Alegre (HCPA), who were able (or had a relative who was able) to understand and answer the study questionnaire and with no other chronic diseases impacting morbidity and mortality (with a life expectancy < 2 years), were deemed eligible. Only patients who agreed with the General Personal Data Protection Law [Lei Geral de Proteção de Dados (LGPD)] were invited to participate in the study. After accepting and signing the Informed Consent Form (ICF), patients were enrolled in the study. This study was submitted to and approved by the Ethical Committee of HCPA (CAAE: 54235721.9.0000.5327).
Hospital and transportation costs
To survey all hospital and transportation costs, the steps from the Time-driven Activity-based Costing (TDABC) method were followed [14, 15], including: mapping of the flow of care with the main activities to which the patient is submitted to; identification of all resources and departments used by the patient; estimation of the overall expenses of every resource identified in the flow of care; estimation of the hourly capacity of every resource or department, and unit cost rate (UCR) calculation; analysis of the time spent on the patient for every resource, and structuring of the time and costs equations; cost per patient calculation; and statistical analyses.
Mapping of the flow of care for the main activities to which the patient is submitted
The mapping of the flow of care was performed by a multidisciplinary team, including physical therapists and physicians from the neurology and medical genetics services of the site participating in the project, as well as researchers from the health technologies evaluation area.
Identification of all hospital resources and departments used by the patient
Based on the review of clinical records in the electronic medical files from patients enrolled in the study, the locations at the reference site where the patient is treated were identified, as well as the equipment and workers involved in the activities to which patients are submitted.
Estimation of the total cost of every resource identified in the hospital flow of care
For each resource identified, annual mean costs were calculated. For the physical structure of the hospital, site data of costs from the departments where the patient received care were surveyed with hospital financial department, considering fixed costs of depreciation, energy, supporting materials, taxes, and system licenses. For labor-costs, the data from 2019 was used, including charges by mean professional class from the institution.
Estimation of the hourly capacity of every resource or department and unit cost rate calculation
The capacity of the ambulatory units where patients with DMD were treated were estimated considering the availability of rooms and service teams and the utilization of the installed capacity. For labor, the workload contracted throughout one month was used. With the information on cost per resource and capacity it is possible to calculate the cost-capacity-rate (CCR) for each resource.
Analysis of the time spent on the patient for every resource and structuring of the time and cost equations
The mean time every worker spends involved with the consultations and procedures performed on patients with DMD was estimated based on reports from healthcare professionals.
Hospital and transportation cost calculation
In order to evaluate the individual cost per patient, the time spent for each resource was multiplied by the time CCR and, subsequently, by the costs with medications and exams. For medications, the individual consumption was extracted from the medical chart, and the cost considered was the one recorded in 2019 in the Health Prices Bank [Banco de Preços em Saúde (BPS)] [16]. Exams were computed from the identification in the clinical records of the exams, with the costs recorded at the site being used as the basis for the financial information.
Finally, the cost with transportation to the reference site per patient was estimated. For residents in the city of Porto Alegre, two round-trip public transportation tickets were accounted for each consultation when transportation costs were not informed by the patient. For residents of other cities, the transportation cost was estimated based on the cost per kilometer (km) covered, simulating the public transportation costs employed by the city halls. In this case, the estimated costs with depreciation, maintenance, vehicle insurances, driver wages, and toll fees were included. Therefore, the transportation cost was established based on the estimation of distances covered from the patients’ city of origin to the hospital where they are treated, considering a round trip.
Home care cost
In order to estimate home care cost with the disease, a validated instrument was applied to the patient or his family via phone call. The questionnaire included questions about costs related to the acquisition of medications and medical materials, health insurance, required changes to the patient’s home, periodic consultations with experts outside the reference site, caretakers, and loss of patient’s productivity and of the relative’s productivity due to the disease (Supplemental material 1) [17].
Cost analysis considering the clinical status of patients
The cost results were shown descriptively, considering the patient’s mobility status (wheelchair user or non-wheelchair user). The costs in the analyzed categories (hospital, home care, transportation, and total costs) for wheelchair users and non-wheelchair users were compared using the Mann-Whitney test, a non-parametric statistical test. Results were collected and are expressed in the Brazilian currency (Brazilian reais).
Results
Sample description
There were 43 follow-up patients identified in 2019 at the study site and, after evaluating for the inclusion criteria and invitation to participate, 27 patients were enrolled in the study (Supplemental Fig. 1). Table 1 shows the clinical characteristics of patients.
Flow of hospital care
To evaluate the hospital costs, firstly the patient flow of care was mapped at the reference site. The follow-up of patients with DMD is conducted primarily in an outpatient setting and includes a series of periodic clinical appointments and exams (Supplemental Fig. 2).
Estimating the costs per time unit and timepoints
The CCR were calculated for all the professionals categories and structural recourses (Supplemental Table 1) and used in the sequence to estimate the cost per resource per activity along the care pathway.
Calculating the costs per patient
The total median cost (hospital, home care, and transportation) per patient/year was R$ 17,122 (IQR R$ 6,786; 25,621), within which 92% was of home care costs, 6% of hospital costs, and 2% of transportation costs. Table 2 shows the cost from all three categories of costs and its components. One patient was treated as an outlier for receiving drug treatment with ataluren and for showing a cost above three standard deviations of the sample. This patient showed an annual cost of R$ 994,758.
The composition of costs within the three classes (hospital, transportation, and home care) for every patient is shown in Supplemental Fig. 3. Among those patients who showed a proportionally higher hospital cost (P13, P20 and P25), two (P13 and P20) did not report home care costs, which contributes to the higher hospital proportion.
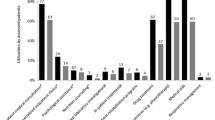
In order to understand the variability of sample costs, the composition of hospital and home care costs were evaluated separately. The cost items with the highest impact on the hospital costs were medications, with a median cost of R$ 767 (IQR R$ 219; 1,371). Among the categories analyzed in terms of home care costs, the median loss of productivity was the most impactful item, with median cost of R$ 5,932 (IQR R$ 0; 15,106), followed by medications, with median cost of R$1,110 (IQR R$ 0; 2,370). Figure 1A and 1B show the composition of hospital and home care costs, respectively. Three patients showed the highest hospital costs: P13, P20 and P25. Among these patients, P13 is a wheelchair user, while P20 and P25 are not wheelchair users, meaning it is not possible to directly associate a higher hospital cost to the fact a patient is a wheelchair user.
In the home care cost analysis, seven participating patients reported holding a health insurance plan (P2, P8, P10, P11, P21, P24, P25), with an annual mean cost of R$ 3,389 (SD R$ 3.331). In addition to the monthly fees of the plan, this group of patients also reported an annual mean cost of R$ 677 (SD R$ 1,312) with private medical and non-medical consultations. Among patients with no health insurance plan, the annual mean cost with private consultations was R$ 105 (SD R$ 282).
Overall, 23 categories of professionals were mentioned among the consultations conducted in 2019 outside the reference site. The professional category with the highest number of consultations attended by patients was motor physical therapist (with 1,840 consultations reported by 22 patients) (Table 3).
Analyses between groups
No significant difference was observed between the median costs for wheelchair users and non-wheelchair users. Descriptively, the median cost for wheelchair users was 22.63% higher than that for non-wheelchair users, with the highest difference being explained by the hospital costs (Fig. 2).
Discussion
The economic burden associated to the care provided to subjects with rare diseases is significant worldwide, and there is still a need for studies that measure the use of resources by these patients in the health system. In addition, researches estimating the economic impact under the perspective of this system vary in terms of methodology, which makes it difficult to use this information to manage resources [19]. This is a pioneer study in Latin America for measuring cost using the micro-costing technique for patients with DMD. The use of human, structural and health technologies resources was measured for patients with the disease over a period of one year, and it was identified that home care costs are more significant than hospital costs, and that wheelchair users have a higher cost to deal with. The information generated may help supporting health policies in rare diseases based on scientific evidence from economic data.
The observed median annual cost of R$ 17,122 (representing a PPP, Purchasing Power Parity of 7,285) is close to the data estimated in a North American study (8,735 dollars a year) that evaluated the expected cost in 20 years for patients with the disease diagnosed at five years of age and with loss of the ability to walk by the age of 11 treated with prednisone [20]. In the payers point of view and using the micro-costing technique, in Germany, the direct yearly costs for patients with DMD were estimated to be 28,951 Euros (PPP 39,604), with 46% of this cost being justified by hospitalizations, rehabilitation services, and investments in housing [17]. Despite the differences in values, which can be partially explained by the different methodologies adopted and pricing policies, the European study also showed an increase in costs as the severity of the patient’s disease increased, with the costs for patients who are no-ambulatory (stage V) being six times higher than those for children in ambulatory transitional phase (stage III). In both studies, the analyses were performed based on payment data, without evaluating the use of resources per patient as in the micro-costing studies, in addition to including distinct variables.
The care for DMD is mostly provided by multidisciplinary teams and, therefore, is concentrated in specialized care sites. In Brazil, patients with DMD have access to treatment by the public health system, SUS, and their families are entitled to the benefits foreseen in law for subjects with disability. The use of the information generated, which shows the financial impact for the patients’ families, indicate the importance of establishing economic strategies for rare diseases involving the State, treatment sites, and organizations that provide therapeutic technologies, so that they can be properly used, resulting in better health outcomes.
The opportunity to understand the variability in the use of health system resources due to the health status of every patient provided by micro-costing methods helps establishing health policies focused on the actual needs of patients. Measuring the incremental costs related to the loss of the ability to walk has a significant contribution to the evaluation process for health technologies in DMD. Technologies that have been recently approved by the Food and Drug Administration (FDA), eteplirsen (Exondys) and golodirsen (Vyondys 53) have the benefit of delaying the loss of the ability to walk [20, 21]. Based on the study conducted using the TDABC method, the national evaluations regarding the incorporation of these technologies in the Brazilian health system may use information that show the actual cost associated with the anticipated worsening of the health status of patients who have no access to this technology.
Some precautions must be considered when using the cost information generated in this study. The inclusion of patients from a single center is the main one. Thus, generalizations about the information presented both nationally and internationally must be made with caution. However, it must be observed that the molecular epidemiology of the patients in this study is similar to that of the largest Brazilian casuistry described recently (Supplemental Table 2) [6].
The presence of a single patient on ataluren treatment in the sample made it impossible to conduct evaluations showing the incremental cost related to the use of new drugs that restore the expression of dystrophin in specific patient profiles. Finally, this study focused on the description of costs from a patient cohort, and future analyses might use the data presented herein to build economic models to evaluate health technologies incorporation and remuneration for DMD in the health system.
Conclusion
The care of patients with DMD represents a significant cost that is primarily justified by family disbursement (92%), followed by hospital costs. This is the first study conducted in Latin America measuring the treatment costs for follow-up patients with DMD in the Brazilian health system, contributing to the international literature, which is scarce in reporting data on the use of resources in the health system by patients with rare diseases. Generating accurate information about costs is crucial to provide health managers with information that make it possible to establish more sustainable policies when managing rare diseases in emerging countries.
Data Availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Caskey CT, Nussbaum RL, Cohan LC, Pollack L. Sporadic occurrence of Duchenne muscular dystrophy: evidence for new mutation. Clin Genet. 1980 Nov;18(5):329–41.
Hoffman EP, Brown RHJ, Kunkel LM. Dystrophin: the protein product of the Duchenne muscular dystrophy locus. Cell 1987 Dec;51(6):919–28.
Birnkrant DJ, Bushby K, Bann CM, Apkon SD, Blackwell A, Brumbaugh D, et al. Diagnosis and management of Duchenne muscular dystrophy, part 1: diagnosis, and neuromuscular, rehabilitation, endocrine, and gastrointestinal and nutritional management. Lancet Neurol. 2018 Mar;17(3):251–67.
Crisafulli S, Sultana J, Fontana A, Salvo F, Messina S, Trifirò G. Global epidemiology of Duchenne muscular dystrophy: an updated systematic review and meta-analysis. Orphanet J Rare Dis. 2020 Jun;15(1):141.
Teixeira M, de SR, Martins GMA, Rodrigues JMM, Marques ER. Epidemiologia da distrofia muscular de Duchenne no Ceará / Epidemiology of Duchenne muscular dystrophy in Ceará. BJD. 2020;6(9):69591–603.
de Almeida PAD, Machado-Costa MC, Manzoli GN, Ferreira LS, Rodrigues MCS, Bueno LSM, et al. Genetic profile of brazilian patients with dystrophinopathies. Clin Genet. 2017 Aug;92(2):199–203.
Broomfield J, Hill M, Guglieri M, Crowther M, Abrams K. Life Expectancy in Duchenne muscular dystrophy: reproduced individual Patient Data Meta-analysis. Neurology. 2021 Dec;97(23):e2304–14.
Bushby K, Finkel R, Birnkrant DJ, Case LE, Clemens PR, Cripe L, et al. Diagnosis and management of Duchenne muscular dystrophy, part 1: diagnosis, and pharmacological and psychosocial management. Lancet Neurol. 2010 Jan;9(1):77–93.
Passamano L, Taglia A, Palladino A, Viggiano E, D’Ambrosio P, Scutifero M, et al. Improvement of survival in Duchenne muscular dystrophy: retrospective analysis of 835 patients. Acta Myol. 2012 Oct;31(2):121–5.
Araujo APQC, de Carvalho AAS, Cavalcanti EBU, Saute JAM, Carvalho E, França MCJ, et al. Brazilian consensus on Duchenne muscular dystrophy. Part 1: diagnosis, steroid therapy and perspectives. Arq Neuropsiquiatr. 2017 Aug;75(8):104–13.
Werneck LC, Lorenzoni PJ, Ducci RDP, Fustes OH, Kay CSK, Scola RH. Duchenne muscular dystrophy: an historical treatment review. Arq Neuropsiquiatr. 2019 Sep;77(8):579–89.
Brasil. Ministério da Saúde. Monitoramento do Horizonte Tecnológico: medicamentos para tratamento da distrofia muscular de Duchenne. Brasília: Ministério da Saúde; 2022. p. 45.
Cannizzo S, Lorenzoni V, Palla I, Pirri S, Trieste L, Triulzi I, et al. Rare diseases under different levels of economic analysis: current activities, challenges and perspectives. RMD Open. 2018;4(Suppl 1):e000794.
Brasil. Ministério da Saúde. Secretaria de Ciência, Tecnologia, Inovação e Insumos Estratégicos em Saúde. Departamento de Gestão e Incorporação de Tecnologias e Inovação em Saúde. Diretriz Metodológica: estudos de microcusteio aplicados a avaliações econômicas em saúde. 2021. 71p.
Keel G, Savage C, Rafiq M, Mazzocato P. Time-driven activity-based costing in health care: a systematic review of the literature. Health Policy. 2017 Jul;121(7):755–63.
BPS - Banco de Preços em Saúde. ; V-3.1.33 [Internet]. [cited 10 de junho de 2022]. Available from: http://bps.saude.gov.br/
Schreiber-Katz O, Klug C, Thiele S, Schorling E, Zowe J, Reilich P, et al. Comparative cost of illness analysis and assessment of health care burden of Duchenne and Becker muscular dystrophies in Germany. Orphanet J Rare Dis. 2014 Dec;9:210.
Araujo APQC, Nardes F, Fortes CPDD, Pereira JA, Rebel MF, Dias CM, et al. Brazilian consensus on Duchenne muscular dystrophy. Part 2: rehabilitation and systemic care. Arq Neuropsiquiatr. 2018 Jul;76(7):481–9.
Garrison S, Kennedy A, Manetto N, Pariser AR, Rutter JL, Yang G. The Economic Burden Of Rare Diseases: Quantifying The Sizeable Collective Burden And Offering Solutions. Health Affairs Forefront. 2022 Feb 01 [cited 2022 May 22]; Available from: https://www.healthaffairs.org/do/https://doi.org/10.1377/forefront.20220128.987667
Beckers P, Caberg JH, Dideberg V, Dangouloff T, den Dunnen JT, Bours V, et al. Newborn screening of duchenne muscular dystrophy specifically targeting deletions amenable to exon-skipping therapy. Sci Rep. 2021 Feb;11(1):3011.
Aartsma-Rus A, Krieg AM. FDA approves Eteplirsen for Duchenne muscular dystrophy: the next chapter in the Eteplirsen Saga. Nucleic Acid Ther. 2017 Feb;27(1):1–3.
Acknowledgements
Not applicable.
Funding
This research was funded by the company Produtos Roche Químicos e Farmacêuticos S/A do Brasil. The Instituto de Avaliação de Tecnologia em Saúde (IATS) was responsible for conducting the study, writing the manuscript and editorial review.
Author information
Authors and Affiliations
Contributions
NBS and ECR collected, analyzed and interpreted data, contributed to manuscript writing and reviewing. ALPS and IPB collected data and contributed to manuscript reviewing. ACA, TCM, NBR and PL participated in study design, data analysis and interpretation, and contributed to manuscript reviewing. JAMS contributed to data analysis and interpretation, manuscript writing and reviewing. APBSE and CAP participated in study design and coordination, contributed to data analysis and interpretation, manuscript writing and reviewing. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Only patients who agreed with the General Personal Data Protection Law [Lei Geral de Proteção de Dados (LGPD)] were invited to participate in the study. All patients included have signed the Informed Consent Form (ICF). This study was submitted to and approved by the Ethical Committee of Hospital de Clínicas de Porto Alegre (CAAE: 54235721.9.0000.5327).
Consent for publication
Not applicable.
Competing interests
The study was an initiative of the company Produtos Roche Químicos e Farmacêuticos S/A do Brasil. The researchers involved in the study had full autonomy in conducting the study and are responsible for the data presented here.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
13023_2023_2767_MOESM1_ESM.docx
Supplementary Material 1: This document includes the “Instrument for collecting household expenses and indirect costs”, and “Equations of loss of patient’s productivity”.


13023_2023_2767_MOESM3_ESM.jpg
Supplementary Material 3: Supplementary Fig. 2: Macro-flow of care for patients with Duchenne Muscular Dystrophy at a specialized site.

13023_2023_2767_MOESM4_ESM.jpg
Supplementary Material 4: Supplementary Fig. 3: Composition of costs for each patient enrolled according to the categories of cost.
13023_2023_2767_MOESM6_ESM.docx
Supplementary Material 6: Supplementary Table 2: Frequency of mutation among the study patients compared to Brazilian population reported by Almeida et al., 2017 [6].
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Schneider, N.B., Roos, E.C., Staub, A.L.P. et al. Estimated costs for Duchenne muscular dystrophy care in Brazil. Orphanet J Rare Dis 18, 159 (2023). https://doi.org/10.1186/s13023-023-02767-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13023-023-02767-6