Abstract
Background
Impairment of bulbar function in adult individuals with spinal muscular atrophy (SMA) usually is not assessed by established motor scores. Measurements of oral function including quantitative muscle and endurance tests are able to detect subtle changes. The aim of this study was to systematically evaluate the measurement of maximum bite force and endurance, maximum tongue pressure and endurance, as well as maximum mouth opening in adult individuals with SMA types 2 and 3.
Methods
Data from oral function tests in 43 individuals were analyzed. Differences in oral function between individuals with different SMA types and numbers of SMN2 copies were tested. Spearman´s rho correlations among oral function measures themselves as well as with established clinical outcome scales were analyzed.
Results
The absolute maximum measures of oral function (maximum bite force, maximum tongue pressure, maximum mouth opening) were able to discriminate between individuals with different SMA types, individuals with a different number of SMN2 copies and with different walking abilities. The pairwise correlations of the absolute maximum measures of oral function were fair to moderate in size; the same was true for their correlations with the established motor scores. All correlations assessing endurance measures of oral function were weaker and statistically insignificant.
Conclusions
Among the oral function tests maximum tongue pressure and maximum mouth opening are particulary promising as clinical and sensitive outcome measures for clinical trials. Oral function tests may supplement existing motor scores, in particular concerning specific questions about bulbar function or in severely affected non-ambulatory individuals where mild (treatment-related) changes would otherwise remain undetected.
Trial registration DRKS, DRKS00015842. Registered 30 July 2019, https://drks.de/search/de/trial/DRKS00015842
Similar content being viewed by others
Background
5q-associated spinal muscular atrophy (SMA) is a rare disease with an incidence of 1 in 7.000 live births in Germany, based on newborn screening [1, 2]. This hereditary autosomal recessive neuromuscular disorder is characterized by a progressive degeneration of motor neurons in the spinal cord. Biallelic deletions and/or point mutations of the survival of motor neuron 1 (SMN1) gene lead to a SMN protein deficiency [3, 4]. The consequences are muscular atrophy and weakness, as described in detail for axial and proximal muscle groups. While early bulbar symptoms have not yet been systematically recorded, bulbar muscles may be affected once the brainstem motor nuclei are involved [5,6,7]. The survival motor neuron 2 (SMN2) gene is a homologous copy of SMN1. Its number of copies inversely correlates with disease severity, and SMN2 is the target of pharmacotherapeutic approaches [8,9,10,11]. Nusinersen, an antisense oligonucleotide drug for SMA therapy was the first causative treatment correcting the splicing of SMN2 pre-mRNA and thus increasing the production of the SMN protein [12, 13].
The clinical spectrum of SMA is very broad. According to age of onset and severity, best achieved motor milestone and life span, the different subtypes 0–4 are classified [14]. Not just since therapy options have been available, there is an urgent need for sensitive clinical tools to observe relevant motor skills including bulbar function in order to precisely represent the functional status of individuals with SMA.
Progressive weakness and degeneration of bulbar muscles in SMA patients become evident in impaired muscle strength and increased fatigability in oral functions. Noticeably, for the patients, it affects their speaking, chewing, swallowing and maximum mouth opening [7, 15, 16]. Clinically, it is observed that oral functions are usually later and less severely affected than axial and proximal muscles in patients with SMA type 1 and 2 [16]. Electrophysiological and video fluoroscopic examinations, questionnaires, and MRI scans confirmed reduced oral functions in these patients [7, 17]. Approaches to quantitatively assess oral function in untreated SMA patients showed that maximum bite force was reduced by 19—50% compared to healthy controls [7, 15]. Reported values of maximum bite force in healthy subjects greatly vary, due to physiological and methodological factors [18], with a landmark of 560 N for the method used in this study (bite force endurance: 65 s; own data on healthy subjects, not reported here). Tongue pressure has already been suggested to be associated with bulbar and upper limb function in SMA and has been presented as a useful biomarker with mean values of 15.3 ± 6.4 kPa in SMA patients versus 37.3 ± 9.6 kPa in healty controls [19]. A physiological active mouth opening can be considered to be about 55 mm and has been shown to be clearly reduced to 38.9 ± 15.3 mm in SMA patients [7].
In patients with SMA, minor bulbar and oral symptoms may occur at earlier stages than currently assumed [19], which is one reason why they should be routinely recorded. There are other important reasons to routinely measure oral function in SMA patients. First, restrictions in oral function have a direct impact on the patient's quality of life [17, 20]. Second, due to a restricted mouth opening and reduced bite force / tongue pressure patients are at risk of serious adverse events, e.g. complications with intubation, severe choking due to impaired mastication or the risk of aspiration pneumonia [16, 21].
In severely affected adult individuals with residual motor function, measurements of oral function can provide valuable information on progressively impaired bulbar function. This may be particularly helpful when existing motor function scores reach their limits due to immobility of the patients. Representing only trunk and extremity function, the Hammersmith Functional Motor Scale (HFMSE) or the Revised Upper Limb Module (RULM), which are probably the most widely used motor scores in late-onset SMA patients, are at risk to miss out possible changes at the extreme ends of the spectrum of physical abilities [22,23,24,25]. Despite its potential, measures of oral function or more general bulbar function in SMA have not yet been validated or standardized. To capture impairment in oral function in different ways, measurement parameters should complement each other.
The Bogenhausener Dysphagia Score (BODS), the Sydney Swallow Questionnaire or the bullbar subscore of the Amyotrophic Lateral Sclerosis Functional Rating Scale Revised (ALSFRS-R) give first insights into parts of the bulbar function. However, these established scales depend on subjective evaluation, produce ordinal data with a limited response range, or are inadequate to cover the whole bulbar spectrum in SMA. A promising avenue to address these shortcomings is the quantitative muscle testing of oral function. The combined measurement of maximum bite force, maximum tongue pressure and maximum mouth opening covers a large part of bulbar muscle function [17]. The complementary measurements can detect small changes in bulbar function providing data over a continuous range. A pilot study confirmed the feasibility of maximum bite force measurements in two severely affected SMA patients during causative treatment, and provided first evidence of changes in bite force over the period of one year [26].
The discriminating power of combined oral function measures can be further increased by measuring muscle endurance. Endurance is defined as the prolonged maintenance of a constant or self-regulated force level [27, 28]. Even though considered less reliable than measures of maximum muscle strength [29], the measurement of oral function endurance is an important additional dimension of physical impairment in SMA. Muscle endurance is also targeted for therapeutic interventions [30,31,32,33,34]. Although the causes of fatigue in SMA patients have not yet been fully elucidated, and the associated disability hinders the patients’ daily life activities, endurance as an outcome measure has received limited attention [30,31,32,33]. Studying endurance in bite force and tongue pressure will help to identify factors associated with fatigability.
The aim of this study was to examine and systematically evaluate oral function tests including maximum bite force and endurance, maximum tongue pressure and endurance, as well as maximum mouth opening in adult SMA patients. We assessed the diagnostic potential of oral function tests (1) by evaluating their ability to differentiate between groups with different SMA-specific characteristics (SMA type, SMN2 copy number, ambulatory status), (2) by identifying the extent to which the different measures of oral function are measuring the same construct, and (3) by examining the degree to which the newly introduced scores are consistent with established instruments measuring motor function.
Material and methods
Subjects
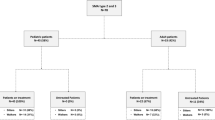
Initially, 44 SMA individuals were recruited into this study. According to the study protocol subjects were excluded if they showed significant respiratory compromise, a maximum mouth opening of less than 8 mm, or multiple missing teeth in the posterior region. The exclusion criteria applied to one out of the 44 individuals, who was excluded due to an extremely restricted mouth opening. A total of 43 adult individuals with genetically confirmed 5q-SMA were included for this study from the Departments of Neurology at the University Hospitals of Cologne and Essen, Germany. The sample size was not calculated, but determined by the number of eligible individuals willing to participate.
The study was approved by the Ethics Committees of the Medical Faculties of the two sites and conducted in accordance with the declaration of Helsinki (Reference Number Cologne: 19–1137; Reference Number Essen: 21–9851-BO). Each subject provided informed consent. Information on SMA type and SMN2 copies were derived from the patients' medical records.
Testing
Maximum bite force, bite force endurance, maximum tongue pressure, tongue pressure endurance, and maximum mouth opening were measured in accordance with the study protocol by Kruse and coworkers [35]. Oral function tests in each SMA patient were performed twice: two measurements were scheduled within one week, with a minimum of two days in between. No nusinersen application or other medical intervention was scheduled within these days. The tests were conducted prospectively at two sites by one of three dentists (DL, AC, TK) who had been previously trained in the method on healthy probands. For both study sites, standardized administration procedures and order of evaluation were set for each measure analyzed. Subjects were evaluated with the established motor scores as part of the routine evaluation during patients’ visits for nusinersen injection or as part of the natural history evaluation: HFMSE, RULM, ALSFRS-R and 6MWT (6-Minute-Walk-Test) were rated by physiotherapists, who had been trained in standardized therapy evaluation. BODS was carried out as part of the study by speech therapists, who were familiar with the evaluation of dysphagia in neuromuscular diseases.
To measure maximum bite force and bite force endurance, a piezoelectric sensor system consisting of a T-Scan sensor covering the entire dental arch and the I-Scan software (Tekscan, Inc., South Boston, MA) was used. Prior to the first measurement, the surface of the sensor was adjusted to the individual’s dental situation using dental silicon as described by Kruse and coworkers [35]. Testing the maximum bite force, individuals were asked to bite three times with maximum force for a duration of three to four seconds, with pauses of at least 30 s to avoid muscle fatigue. The highest score of maximum bite force (in Newton) was used for analysis. For the endurance test, individuals were asked to hold the adduction at 60% of the previously determined maximum bite force for as long as they could. The time (in seconds) until bite force dropped below 30% of the previously determined maximum bite force was used as an outcome value for further analysis.
Maximum tongue pressure and tongue pressure endurance were measured using a handheld device (IOPI Medical LLC, Carnation, WA: Iowa Oral Performance Instrument) with a single air-filled bulb tongue array, which was placed on the tongue blade in a predefined position: 10 mm posterior of the tongue tip and 10 mm anterior to the circumvallata papilla [36]. Individuals were advised to press their tongue against the air-filled bulb three times with maximum force for a duration of three to four seconds, with a 30 s pause between each repetition. The highest score of maximum tongue pressure (in kilopascal) was used for analysis. For the endurance test, individuals were asked to hold the muscle force at 60% of the previously determined maximum tongue pressure as long as they could. Again, time (in seconds) was recorded in which patients’ values dropped from 60% to 30% of their maximum value. Active mouth opening was measured at the mesioincisal angle of the upper and lower front teeth by a ruler registering the maximum distance (in millimeter) without reported pain.
Inter- and intra-rater reliability
During additional measurements on healthy subjects inter- and intra-rater reliability was established using intraclass correlation coefficients (ICC). Inter-rater reliability was determined based on measurements by two raters (trained dentists DL and AC) alternately rating the same subjects within a one-week period (14 subjects overall for bite force and 7 subjects overall for tongue pressure). Intra-rater reliability was determined based on measurements by three raters (trained dentists DL, AC, and TK), each of whom rated the subjects twice within a given week (43 subjects overall for bite force and 33 subjects overall for tongue pressure).
Statistical analysis
The outlined testing procedure resulted in patient-specific data on five outcomes from two visits. Distribution of the data was examined for normality. As all outcome variables failed to withstand the Kolmogorov Smirnoff or Shapiro–Wilk test, no normal distribution could be confirmed. For each outcome, the mean across the first and second measurement was used for analysis in order to reduce bias due to training effects or fluctuations depending on daily form [38, 39].
Discriminant power of oral function tests was examined via Wilcoxon rank-sum tests assessing distributional differences between different patient groups (SMA type 2 vs. 3, 3 vs. 4 SMN2 copies, non-ambulatory vs. ambulatory). After alpha adjustment (Bonferroni correction), results were deemed statistically significant at a level of p < 0.017.
Correlations between the outcomes of the different oral function tests were assessed by means of Spearman´s rho (ρ). Results were deemed statistically significant at a level of p < 0.05.
Correlations among oral function tests and the clinical outcome scales BODS, HFMSE, RULM, ALSFRS-R and 6MWT were assessed by means of Spearman´s rho (ρ) and the treshold for statistical significance was set at p < 0.01 applying a Bonferroni correction for multiple testing. Since the 6MWT is only measured for ambulatory patients, non-ambulatory patients were defined as reaching a distance of 0 m for statistical analysis. The strength of all correlations was classified as none, poor, fair, moderate, very strong or perfect according to the definition introduced by Chan Y [40]. All statistical analyses were conducted using the statistical software SPSS 28.0.1.0 (IBM, SPSS statistics version 28.0.1.0, Chicago, IL, USA).
Results
Inter- and intra-rater reliability
Repeated measurements in healthy subjects indicated very good inter- and intra-rater reliability of the oral function measures, according to conventional guidances [37]. Intraclass correlation coefficients assessing interrater reliability were ‘almost perfect’ to ‘excellent’, ranging at 0.81 for maximum bite force, 0.95 for bite force endurance and 0.93 for maximum tongue pressure. For tongue pressure endurance the ICC of 0.67 showed ‘substantial agreement’. The intra-rater reliability of maximum bite force, bite force endurance, maximum tongue pressure and tongue pressure endurance were ‘excellent’: ICC values ranged at 0.94 for maximum bite force, 0.92 for bite force endurance, 0.95 for maximum tongue pressure and 0.96 for tongue pressure endurance.
Main results
Of the 43 individuals included, 25 were male and 18 were female. The mean age of the individuals at first testing was 39.7 ± 12.0 years (ranging between 20 and 65 years). Overall 60.5% of the individuals carried 4 SMN2 copies, 34.9% carried 3 SMN2 copies and one patient (2.3%) carried 2 SMN2 copies. For one untreated patient information on the number of SMN2 copies was missing (2.3%). According to clinical criteria, 12 individuals were diagnosed with SMA type 2 (one with 2 SMN2 and 10 with 3 SMN2 copies, one without information on the number of SMN2 copies), and 31 with SMA type 3 (five with 3 SMN2, 26 with 4 SMN2 copies). The mean age of individuals for type 2 was 33.8 ± 8.3 years and for type 3 42.0 ± 12.5 years. The majority of the sample (n = 35) consisted of patients on nusinersen therapy who had received at least the first three doses according to routine clinical practice. Eight individuals were treatment-naive at the time of data collection, 28 individuals were non-ambulatory, and 15 were ambulatory at the time of examination (Table 1). In one case, a single motor score (HFMSE) could not be performed. Depending on the respective analysis, this missing information results in a sample size ranging between 41 and 43 (Fig. 1, Tables 2, 3 and 4).
Differences in oral function across SMA type, SMN2 copy number and ambulatory status. Box-plots including medians (thick lines), first and third quartiles (lower and upper limits of each box), minimum and maximum values (whiskers), as well as outliers (filled circles). Statistically significant distributional differences are indicated by an asterisk (p < 0.017; Bonferroni corrected). Top left: max. bite force, top right: bite force endurance, center left: max. tongue pressure, center right: tongue pressure endurance, bottom: max. mouth opening
Table 2 gives a first impression of the measurements indicating the sample median values of the oral function tests and the clinical outcome scales. Minimum and maximum values show that both the oral function tests and the clinical outcome scales are subject to considerable variation in the sample of SMA patients.
Discriminant power was supported by distributional differences in the expected direction along SMA type, SMN2 copy number and ambulatory status (Fig. 1). With the exception of tongue pressure endurance, all oral function measures tended toward higher values in patients with SMA type 3 relative to SMA type 2 (n = 43), in individuals with 4 relative to 3 SMN2 copies (n = 41; one individual with 2 SMN2 copies excluded for this analysis), and in ambulatory relative to non-ambulatory individuals (n = 43).
For maximum bite force, statistically significant distributional differences after correction for multiple comparison were observable between individuals with different SMN2 copies (z = 2.653, p = 0.007) and different ambulatory status (z = 2.421, p = 0.015). Between individuals with SMA types 2 and 3, distributional differences in maximum bite force were statistically insignificant after Bonferroni correction (z = 2.031, p = 0.043). For maximum tongue pressure, statistically significant distributional differences after correction for multiple comparison were observable between individuals with different SMA types (z = 3.169, p = 0.001), different SMN2 copies (z = 3.749, p < 0.001), and different ambulatory status (z = 4.078, p < 0.001).
For bite force endurance, all comparisons of distributional differences failed to reach statistical significance at a level of p < 0.017 Bonferroni corrected (all z ≤ 1.899). The same applied to all comparisons of distributional differences of tongue pressure endurance (all z ≤ 1.164).
For maximum mouth opening, statistically significant distributional differences after correction for multiple comparison were observable between individuals with different SMA type (z = 3.712, p < 0.001), different SMN2 copies (z = 4.631, p < 0.001), and ambulatory status (z = 3.200, p = 0.001).
The correlation between maximum bite force and maximum tongue pressure was fair in size and statistically significant at the 0.05 level (ρ = 0.439, p = 0.003). Maximum mouth opening correlated fairly and statistically significantly with maximum bite force (ρ = 0.415, p = 0.006) and moderately with maximum tongue pressure (ρ = 0.558, p < 0.001). The two endurance-related measures showed little assessment agreement: Correlations between bite force endurance and tongue pressure endurance were fair, but statistically insignificant at the 0.05 level (ρ = 0.274, p = 0.075). The correlations between endurance measures and absolute maximum measures of muscle strength were poor to fair and statistically insignificant (p > 0.05; Table 3).
Maximum mouth opening correlated fairly with the 6MWT (ρ = 0.482), moderately with BODS, HFMSE, RULM and ALSFRS-R (all |ρ| ≥ 0.557). The correlations found between maximum mouth opening and all clinical outcome scales were statistically significant including the negative correlation with the Bogenhausener Dysphagia Score (BODS: higher scores indicate more severe dysphagia; all p < 0.01 Bonferroni corrected). The correlation between maximum tongue pressure and RULM was moderate and statistically significant (ρ = 0.668, p < 0.01 Bonferroni corrected). The correlations between maximum tongue pressure and BODS, HFMSE, ALSFRS-R, 6 MWT were fair and statistically significant after Bonferroni correction (all |ρ| ≥ 0.571, p < 0.01). Maximum bite force correlated fairly and statistically significantly at the 0.05 level with the HFMSE, RULM, ALSFRS-R, but statistically insignificantly after Bonferroni correction (all ρ ≥ 0.372, p ≥ 0.01). The correlations between maximum bite force and BODS as well as 6MWT were fair and still statistically significant at the 0.01 level after Bonferroni correction (both |ρ| ≥ 0.409, p ≤ 0.006) . Correlations between tongue pressure endurance and clinical outcome scales were poor or almost non-existent and statistically insignificant (all |ρ| ≤ 0.149, p ≥ 0.341). Correlations between bite force endurance and clinical outcome scales were poor to fair (all |ρ| ≤ 0.277, p ≥ 0.072). All respective correlation coefficients (ρ) are shown in Table 4.
Discussion
In this prospective, cross-sectional multicenter study, we for the first time addressed the evaluation of a set of oral function tests in adult SMA patients. New disease-modifying treatment options call for objective and sensitive methods to identify motor improvement in adult SMA patients. In recent years, the evaluation of bulbar function gained more attention and increasing interest in research [7, 16, 17, 26, 41]. Measuring small changes in adult SMA patients is complex and the combined use of several outcome measures is recommended—particularly so for specific subgroups. Oral function tests are suitable for complementing established motor scores in order to address their methodological limitations, but also to appropriately describe bulbar function in individuals at different functional levels and ages. The advantages of quantitative strength measures have been shown in ambulatory and non-ambulatory SMA patients [42,43,44,45]. Practically, the presented quantitative oral function tests have shown to be time-efficient given that they are bedside functional scores, and that muscle strength and endurance can be recorded with the same instruments.
In this study, we demonstrated that absolute maximum measures of oral function tests can discriminate between the various diagnostic types of SMA and between ambulatory and non-ambulatory individuals. They could differentiate between individuals with 3 or 4 SMN2 copies, a time-constant parameter (i.e., irrespective of patients’ age and progression of the disease) that has been used in previous validation studies [46, 47]. The correlations between the absolute maximum measures and the clinical outcome scales were fair to moderate and therefore in expected ranges, notably because at best moderate correlations can be achieved when comparing survey methods that do not measure the same constructs [48]. The highest correlation coefficients were found for maximum mouth opening, confirming that maximum mouth opening is strongly correlated with SMA type, number of SMN2 copies, maximum bite force, maximum tongue pressure and all clinical outcome scales. Restricted mouth opening is known to be associated with atrophy and fatty infiltration of the lateral pterygoid muscle [17] as well as (self reported) bulbar problems in individuals with SMA [17, 49]. Our findings support the idea that bulbar involvement is particularly well reflected in the measurement of maximal mouth opening. This may be because mouth opening is, compared to swallowing and chewing, mediated by a relatively limited number of muscles [17]. Similarly promising is the measurement of maximum tongue pressure, given the outlined correlations with established motor scores. From a clinical perspective, both methods are cost-effective and rather easy to handle for routine use.
In our data, there was little variation in the HFMSE values of weakest sitters (HFMSE < 5). These known problems of discrimination, not only of HFMSE but also of other motor scores [44, 50, 51], cannot yet be compensated by any valid outcome measure. This problem becomes particularly obvious during the survey of the 6MWT, where an evaluation is impossible for non-walkers. In contrast, oral function tests could be performed without any difficulties in this subgroup (e.g., maximum bite force ranging between 2.7 N and 1394.1 N in this study). The limited variability of the established motor scores at the lower end of the scales may reduce the statistical power of the data and could hence be the reason that not even higher correlations between the results of oral function tests and for example HFMSE, RULM or 6MWT could be achieved. The same holds true for the limited variability in BODS, where 84% of the individuals scored at 2. A more detailed analysis of ceiling and floor effects of oral function tests compared to established motor scores may provide further insights in this regard.
The relatively weak correlations observed between oral function endurance tests themselves and with established motor scores should be interpreted with some caution. Constructs tested by established motor scores are related but not identical to fatigability in bulbar function not least due to the time-lagged degeneration of bulbar function. Previous work assessing other neuromuscular diseases underlined that fatigability and weakness are distinct features of motor (dys)function [52, 53]. Endurance has been shown to be weakly associated with strength in some muscles [52]. Anatomical differences might explain the unexpected weak association between our endurance tests and the 6MWT, which sensitively detects fatigue-related changes in ambulatory SMA patients, but aims at different muscle groups [54]. Endurance measurements had been shown to be less reliable and less meaningful than absolute strength measurements [15, 39]. But in less impaired individuals (i.e., without oral dysfunction), they may be more sensitive to first constraints. Changes in bite force endurance or tongue pressure endurance could possibly contribute to a better discrimination in some subgroups, as prominent fatigability despite preserved muscle function has recently been detected in SMA patients [55]. The etiology of fatigability is complex. Fatigability and fatigue in SMA have been discussed as a secondary manifestation of impaired motor neurons and muscle loss, to be caused by neuromuscular transmission failure or by metabolic dysfunction [55,56,57,58,59,60,61]. Further attempts to determinate the reliability and to establish endurance tests are crucial steps to better understand fatigability (of bulbar function) in SMA and should be pursued.
Anatomical conditions of SMA patients and methodological challenges complicated the measurement of bite force and tongue pressure and especially endurance measurements. Similar to other neuromuscular diseases, the altered craniofacial development in SMA patients leads to a vertical growth pattern with an anterior open bite and a narrow and deep palate [15, 62]. In the case of an anterior open bite or other malocclusions, the measurement of maximum mouth opening may have been compromised and could have led to overly high values in our examinations. Due to the high palate, SMA patients may not have been able to apply full tongue pressure to the air-filled bulb or, more important, hold the tongue position for a longer time period. The challenging positioning of the air-filled bulb itself may also have added inaccuracies, as the bulb tended to slide on the tongue surface [63]. Just as bite force decreases with greater interocclusal distance [64], tongue pressure and endurance may have been influenced by the interincisal separation which is caused by the thin flexible connector tube of the IOPI device [65, 66]. To reduce interincisal separation, a thin intraoral sensor was chosen for maximum bite force measurements. While the adjusted soft sensor surface improved the area under load and prevented subconscious inhibition due to periodontal or temporomandibular joint sensibility [35, 67], it resulted in a slightly increased interocclusal distance. This distance unavoidably varied between patients which may have led to minor inaccuracies. For endurance measurements, we chose a target value of 60% of the previously determined force. Although this procedure was in line with previous approaches [15], it can be questioned for two reasons. First, methodologically inaccurate maximum values can translate into biased endurance measurements. Second, if patients had been asked to generate an absolute predefined force level, interindividual differences would potentially have been more pronounced.
The heterogeneity of patients, sometimes seen as a limitation reducing the analytic power of the data, allowed us to assess the reliability and applicability of oral function tests across a wide range of individuals differing in SMA type and SMN2 copies. These wide-ranging data on oral function in SMA have the potential to reveal additional insights into bulbar involvement in SMA through further subgroup analyses.
As only cross-sectional data were analyzed in this study, there was no possibility to examine the relative sensitivity of oral function tests to changes over time. Further research is necessary to confirm oral function tests as a standardized outcome measure in the clinical evaluation of SMA patients.
Conclusions
This study provides support for continued investigation on oral function using maximum bite force and maximum tongue pressure measurements, complemented by endurance tests and the evaluation of maximum mouth opening. The measurement of maximum mouth opening and tongue pressure, and to a lesser degree also maximum bite force have shown to be appropriate instruments both as clinical measures as well as outcome measures for clinical trials in individuals with SMA. In particular, they are able to meaningfully supplement existing motor scores in specific questions about bulbar function or in certain subgroups as severely affected, non-ambulatory individuals.
Availability of data and materials
The datasets used and/or analyzed for the current study are available from the corresponding author on reasonable request.
References
Muller-Felber W, Vill K, Schwartz O, Glaser D, Nennstiel U, Wirth B, et al. Infants diagnosed with spinal muscular atrophy and 4 SMN2 copies through newborn screening - opportunity or burden? J Neuromuscul Dis. 2020. https://doi.org/10.3233/JND-200475.
Vill K, Schwartz O, Blaschek A, Glaser D, Nennstiel U, Wirth B, et al. Newborn screening for spinal muscular atrophy in Germany: clinical results after 2 years. Orphanet J Rare Dis. 2021. https://doi.org/10.1186/s13023-021-01783-8.
Wirth B, Herz M, Wetter A, Moskau S, Hahnen E, Rudnik-Schoneborn S, et al. Quantitative analysis of survival motor neuron copies: identification of subtle SMN1 mutations in patients with spinal muscular atrophy, genotype-phenotype correlation, and implications for genetic counseling. Am J Hum Genet. 1999. https://doi.org/10.1086/302369.
Lefebvre S, Burglen L, Reboullet S, Clermont O, Burlet P, Viollet L, et al. Identification and characterization of a spinal muscular atrophy-determining gene. Cell. 1995. https://doi.org/10.1016/0092-8674(95)90460-3.
Willig TN, Paulus J, Lacau Saint Guily J, Beon C, Navarro J. Swallowing problems in neuromuscular disorders. Arch Phys Med Rehabil. 1994. https://doi.org/10.1016/0003-9993(94)90001-9.
Mercuri E, Bertini E, Iannaccone ST. Childhood spinal muscular atrophy: controversies and challenges. Lancet Neurol. 2012. https://doi.org/10.1016/S1474-4422(12)70061-3.
van Bruggen HW, Wadman RI, Bronkhorst EM, Leeuw M, Creugers N, Kalaykova SI, et al. Mandibular dysfunction as a reflection of bulbar involvement in SMA type 2 and 3. Neurology. 2016. https://doi.org/10.1212/WNL.0000000000002348.
Lefebvre S, Burlet P, Liu Q, Bertrandy S, Clermont O, Munnich A, et al. Correlation between severity and SMN protein level in spinal muscular atrophy. Nat Genet. 1997. https://doi.org/10.1038/ng0797-265.
Calucho M, Bernal S, Alias L, March F, Vencesla A, Rodriguez-Alvarez FJ, et al. Correlation between SMA type and SMN2 copy number revisited: an analysis of 625 unrelated Spanish patients and a compilation of 2834 reported cases. Neuromuscul Disord. 2018. https://doi.org/10.1016/j.nmd.2018.01.003.
Feldkotter M, Schwarzer V, Wirth R, Wienker TF, Wirth B. Quantitative analyses of SMN1 and SMN2 based on real-time lightCycler PCR: fast and highly reliable carrier testing and prediction of severity of spinal muscular atrophy. Am J Hum Genet. 2002. https://doi.org/10.1086/338627.
Wirth B. An update of the mutation spectrum of the survival motor neuron gene (SMN1) in autosomal recessive spinal muscular atrophy (SMA). Hum Mutat. 2000. https://doi.org/10.1002/(SICI)1098-1004(200003)15:3%3c228::AID-HUMU3%3e3.0.CO;2-9.
Finkel RS, Mercuri E, Darras BT, Connolly AM, Kuntz NL, Kirschner J, et al. Nusinersen versus sham control in infantile-onset spinal muscular atrophy. N Engl J Med. 2017. https://doi.org/10.1056/NEJMoa1702752.
Mercuri E, Darras BT, Chiriboga CA, Day JW, Campbell C, Connolly AM, et al. Nusinersen versus sham control in later-onset spinal muscular atrophy. N Engl J Med. 2018. https://doi.org/10.1056/NEJMoa1710504.
Mercuri E, Finkel RS, Muntoni F, Wirth B, Montes J, Main M, et al. Diagnosis and management of spinal muscular atrophy: Part 1: recommendations for diagnosis, rehabilitation, orthopedic and nutritional care. Neuromuscul Disord. 2018. https://doi.org/10.1016/j.nmd.2017.11.005.
Granger MW, Buschang PH, Throckmorton GS, Iannaccone ST. Masticatory muscle function in patients with spinal muscular atrophy. Am J Orthod Dentofacial Orthop. 1999;115:697–702.
van den Engel-Hoek L, Erasmus CE, van Bruggen HW, de Swart BJ, Sie LT, Steenks MH, et al. Dysphagia in spinal muscular atrophy type II: more than a bulbar problem? Neurology. 2009. https://doi.org/10.1212/WNL.0b013e3181c34aa6.
Wadman RI, van Bruggen HW, Witkamp TD, Sparreboom-Kalaykova SI, Stam M, van den Berg LH, et al. Bulbar muscle MRI changes in patients with SMA with reduced mouth opening and dysphagia. Neurology. 2014. https://doi.org/10.1212/WNL.0000000000000796.
Hagberg C. Assessment of bite force: a review. J Craniomandib Disord. 1987;1:162–9.
Mano T, Katsuno M, Banno H, Suzuki K, Suga N, Hashizume A, et al. Tongue pressure as a novel biomarker of spinal and bulbar muscular atrophy. Neurology. 2014. https://doi.org/10.1212/WNL.0000000000000041.
van der Heul AMB, van Eijk RPA, Wadman RI, Asselman F, Cuppen I, Nievelstein RAJ, et al. Mastication in patients with spinal muscular atrophy types 2 and 3 is characterized by abnormal efficiency, reduced endurance, and fatigue. Dysphagia. 2021. https://doi.org/10.1007/s00455-021-10351-y.
Atsuta N, Watanabe H, Ito M, Banno H, Suzuki K, Katsuno M, et al. Natural history of spinal and bulbar muscular atrophy (SBMA): a study of 223 Japanese patients. Brain. 2006. https://doi.org/10.1093/brain/awl096.
Mazzone E, De Sanctis R, Fanelli L, Bianco F, Main M, van den Hauwe M, et al. Hammersmith functional motor scale and motor function measure-20 in non ambulant SMA patients. Neuromuscul Disord. 2014. https://doi.org/10.1016/j.nmd.2014.01.003.
Stam M, Wadman RI, Bartels B, Leeuw M, Westeneng HJ, Wijngaarde CA, et al. A continuous repetitive task to detect fatigability in spinal muscular atrophy. Orphanet J Rare Dis. 2018. https://doi.org/10.1186/s13023-018-0904-5.
Finkel R, Bertini E, Muntoni F, Mercuri E, Group ESWS. 209th ENMC International Workshop: Outcome Measures and Clinical Trial Readiness in Spinal Muscular Atrophy 7–9 November 2014, Heemskerk, The Netherlands. Neuromuscul Disord. 2015; doi:https://doi.org/10.1016/j.nmd.2015.04.009.
Hagenacker T, Wurster CD, Gunther R, Schreiber-Katz O, Osmanovic A, Petri S, et al. Nusinersen in adults with 5q spinal muscular atrophy: a non-interventional, multicentre, observational cohort study. Lancet Neurol. 2020. https://doi.org/10.1016/S1474-4422(20)30037-5.
Kruse T, Heller R, Wirth B, Gloggler J, Wurster CD, Ludolph AC, et al. Maximum bite force in patients with spinal muscular atrophy during the first year of nusinersen therapy-a pilot study. Acta Myol. 2020. https://doi.org/10.36185/2532-1900-010.
Kluger BM, Krupp LB, Enoka RM. Fatigue and fatigability in neurologic illnesses: proposal for a unified taxonomy. Neurology. 2013. https://doi.org/10.1212/WNL.0b013e31827f07be.
Pageaux B, Lepers R. Fatigue induced by physical and mental exertion increases perception of effort and impairs subsequent endurance performance. Front Physiol. 2016. https://doi.org/10.3389/fphys.2016.00587.
Adams V, Mathisen B, Baines S, Lazarus C, Callister R. A systematic review and meta-analysis of measurements of tongue and hand strength and endurance using the Iowa Oral Performance Instrument (IOPI). Dysphagia. 2013. https://doi.org/10.1007/s00455-013-9451-3.
McGraw S, Qian Y, Henne J, Jarecki J, Hobby K, Yeh WS. A qualitative study of perceptions of meaningful change in spinal muscular atrophy. BMC Neurol. 2017. https://doi.org/10.1186/s12883-017-0853-y.
Bartels B, Habets LE, Stam M, Wadman RI, Wijngaarde CA, Schoenmakers M, et al. Assessment of fatigability in patients with spinal muscular atrophy: development and content validity of a set of endurance tests. BMC Neurol. 2019. https://doi.org/10.1186/s12883-019-1244-3.
Montes J, Dunaway S, Montgomery MJ, Sproule D, Kaufmann P, De Vivo DC, et al. Fatigue leads to gait changes in spinal muscular atrophy. Muscle Nerve. 2011. https://doi.org/10.1002/mus.21917.
Mongiovi P, Dilek N, Garland C, Hunter M, Kissel JT, Luebbe E, et al. Patient reported impact of symptoms in spinal muscular atrophy (PRISM-SMA). Neurology. 2018. https://doi.org/10.1212/WNL.0000000000006241.
Bartels B, de Groot JF, Habets LE, Wijngaarde CA, Vink W, Stam M, et al. Fatigability in spinal muscular atrophy: validity and reliability of endurance shuttle tests. Orphanet J Rare Dis. 2020. https://doi.org/10.1186/s13023-020-1348-2.
Kruse T, Lehmann HC, Braumann B, Fink GR, Wunderlich G. The maximum bite force for treatment evaluation in severely affected adult SMA patients-protocol for a longitudinal study. Front Neurol. 2020. https://doi.org/10.3389/fneur.2020.00139.
Robbins J, Levine R, Wood J, Roecker EB, Luschei E. Age effects on lingual pressure generation as a risk factor for dysphagia. J Gerontol A Biol Sci Med Sci. 1995. https://doi.org/10.1093/gerona/50a.5.m257.
Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33:159–74.
Thompson DJ, Throckmorton GS, Buschang PH. The effects of isometric exercise on maximum voluntary bite forces and jaw muscle strength and endurance. J Oral Rehabil. 2001;28:909–17.
Adams V, Mathisen B, Baines S, Lazarus C, Callister R. Reliability of measurements of tongue and hand strength and endurance using the Iowa Oral Performance Instrument with elderly adults. Disabil Rehabil. 2015. https://doi.org/10.3109/09638288.2014.921245.
Chan Y. Biostatistics 104: correlational analysis. Singapore Med J. 2003;44:614–9.
Brakemeier S, Stolte B, Thimm A, Kizina K, Totzeck A, Munoz-Rosales J, et al. Assessment of bulbar function in adult patients with 5q-SMA type 2 and 3 under treatment with nusinersen. Brain Sci. 2021. https://doi.org/10.3390/brainsci11091244.
Elsheikh B, King W, Peng J, Swoboda KJ, Reyna SP, LaSalle B, et al. Outcome measures in a cohort of ambulatory adults with spinal muscular atrophy. Muscle Nerve. 2020. https://doi.org/10.1002/mus.26756.
Querin G, Lenglet T, Debs R, Stojkovic T, Behin A, Salachas F, et al. Development of new outcome measures for adult SMA type III and IV: a multimodal longitudinal study. J Neurol. 2021. https://doi.org/10.1007/s00415-020-10332-5.
Wijngaarde CA, Stam M, Otto LAM, Bartels B, Asselman FL, van Eijk RPA, et al. Muscle strength and motor function in adolescents and adults with spinal muscular atrophy. Neurology. 2020. https://doi.org/10.1212/WNL.0000000000010540.
Beck M, Giess R, Wurffel W, Magnus T, Ochs G, Toyka KV. Comparison of maximal voluntary isometric contraction and Drachman’s hand-held dynamometry in evaluating patients with amyotrophic lateral sclerosis. Muscle Nerve. 1999. https://doi.org/10.1002/(sici)1097-4598(199909)22:9%3c1265::aid-mus15%3e3.0.co;2-f.
Glanzman AM, O’Hagen JM, McDermott MP, Martens WB, Flickinger J, Riley S, et al. Validation of the expanded hammersmith functional motor scale in spinal muscular atrophy type II and III. J Child Neurol. 2011. https://doi.org/10.1177/0883073811420294.
Glanzman AM, McDermott MP, Montes J, Martens WB, Flickinger J, Riley S, et al. Validation of the children’s hospital of philadelphia infant test of neuromuscular disorders (CHOP INTEND). Pediatr Phys Ther. 2011. https://doi.org/10.1097/PEP.0b013e3182351f04.
Godwin M, Pike A, Bethune C, Kirby A, Pike A. Concurrent and convergent validity of the simple lifestyle indicator questionnaire. ISRN Family Med. 2013. https://doi.org/10.5402/2013/529645.
van der Heul AMB, Wijngaarde CA, Wadman RI, Asselman F, van den Aardweg MTA, Bartels B, et al. Bulbar problems self-reported by children and adults with spinal muscular atrophy. J Neuromuscul Dis. 2019. https://doi.org/10.3233/JND-190379.
Vázquez-Costa JF, Povedano M, Nascimiento-Osorio AE, Escribano AM, Garcia SK, Dominguez R, et al. Validation of motor and functional scales for the evaluation of adult patients with 5q spinal muscular atrophy. medRxiv. 2021. https://doi.org/10.1101/2021.06.12.21258357.
Pera MC, Coratti G, Mazzone ES, Montes J, Scoto M, De Sanctis R, et al. Revised upper limb module for spinal muscular atrophy: 12 month changes. Muscle Nerve. 2019. https://doi.org/10.1002/mus.26419.
Sanjak M, Brinkmann J, Belden DS, Roelke K, Waclawik A, Neville HE, et al. Quantitative assessment of motor fatigue in amyotrophic lateral sclerosis. J Neurol Sci. 2001. https://doi.org/10.1016/s0022-510x(01)00624-4.
Schwid SR, Thornton CA, Pandya S, Manzur KL, Sanjak M, Petrie MD, et al. Quantitative assessment of motor fatigue and strength in MS. Neurology. 1999. https://doi.org/10.1212/wnl.53.4.743.
Montes J, McDermott MP, Martens WB, Dunaway S, Glanzman AM, Riley S, et al. Six-minute walk test demonstrates motor fatigue in spinal muscular atrophy. Neurology. 2010. https://doi.org/10.1212/WNL.0b013e3181d3e308.
Bartels B, de Groot JF, Habets LE, Wadman RI, Asselman FL, Nieuwenhuis EES, et al. Correlates of fatigability in patients with spinal muscular atrophy. Neurology. 2021. https://doi.org/10.1212/WNL.0000000000011230.
Wadman RI, Vrancken AF, van den Berg LH, van der Pol WL. Dysfunction of the neuromuscular junction in spinal muscular atrophy types 2 and 3. Neurology. 2012. https://doi.org/10.1212/WNL.0b013e3182749eca.
Kariya S, Park GH, Maeno-Hikichi Y, Leykekhman O, Lutz C, Arkovitz MS, et al. Reduced SMN protein impairs maturation of the neuromuscular junctions in mouse models of spinal muscular atrophy. Hum Mol Genet. 2008. https://doi.org/10.1093/hmg/ddn156.
Kong L, Wang X, Choe DW, Polley M, Burnett BG, Bosch-Marce M, et al. Impaired synaptic vesicle release and immaturity of neuromuscular junctions in spinal muscular atrophy mice. J Neurosci. 2009. https://doi.org/10.1523/JNEUROSCI.4434-08.2009.
Arnold AS, Gueye M, Guettier-Sigrist S, Courdier-Fruh I, Coupin G, Poindron P, et al. Reduced expression of nicotinic AChRs in myotubes from spinal muscular atrophy I patients. Lab Invest. 2004. https://doi.org/10.1038/labinvest.3700163.
Goulet BB, Kothary R, Parks RJ. At the “junction” of spinal muscular atrophy pathogenesis: the role of neuromuscular junction dysfunction in SMA disease progression. Curr Mol Med. 2013. https://doi.org/10.2174/15665240113139990044.
Ripolone M, Ronchi D, Violano R, Vallejo D, Fagiolari G, Barca E, et al. Impaired muscle mitochondrial biogenesis and myogenesis in spinal muscular atrophy. JAMA Neurol. 2015. https://doi.org/10.1001/jamaneurol.2015.0178.
Houston K, Buschang PH, Iannaccone ST, Seale NS. Craniofacial morphology of spinal muscular atrophy. Pediatr Res. 1994. https://doi.org/10.1203/00006450-199408000-00020.
Arakawa I, Igarashi K, Imamura Y, Muller F, Abou-Ayash S, Schimmel M. Variability in tongue pressure among elderly and young healthy cohorts: a systematic review and meta-analysis. J Oral Rehabil. 2021. https://doi.org/10.1111/joor.13076.
Arima T, Takeuchi T, Honda K, Tomonaga A, Tanosoto T, Ohata N, et al. Effects of interocclusal distance on bite force and masseter EMG in healthy participants. J Oral Rehabil. 2013. https://doi.org/10.1111/joor.12097.
Solomon NP, Munson B. The effect of jaw position on measures of tongue strength and endurance. J Speech Lang Hear Res. 2004. https://doi.org/10.1044/1092-4388(2004/045).
Arakawa I, Abou-Ayash S, Genton L, Tsuga K, Leles CR, Schimmel M. Reliability and comparability of methods for assessing oral function: chewing, tongue pressure and lip force. J Oral Rehabil. 2020. https://doi.org/10.1111/joor.12976.
Serra CM, Manns AE. Bite force measurements with hard and soft bite surfaces. J Oral Rehabil. 2013. https://doi.org/10.1111/joor.12068.
Acknowledgements
The authors thank all patients with SMA who have been participating in our study. The authors thank Velia Hullmann and all other members of the speech therapy team of the UniReha GmbH at the University Hospital of Cologne as well as Jaqueline Lipka (study assistant at the Center for Neuromuscular Diseases, Department of Neurology, University Hospital Essen) for their support. For technical support, the authors would like to thank the company CMV Hoven GmbH, Mönchengladbach (Germany).
Funding
Open Access funding enabled and organized by Projekt DEAL. This study was funded by Biogen (ID 228101 / PO 254116) (Cambridge, MA, USA). We acknowledge support for the Article Processing Charge from the DFG (German Research Foundation, 491454339).
Author information
Authors and Affiliations
Contributions
Initiation: BW, RH; conceptualization: TK, BB and GW; methodology and formal analysis: TK; patient recruitment: HCL, TH, GW, SB and NS; investigation: TK, SS, DL, HCL, TH, GW, SB and NS; resources: TK, HCL, TH, BB and GW; writing—original draft preparation: TK, BB and GW; writing—review and editing: TK, SS, DL, BW, RH, NS, HCL, SB, TH, BB and GW. All authors have read and agreed to the final version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was approved by the Ethics Committee of the Medical Faculty of the University of Cologne (Reference Number: 19–1137) and by the Ethics Committee of the Medical Faculty of the University of Essen (Reference Number: 21–9851-BO). Each subject provided informed consent.
Consent for publication
Not applicable.
Competing interests
TK, and SS have received honoraria from Biogen for lectures. TK received financial research support from Biogen. TH received advisory and lecture honoraria and research support from Biogen, Roche and Novartis. DL, BB, SB, NS and GW have no conflicts of interests. BW received honoraria for lectures, workshops and interviews from Biogen and AveXis/Novartis. The funder had no role in the design of the study, in the collection, analyses, or interpretation of data, in the writing of the manuscript, or in the decision to publish the results.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Kruse, T., Shamai, S., Leflerovà, D. et al. Objective measurement of oral function in adults with spinal muscular atrophy. Orphanet J Rare Dis 18, 103 (2023). https://doi.org/10.1186/s13023-023-02688-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13023-023-02688-4