Abstract
Acute hepatic porphyrias (AHPs) are a family of four rare genetic diseases resulting from a deficiency in one of the enzymes involved in heme biosynthesis. AHP patients can experience potentially life-threatening acute attacks, characterized by severe abdominal pain, along with other signs and symptoms including nausea, mental confusion, hyponatraemia, hypertension, tachycardia and muscle weakness. Some patients also experience chronic manifestations and long-term complications, such as chronic pain syndrome, neuropathy and porphyria-associated kidney disease. Most symptomatic patients have only a few attacks in their lifetime; nevertheless, some experience frequent attacks that result in ongoing symptoms and a significant negative impact on their quality of life (QoL). Initial diagnosis of AHP can be made with a test for urinary porphobilinogen, \(\delta\)-aminolaevulinic acid and porphyrins using a single random (spot) sample. However, diagnosis is frequently missed or delayed, often for years, because the clinical symptoms of AHP are non-specific and mimic other more common disorders. Delayed diagnosis is of concern as some commonly used medications can trigger or exacerbate acute attacks, and untreated attacks can become severe, potentially leading to permanent neurological damage or fatality. Other attack triggers include hormonal fluctuations in women, stress, alcohol and low-calorie diets, which should be avoided in patients where possible. For the management of attacks, intravenous hemin is approved, whereas new therapeutic approaches are currently being investigated as a baseline therapy for prevention of attacks and improvement of QoL. Among these, a novel siRNA-based agent, givosiran, has shown very promising results in a recently concluded Phase III trial and has been approved for the management of AHPs. Here, we propose a challenging case study-with a very unusual pediatric onset of variegate porphyria-as a starting point to summarize the main clinical aspects (namely, clinical manifestations, diagnostic challenges, and therapeutic management) of AHPs, with a focus on the latest therapeutic innovations.
Similar content being viewed by others
Introduction
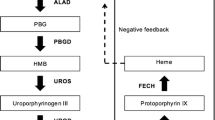
Acute hepatic porphyrias (AHPs) are a family of four rare genetic diseases characterized by potentially life-threatening acute neurovisceral attacks (acute porphyric attacks, APAs) and, for some patients, chronic debilitating manifestations whose burden negatively impacts their quality of life (QoL) [1,2,3]. The diseases result from a genetic defect leading to a functional impairment in four of the eight enzymes of the pathway of heme biosynthesis (Fig. 1) [4]. The four types of AHP are acute intermittent porphyria (AIP, the most common type), variegate porphyria (VP), hereditary coproporphyria (HCP) and the ultra-rare ALA dehydratase deficiency porphyria (ADP), each resulting from a different enzyme deficiency (Table 1) [5,6,7].
Although most heme in the human body is synthesized in the bone marrow for hemoglobin synthesis (75–80%), \(\sim\)15–20% is synthesised in the liver as a cofactor for numerous hemoproteins (e.g., myoglobin, cytochrome P450s, catalase and peroxidase) [1]. The body’s need for hepatic heme therefore fluctuates depending on exogenous factors, such as medication or alcohol intake, or endogenous factors such as hormone levels [8]. By engendering an increased requirement of heme, these factors can act to up-regulate hepatic \(\delta\)-aminolaevulinic acid (ALA) synthase (ALA synthase 1, ALAS1), the first and rate-limiting enzyme in the eight-step pathway of heme biosynthesis in the liver (Fig. 1) [7, 8]. In patients with AHP, whose heme biosynthesis is dysfunctional, the upregulation of ALAS1 can lead to increased levels of heme intermediates such as ALA and porphobilinogen (PBG) (Fig. 1) [6]. Accumulation of ALA, and possibly PBG, is believed to be neurotoxic [9] and the primary cause of the disease manifestations of AHP [8, 10]. A variety of triggers can increase heme requirements and activate this process leading to acute attacks, including hormonal fluctuations in women, infections, stress, use of certain medications, alcohol and fasting/low-calorie diets [4, 11].
The prevalence of symptomatic patients with AHP in Europe is estimated at 1:100,000 [12]. Most symptomatic patients have only a few APAs in their lifetime; however, up to 8% will suffer from ongoing attacks (typically defined as at least four APAs per year) [13]. The age of onset of clinical manifestation varies, but is most common between the second and fourth decades of life, whereas onset before puberty is unusual [8, 14], with most reports focusing on pediatric presentations of AIP [15,16,17,18]. Although both sexes inherit mutations with equal frequency, women are predominantly affected, likely related to the effect of hormonal changes (e.g., menstrual cycle) [8, 19].
With regard to the most common among AHPs, there is a relatively high incidence of mutations associated with AIP (\(\sim\)1/1600 Caucasians) but clinically manifest disease occurs in < 10% of the at-risk population, indicating the importance of genetic modifiers and environmental factors [20, 21].
In recent years, the management of acute hepatic porphyrias has been revolutionised by small interfering RNA (siRNA) technology: givosiran (Givlaari\(\circledR\)), a siRNA-based agent specifically targeting ALAS1 in the liver, has shown excellent results in lowering the annual rate of APAs and improving the overall QoL of patients.
Here, we present a case study as a starting point for discussion of the main clinical aspects (namely, clinical manifestations, diagnostic challenges, and therapeutic management) of AHPs, with a focus on the latest therapeutic innovations.
Case study
A 14-year-old woman presented to the Emergency Department (ED) due to progressive loss of strength in the limbs with difficulty in standing, mental confusion, crampy severe lower abdominal pain, nausea and vomiting. All symptoms had been progressive in their onset; they started about 30 days after the onset of fever, due to a documented viral infection (mononucleosis). The patient had a history of ED admissions due to recurrent episodes of abdominal pain (with negative radiography, ultrasound and computerized tomography [CT] scans) that were variably responsive to analgesic medications. The patient reported that during past episodes of abdominal pain, she had developed nausea, darkening of urine, fatigue, limb muscle weakness, difficulty with concentration and worsening of constipation, which for her was a chronic problem. The patient mentioned previous episodes of anorexia and weight loss. She also had a history of epilepsy (absence seizures, treated with sodium valproate 600 mg/day and ethosuximide 750 mg/day) and left subcortical parietal cavernoma with venous dysgenesis. No history of sunlight intolerance or skin lesions in sunlight exposed regions was referred by the patient.
In the ED, the body mass index was low (17.2 kg/m\(^{2}\); reference range 18.5–25 kg/m\(^{2}\)), whereas temperature, blood pressure, heart rate and respiratory rate were all within the normal range. Laboratory studies revealed an increase in white blood cells (12.7\(\times\)10\(^{3}\)/mm3, reference range 4–10.9 \(\times\)10\(^{3}\)/mm\(^{3}\) , 75% neutrophils), normocytic anemia (hemoglobin 10.5 g/dL, reference range 13.5–17.5 g/dL, mean corpuscular volume 91 fL, reference range 80–99 fL), decreased serum sodium (110 mEq/L, reference range 135–145 mEq/L) and increased blood urea nitrogen (48 mg/dL, reference range 15–45 mg/dL). Potassium, chloride, bicarbonate, blood sugar and creatinine were all within the normal range. Urinalysis revealed scant white and red blood cells (< 5 per high-power field), trace protein and positive ketones, with urinary glucose not detected. Serum alanine transaminase was 48 units/L (reference range 1–37 units/L) and aspartate transaminase 45 units/L (reference range 1–40 units/L), while serum total protein, bilirubin, lipase and amylase were within the normal range; serum Helicobacter pylori antibody and coeliac testing were non-reactive.
The patient looked anxious, lethargic and responded slowly to questions. She was variably oriented to person but not to place and time. She continuously complained about pain and requested pain relief. The abdomen was soft and non-distended with absent bowel sounds. No complaints of increased pain, nor any voluntary guarding could be elicited by deep palpation. A cardiopulmonary examination was normal. A neurological examination excluded any focal deficits, albeit disclosing generalized significant weakness (especially in the lower limbs) with hyporeflexia. She was treated with intravenous (IV) saline and hydromorphone, with only a partial improvement in her symptoms, and was admitted for additional observation.
A contrast-enhanced CT scan of the abdomen and pelvis was normal, showing only retained stool in the colon. The findings were similar to those of three CT scans made during previous ED admissions. Nuclear magnetic resonance imaging of the head did not show any significant abnormalities (except for the known left parietal cavernoma), hypophysis imaging was normal. An electroencephalogram showed slow, irritative diffuse abnormalities.
In light of her gender, young age, recurrent abdominal pain, hyponatremia and seizures, a diagnostic hypothesis of acute hepatic porphyria (AHP) was proposed. Thus, urinary analysis of \(\delta\)-aminolaevulinic acid (ALA), porphobilinogen (PBG) and porphyrins was undertaken. Urinary PBG was 31.6 \(\upmu\)mol/mmol creatinine (reference range 0–1.5 \(\upmu\)mol/mmol creatinine), urinary ALA was 23.3 \(\upmu\)mol/mmol creatinine (reference range 0–5 \(\upmu\)mol/mmol creatinine) and total urinary porphyrins were 4414 \(\upmu\)mol/mmol creatinine (reference range 0–82 \(\upmu\)mol/mmol creatinine) with coproporphyrins prevalent. As a result, a diagnosis of AHP was formulated. Additionally, fecal porphyrins were assessed (1558 nmol/g, reference range < 200 nmol/g) and a plasma fluorescence scan was performed (positive peak at 625 nm), suggesting that the type of AHP was variegate porphyria (VP).
Sodium valproate and ethosuximide, both drugs known to be triggers for attacks [5], were gradually reduced and stopped, and treatment with IV 10% dextrose and hemin (marketed in Europe as Normosang\(\circledR\)) was started and continued for 4 days. Hemin was reconstituted with human serum albumin and administered by central venous catheter into a high-flow, large-bore vein, in order to decrease the likelihood of venous side effects (such as thrombosis or thrombophlebitis) [5]. IV caloric supplementation (50% carbohydrate input) was also started. Treatment resulted in a dramatic improvement in the patient’s symptoms (complete remission of pain and neurological symptoms after 3 days), together with progressive normalization of serum sodium, and urinary ALA and PBG levels. Genetic testing showed a heterozygous mutation (c.807 G>A) in the protoporphyrinogen oxidase gene, confirming the diagnosis of VP.
Clinical manifestations of acute hepatic porphyrias
The most dramatic manifestations of AHPs are acute neurovisceral attacks, which often require hospitalization and, in the severest cases, a critical care setting [22]. As highlighted in the case study, the most common symptom in AHPs is severe, diffuse abdominal pain; other signs and symptoms can include nausea, weakness, tachycardia, hyponatremia, mental status changes, hypertension and changes in urine colour (Fig. 2) [3, 23]. If the attack is particularly severe, treatment is not initiated promptly or exposure to triggers is prolonged, patients can also experience seizures, delirium and paralysis, posterior reversible encephalopathy syndrome (PRES) along with permanent neurological damage or fatality [1, 5, 24].
Constellation of clinical characteristics and associated conditions for acute hepatic porphyria. a Only occurs in severe attacks. b Only occurs in variegate porphyria and hereditary coproporphyria. ANS: autonomic nervous system; CNS: central nervous system; PNS: peripheral nervous system; HCC: hepatocellular carcinoma; CKD: chronic kidney disease. Created with BioRender.com (last accessed 9 January 2022)
Several hypotheses have been proposed to explain the pathogenesis of APAs; some mechanisms of neuronal damage are likely related to the neurotoxic effects of the accumulation of non-porphyrin precursors (PBG, but especially ALA) on the central, peripheral and autonomic nervous systems [2, 9, 25]. Otherwise, additional mechanisms of damage have been hypothesized, such as those supposedly related to relative dysfunctions of the secondary routes of heme utilization (e.g. for the functioning of cytochromes, nitric oxide synthases, or enzymes involved in tryptophan metabolism) [9].
Research has also highlighted that some patients experience chronic symptoms (such as pain, fatigue and nausea) that affect daily functioning and QoL [3, 23, 26]. Additionally, patients with AHP can be at risk of experiencing numerous, multi-system, long-term complications and comorbidities as a result of the disease [22]. Long-term complications related to AHP and its treatment can include liver disease (e.g., hepatocellular carcinoma, fibrosis and cirrhosis), chronic kidney disease, peripheral neuropathy, chronic pain and systemic arterial hypertension [23, 27,28,29,30,31,32]. Comorbidities related to AHP can include anxiety, depression, elevated lipase/amylase levels, pancreatitis, hypertension, tachycardia and cardiac arrhythmias [3, 22, 31, 33]. In addition, the overproduction of porphyrins in HCP and especially in VP may cause chronic, blistering, photosensitive skin rashes [7, 11].
Impact on QoL and financial burden
For patients who experience ongoing attacks, AHP can have a substantial negative impact on QoL [26]. Patients have reported diminished QoL compared with population norms, with impacts on multiple aspects of their lives including pain and discomfort, anxiety and depression, the ability to perform usual activities and sleep disorders [3]. Patients have also reported a considerable impact on their social lives, including isolation, missing important occasions and limiting travel [26, 34, 35]. In addition, patients can experience substantial economic burden: many are not fully employed (and receiving disability payments), whereas those who are employed often miss many days of work due to AHP [35]. Patients with symptomatic AHP may also have increased levels of healthcare utilization, notably ED visits and hospital stays [3, 36, 37].
Diagnosis
As highlighted in the prior case study, the diagnosis of AHP is challenging for several reasons [38]. As a group of rare diseases, AHP is often not considered as part of the differential diagnosis when assessing for acute abdominal pain (and other common symptoms) [25]. Patients experiencing acute attacks often present to EDs where rare diseases may go unnoticed due to time constraints and the priority placed on stabilizing patients [39]. Furthermore, AHP has a variable presentation with many of its symptoms mimicking other, more prevalent conditions, thus making it a difficult disease to identify, even when symptoms are severe [4, 27]. This may be especially true for specialists who focus on a specific organ system, as often, for AHP to be suspected, the totality of a patient’s clinical presentation needs to be considered. This symptom variability and lack of specificity can lead to missed diagnosis or misdiagnosis, often for years, with one study finding a mean delay from onset of symptoms to diagnosis of \(\sim\)15 years [23].
It should be emphasized that our Case Study features a remarkably early age of onset for variegate porphyria: pediatric presentation of heterozygous AHPs is unusual [14], and most commonly described for AIPs [15, 16]. The exceedingly rare homozygous variants of AHPs, on the contrary, usually present in childhood and tend to manifest with chronic neuropathy, growth retardation, and/or other symptoms of variable severity [14]. It is worth noting that we could not retrieve any explicit report of a case of heterozygous variegate porphyria with pediatric onset from the scientific literature.
A timely diagnosis of AHP is crucial as untreated acute attacks can progress, become more severe and potentially lead to permanent neurological damage, or even be life-threatening [25, 40]. Also of concern is that many commonly used drugs can increase hepatic heme requirements, and may trigger acute attacks or exacerbate symptoms (a concern highlighted in the case study) [8]. Undiagnosed patients may therefore inadvertently be prescribed medications that induce or worsen attacks [27]. In addition, undiagnosed patients can be misdiagnosed and given unnecessary medical treatments, and even surgery [5, 23]. AHP should be considered in patients with severe unexplained abdominal pain (which occurs in >90% of acute attacks [3]), particularly if present alongside any of the following signs and symptoms: pain in other parts of the body, nausea, constipation, mental confusion, change in urine color, muscle weakness, hyponatremia, tachycardia and hypertension (Fig. 3).
Biochemical diagnosis of the disease can be undertaken using a random (spot) urine test for PBG, ALA and porphyrins. The optimal time to take a urine sample is during or shortly after an attack, when ALA or PBG levels will be most elevated [25]. PBG levels are particularly useful as they are elevated during AHP attacks (often many orders of magnitude above normal) but not in any other medical condition, allowing the diagnosis or exclusion of AHP [6, 25]. The exception to this is ADP, where ALA but not PBG levels are typically elevated (this can also occur with lead intoxication), although ADP is ultra-rare with < 10 documented cases worldwide [6, 27]. Measurement of urine porphyrins may be important to ensure that a diagnosis of VP or HCP is not missed, as urine PBG excretion can return to normal within a few days of clinical presentation in these two porphyrias (Table 1) [41]. However, a test of urine porphyrins by itself cannot diagnose AHP [6]. Genetic testing of the AHP genes should be undertaken to determine AHP type [42]. All AHP attacks are treated in the same manner, so there is no need to wait for results of genetic testing before initiating the treatment of an attack [6].
Current treatment approaches
All potentially precipitating factors such as porphyrinogenic medications (a database of safe and unsafe medications can be found here: www.drugs-porphyria.org), reduced calorie intake, smoking and alcohol should be eliminated during an acute attack, and minimized to prevent future attacks [4]. As suggested by the case study, infections may also deteriorate the patient’s clinical condition and should be treated properly [22]. For the management of attacks, IV hemin is approved, due its effectiveness in replacing the heme pool and down-regulating heme biosynthesis [43]. It is usually infused daily (3–4 mg/kg) into a large peripheral vein or venous access port for 3–4 consecutive days, but a repetitive course may be required if AHP symptoms are ongoing [22, 44]. Reconstitution with human serum albumin may reduce the risk of side effects [5]. The treatment should be started immediately during a severe or moderate acute attack after the demonstration of typical symptoms of acute porphyria and an elevation in urine PBG [22]. Side effects of hemin utilisation include thrombophlebitis (for which infusion through a large-bore venous catheter is usually needed), headache, dizziness (ethanol is one of the main excipients) and (hepatic) iron accumulation due to repeated infusions. As presented in the case study, IV glucose may also down-regulate the heme biosynthesis pathway and may be effective, especially in patients who are malnourished or in whom dietary restrictions have contributed to an attack [6]. Being more readily available in most emergency settings, IV glucose may be started early in the attempt to stop the progression of mild attacks, even though great care should be taken not to worsen hyponatremia with the sodium-free content of glucose infusions. Hemin is more effective than glucose in the treatment of acute attacks and should be promptly requested from a (previously designed) pharmacy of reference when a known porphyric patient presents to the ED. In the most severe cases, glucose and hemin infusions may be used together. Finally, it is paramount to strictly monitor the clinical course of the attack, since patients with deteriorating conditions should be promptly moved to a critical care setting.
As pain is a cardinal symptom of AHP, patients often require the use of analgesic regimes, with many patients relying on opioids to manage acute and chronic pain [5, 22]. However, their use, particularly in a chronic setting, needs to be weighed against the risks of addiction, somnolence and apnea [4, 22]. Patients have also expressed concerns around being labelled as malingerers and drug-seekers due to their substantial need for pain relief [26, 35]. Other symptomatic therapy for hypertension, tachycardia, nausea and vomiting is commonly required. [22]
A particular challenge is the treatment of patients who experience ongoing attacks [5]. Current treatment options focus on acute attack management and the resolution of symptoms. Prophylactic approaches remain limited, are used variably and are highly dependent on clinical experience [4]. Off-label prophylactic hemin infusions have been used successfully in some patients. This often requires indwelling central venous catheters, may induce dependence on endogenous heme, and may lead to tachyphylaxis and side effects such iron overload, thrombosis or phlebitis [3, 5, 22, 45]. Chemically induced menopause with gonadotropin-releasing hormone agonists has been used successfully in some young women experiencing acute attacks related to their menstrual cycles [46, 47]. One treatment used in severely affected patients is liver transplantation. Although this is potentially curative, it is rarely used due to the highly invasive nature of the surgery, the need for lifelong immunosuppression and a shortage of donors [48]. Thus, there remains a high unmet medical need for effective treatments for these patients.
Emerging therapies
Given the limitations of the current therapeutic landscape, novel approaches for the development of efficacious and safe AHP therapies are needed. An investigational RNA interference therapeutic, givosiran, that specifically targets ALAS1 in hepatocytes has been recently approved for the treatment of AHP in adults (in the US and Brazil) or in patients who are older than 12 years (in Europe) [49,50,51]. Givosiran is subcutaneously administered and has been developed to reduce the overproduction of potentially neurotoxic heme intermediates in the liver [39, 52]. Results from a Phase III clinical study (ENVISION) in AHP patients with recurrent attacks showed, compared to placebo, a lower attack rate, less debilitating symptoms and an improved QoL between attacks, significantly decreased levels of ALA and PBG, and an acceptable safety profile [53, 54]. Givosiran has been approved for the treatment of patients with AHP, regardless of their annualized attack rate, albeit its efficacy has been tested mainly in patients with a clinically active disease and more frequent and severe APAs: in this population and to the authors’ opinion, givosiran has represented a real breakthrough in the prevention of potentially life-threatening attacks. Among the most frequently reported adverse effects, special attention should be paid to hyperhomocysteinemia (however responsive to vitamin supplementation therapy) [55,56,57]. Also reported were injection-site reactions and elevations in liver transaminases and pancreatic enzymes. Recently, a decline in renal function was detected in a minority of patients under siRNA-based therapy, which was worse than expected given the natural course of porphyria-associated kidney disease [58]. For these reasons, we suggest that every patient should have a blood chemistry check-up inclusive of liver transaminases, pancreatic enzimes, kidney function, and homocysteine, before starting therapy with givosiran and periodically (i.e. every few weeks) thereafter. In particular, increases in homocysteine should be promptly treated as already described [57].
In another approach to find a treatment for AHP, gene therapy with a viral vector delivering a normal hydroxymethylbilane synthase (HMBS) gene to hepatocytes was assessed in a Phase I clinical trial. Despite an acceptable safety profile, this investigational agent did not show efficacy in preventing frequent attacks [59]. Further research is underway to optimize the viral vector [60].
Finally, in a pre-clinical study, intravenous human HMBS messenger RNA encapsulated in lipid nanoparticles was used to transduce hepatocytes. In AIP mice, this approach reduced urinary heme intermediates when acute attacks were induced, suggesting that it may be a potential therapy for AIP [61]. A clinical trial is necessary to demonstrate the safety, feasibility and efficacy of this therapy in humans.
Conclusion
AHP is a family of rare, serious diseases resulting from a genetic defect in the heme biosynthesis pathway enzymes in the liver. Patients can experience potentially life-threatening acute attacks, chronic manifestations and long-term complications. Accumulation of heme precursors ALA, and possibly PBG, are believed to be neurotoxic and the primary cause of disease manifestations.
Diagnosis is challenging due to a non-specific, variable presentation with many of the symptoms mimicking other, more prevalent conditions. However, a relatively straightforward biochemical test for urinary PBG, ALA and porphyrins using a single random (spot) sample can exclude or provide an initial diagnosis of AHP. Most symptomatic patients have only a few attacks in their lifetime; nonetheless, some experience frequent attacks with up to 8% having four or more attacks per year. As a result, these patients experience a strong negative effect on their QoL and have high unmet needs for new treatment options. Results of the Phase III trial suggest that givosiran may be an effective treatment to reduce attacks in these patients.
Availability of data and materials
Not applicable
Abbreviations
- ALA:
-
\(\delta\)-aminolaevulinic acid
- ALAS1:
-
ALA synthase 1
- AHP:
-
acute hepatic porphyria
- APA:
-
acute porphyric attacks
- CT:
-
computerised tomography
- ED:
-
Emergency Department
- IV:
-
intravenous
- PBG:
-
porphobilinogen
- QoL:
-
quality of life
- RNA:
-
ribonucleic acid
- siRNA:
-
small interfering RNA
- VP:
-
variegate porphyria
References
Puy H, Gouya L, Deybach J-C. Porphyrias. The Lancet. 2010;375(9718):924–37.
Balwani M, Desnick RJ. The porphyrias: advances in diagnosis and treatment. Blood J Am Soc Hematol. 2012;120(23):4496–504.
Gouya L, Ventura P, Balwani M, Bissell DM, Rees DC, Stölzel U, Phillips JD, Kauppinen R, Langendonk JG, Desnick RJ, et al. Explore: a prospective, multinational, natural history study of patients with acute hepatic porphyria with recurrent attacks. Hepatology. 2020;71(5):1546–58.
Balwani M, Wang B, Anderson KE, Bloomer JR, Bissell DM, Bonkovsky HL, Phillips JD, Desnick RJ. Of the rare diseases clinical research network P.C.: acute hepatic porphyrias: recommendations for evaluation and long-term management. Hepatology. 2017;66(4):1314–22.
Wang B, Rudnick S, Cengia B, Bonkovsky HL. Acute hepatic porphyrias: review and recent progress. Hepatol Commun. 2019;3(2):193–206.
Anderson KE. Acute hepatic porphyrias: current diagnosis & management. Mol Genet Metab. 2019;128(3):219–27.
Bonkovsky HL, Dixon N, Rudnick S. Pathogenesis and clinical features of the acute hepatic porphyrias (ahps). Mol Genet Metab. 2019;128(3):213–8.
Besur S, Hou W, Schmeltzer P, Bonkovsky HL. Clinically important features of porphyrin and heme metabolism and the porphyrias. Metabolites. 2014;4(4):977–1006.
Ricci A, Di Pierro E, Marcacci M, Ventura P. Mechanisms of neuronal damage in acute hepatic porphyrias. Diagnostics. 2021;11(12):2205.
Bissell DM, Lai JC, Meister RK, Blanc PD. Role of delta-aminolevulinic acid in the symptoms of acute porphyria. Am J Med. 2015;128(3):313–7.
Ramanujam V-MS, Anderson KE. Porphyria diagnostics-part 1: a brief overview of the porphyrias. Curr Protoc Hum Genet. 2015;86(1):17–20.
Elder G, Harper P, Badminton M, Sandberg S, Deybach J-C. The incidence of inherited porphyrias in europe. J Inherit Metab Dis. 2013;36(5):849–57.
Schmitt C, Lenglet H, Yu A, Delaby C, Benecke A, Lefebvre T, Letteron P, Paradis V, Wahlin S, Sandberg S, et al. Recurrent attacks of acute hepatic porphyria: major role of the chronic inflammatory response in the liver. J Int Med. 2018;284(1):78–91.
Elder G. Hepatic porphyrias in children. J Inherit Metab Dis. 1997;20(2):237–46.
Kaplan PW, Lewis DV. Juvenile acute intermittent porphyria with hypercholesterolemia and epilepsy: a case report and review of the literature. J Child Neurol. 1986;1(1):38–45.
Hultdin J, Schmauch A, Wikberg A, Dahlquist G, Andersson C. Acute intermittent porphyria in childhood: a population-based study. Acta Paediatr. 2003;92(5):562–8.
Pierro E, Granata F, Rosafio C, Marchini S, Guerra A, et al. Acute intermittent porphyria in a child with severe neuropathy. J Blood Lymph. 2017;8(195):2.
Fatima SA, Jurair H, Abbas Q, Rehman AJ. Paediatric porphyria and human hemin: a treatment challenge in a lower middle income country. BMJ Case Rep CP 2020; 13(1) . https://doi.org/10.1136/bcr-2019-232236. https://casereports.bmj.com/content/13/1/e232236.full.pdf
Andersson C, Innala E, Bäckström T. Acute intermittent porphyria in women. clinical expression, use and experience of exogenous sex hormones. a population-based study in northern sweden. J Int Med. 2003;254(2):176–83.
Chen B, Solis-Villa C, Hakenberg J, Qiao W, Srinivasan RR, Yasuda M, Balwani M, Doheny D, Peter I, Chen R, et al. Acute intermittent porphyria: predicted pathogenicity of hmbs variants indicates extremely low penetrance of the autosomal dominant disease. Hum Mutat. 2016;37(11):1215–22.
Lenglet H, Schmitt C, Grange T, Manceau H, Karboul N, Bouchet-Crivat F, Robreau A-M, Nicolas G, Lamoril J, Simonin S, et al. From a dominant to an oligogenic model of inheritance with environmental modifiers in acute intermittent porphyria. Hum Mol Genet. 2018;27(7):1164–73.
Pischik E, Kauppinen R. An update of clinical management of acute intermittent porphyria. Appl Clin Genet. 2015;8:201.
Bonkovsky HL, Maddukuri VC, Yazici C, Anderson KE, Bissell DM, Bloomer JR, Phillips JD, Naik H, Peter I, Baillargeon G, et al. Acute porphyrias in the usa: features of 108 subjects from porphyrias consortium. Am J Med. 2014;127(12):1233–41.
Jaramillo-Calle DA, Solano JM, Rabinstein AA, Bonkovsky HL. Porphyria-induced posterior reversible encephalopathy syndrome and central nervous system dysfunction. Mol Genet Metab. 2019;128(3):242–53.
Anderson KE, Bloomer JR, Bonkovsky HL, Kushner JP, Pierach CA, Pimstone NR, Desnick RJ. Recommendations for the diagnosis and treatment of the acute porphyrias. Ann Int Med. 2005;142(6):439–50.
Simon A, Pompilus F, Querbes W, Wei A, Strzok S, Penz C, Howe DL, Hungate JR, Kim JB, Agarwal S, et al. Patient perspective on acute intermittent porphyria with frequent attacks: a disease with intermittent and chronic manifestations. Patient Patient-Centered Outcomes Res. 2018;11(5):527–37.
Stein PE, Badminton MN, Rees DC. Update review of the acute porphyrias. Br J Haematol. 2017;176(4):527–38.
Willandt B, Langendonk JG, Biermann K, Meersseman W, D’Heygere F, George C, Verslype C, Monbaliu D, Cassiman D. Liver fibrosis associated with iron accumulation due to long-term heme-arginate treatment in acute intermittent porphyria: a case series. JIMD Rep. 2015;25:77–81.
Baravelli C, Sandberg S, Aarsand A, Nilsen R, Tollånes M. Acute hepatic porphyria and cancer risk: a nationwide cohort study. J Int Med. 2017;282(3):229–40.
Andersson C, Bjersing L, Lithner F. The epidemiology of hepatocellular carcinoma in patients with acute intermittent porphyria. J Int Med. 1996;240(4):195–201.
Pallet N, Karras A, Thervet E, Gouya L, Karim Z, Puy H. Porphyria and kidney diseases. Clin Kidney J. 2018;11(2):191–7.
Ricci A, Guida CC, Manzini P, Cuoghi C, Ventura P. Kidney involvement in acute hepatic porphyrias: pathophysiology and diagnostic implications. Diagnostics. 2021;11(12):2324.
Duque-Serrano L, Patarroyo-Rodriguez L, Gotlib D, Molano-Eslava JC. Psychiatric aspects of acute porphyria: a comprehensive review. Curr Psychiatry Rep. 2018;20(1):1–7.
Naik H, Stoecker M, Sanderson SC, Balwani M, Desnick RJ. Experiences and concerns of patients with recurrent attacks of acute hepatic porphyria: a qualitative study. Mol Genet Metab. 2016;119(3):278–83.
American Porphyria Foundation. The voice of the patient: patient-focused drug development meeting - acute porphyrias. 2017. https://www.fda.gov/media/130386/download (accessed: 4 January 2022)
Neeleman RA, Wagenmakers MA, Koole-Lesuis RH, Mijnhout GS, Wilson JP, Friesema EC, Langendonk JG. Medical and financial burden of acute intermittent porphyria. J Inherited Metab Dis. 2018;41(5):809–17.
Gouya L, Bloomer J, Balwani M, Bissell D, Rees D, Stölzel U, Phillips J, Kauppinen R, Langendonk J, Desnick R, Deybach J. An analysis of healthcare utilization and costs associated with patients with acute hepatic porphyrias (ahp) with recurrent attacks in explore: a prospective, multinational natural history study of patients with ahp. Int Congress Porphyria and Porphyrias (ICPP); 25–28 June 2017; Paris, France
Ventura P, Cappellini MD, Biolcati G, Guida CC, Rocchi E, Porfiria GI. A challenging diagnosis for potential fatal diseases: recommendations for diagnosing acute porphyrias. Eur J Int Med. 2014;25(6):497–505.
Bissell DM. The porphyrias. Rosenberg’s Molecular and Genetic Basis of Neurological and Psychiatric Disease. 2015;731–749.
Stein P, Badminton MN, Barth J, Rees D, Sarkany R, Stewart M, Cox T. Acute intermittent porphyria: fatal complications of treatment. Clin Med. 2012;12(3):293.
Woolf J, Marsden JT, Degg T, Whatley S, Reed P, Brazil N, Stewart MF, Badminton M. Best practice guidelines on first-line laboratory testing for porphyria. Ann Clin Biochem. 2017;54(2):188–98.
Whatley SD, Mason NG, Woolf JR, Newcombe RG, Elder GH, Badminton MN. Diagnostic strategies for autosomal dominant acute porphyrias: retrospective analysis of 467 unrelated patients referred for mutational analysis of the hmbs, cpox, or ppox gene. Clin Chem. 2009;55(7):1406–14.
Orphan Europe. Summary of product characteristics: Normosang 25 mg/ml, concentrate for solution for infusion (2007). https://www.mhlw.go.jp/shingi/2008/03/dl/s0326-10n.pdf (accessed 4 January 2022.)
Open-label study of hemin for acute porphyria. Clinical practice implications. The American Journal of Medicine. 2006;119(9):801–18016.
Marsden JT, Guppy S, Stein P, Cox TM, Badminton M, Gardiner T, Barth JH, Stewart MF, Rees DC. Audit of the use of regular haem arginate infusions in patients with acute porphyria to prevent recurrent symptoms. JIMD Rep. 2015;22:57–65.
Schulenburg-Brand D, Gardiner T, Guppy S, Rees DC, Stein P, Barth J, Stewart MF, Badminton M. An audit of the use of gonadorelin analogues to prevent recurrent acute symptoms in patients with acute porphyria in the united kingdom. JIMD Rep. 2017;36:99–107.
Innala E, Bäckström T, Bixo M, Andersson C. Evaluation of gonadotropin-releasing hormone agonist treatment for prevention of menstrual-related attacks in acute porphyria. Acta Obstet Gynecol Scand. 2010;89(1):95–100.
Dowman JK, Gunson BK, Mirza DF, Bramhall SR, Badminton MN, Newsome PN, Selection UL, Party AW. Liver transplantation for acute intermittent porphyria is complicated by a high rate of hepatic artery thrombosis. Liver Transpl. 2012;18(2):195–200.
Scott LJ. Givosiran: first approval. Drugs. 2020;80(3):335–9.
Alnylam Pharmaceuticals. Alnylam announces approval of GIVLAARI® (givosiran) in Brazil for the treatment of acute hepatic porphyria (AHP) in adults (press release) Sao Paulo, Brazil 2020. https://investors.alnylam.com/sites/default/files/GIVLAARI-Brazil-Approval-Press-Release.pdf
European Medicines Agency EMA. Givlaari (summary of product characteristics) 2021. https://www.ema.europa.eu/en/documents/product-information/givlaari-epar-product-information_en.pdf
Chan A, Liebow A, Yasuda M, Gan L, Racie T, Maier M, Kuchimanchi S, Foster D, Milstein S, Charisse K, et al. Preclinical development of a subcutaneous alas1 rnai therapeutic for treatment of hepatic porphyrias using circulating rna quantification. Mol Therapy Nucl Acids. 2015;4:263.
Balwani M, Sardh E, Ventura P, Peiró PA, Rees DC, Stölzel U, Bissell DM, Bonkovsky HL, Windyga J, Anderson KE, et al. Phase 3 trial of rnai therapeutic givosiran for acute intermittent porphyria. N Engl J Med. 2020;382(24):2289–301.
Ventura P, Bonkovsky HL, Gouya L, Aguilera-Peiró P, Montgomery Bissell D, Stein PE, Balwani M, Anderson DKE, Parker C, Kuter DJ, Monroy S, Oh J, Ritchie B, Ko JJ, Hua Z, Sweetser MT, Sardh E. ENVISION Investigators: Efficacy and safety of givosiran for acute hepatic porphyria: 24-month interim analysis of the randomized phase 3 envision study. Liver Int. 2022. https://doi.org/10.1111/liv.15090.
To-Figueras J, Wijngaard R, García-Villoria J, Aarsand AK, Aguilera P, Deulofeu R, Brunet M, Gomez-Gomez A, Pozo OJ, Sandberg S. Dysregulation of homocysteine homeostasis in acute intermittent porphyria patients receiving heme arginate or givosiran. J Inherit Metab Dis. 2021. https://doi.org/10.1002/jimd.12391.
Petrides PE, Klein M, Schuhmann E, Torkler H, Molitor B, Loehr C, Obermeier Z, Beykirch MK. Severe homocysteinemia in two givosiran-treated porphyria patients: is free heme deficiency the culprit? Ann Hematol. 2021. https://doi.org/10.1007/s00277-021-04547-3.
Ricci A, Marcacci M, Cuoghi C, Pietrangelo A, Ventura P. Hyperhomocysteinemia in patients with acute porphyrias: a possible effect of alas1 modulation by sirnam therapy and its control by vitamin supplementation. Eur J Int Med. 2021;92:121–3.
Lazareth H, Poli A, Bignon Y, Mirmiran A, Rabant M, Cohen R, Schmitt C, Puy H, Karras A, Gouya L, Pallet N. Renal Function Decline Under Therapy With Small Interfering RNA Silencing ALAS1 for Acute Intermittent Porphyria. Kidney Int Rep. 2021;6(7):1904–11. https://doi.org/10.1016/j.ekir.2021.04.004.
D’Avola D, López-Franco E, Sangro B, Pañeda A, Grossios N, Gil-Farina I, Benito A, Twisk J, Paz M, Ruiz J, et al. Phase i open label liver-directed gene therapy clinical trial for acute intermittent porphyria. J Hepatol. 2016;65(4):776–83.
Serrano-Mendioroz I, Sampedro A, Alegre M, Enriquezde Salamanca R, Berraondo P, Fontanellas A. An inducible promoter responsive to different porphyrinogenic stimuli improves gene therapy vectors for acute intermittent porphyria. Hum Gene Ther. 2018;29(4):480–91.
Jiang L, Berraondo P, Jericó D, Guey LT, Sampedro A, Frassetto A, Benenato KE, Burke K, Santamaría E, Alegre M, et al. Systemic messenger rna as an etiological treatment for acute intermittent porphyria. Nat Med. 2018;24(12):1899–909.
Acknowledgements
Editorial assistance was provided by Dalia Cahana-Amitay (Alnylam Pharmaceuticals, Cambridge, MA, USA) and medical writing services provided by Stephen Whiting (Adelphi Communications Ltd, Macclesfield, UK), funded by Alnylam Pharmaceuticals, Cambridge, MA, USA, in accordance with Good Publication Practice (GPP3) guidelines.
Funding
Supported by Alnylam Pharmaceuticals, Cambridge, MA, USA.
Author information
Authors and Affiliations
Contributions
All authors have been variously involved in the clinical management of the Case Study, the biochemical measurements of porphyrins and porphyrin precursors, and/or writing and final editing of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The patient (who at the time of writing has reached legal age) has provided written consent for publication of the Case Study.
Consent for publication
The patient (who at the time of writing has reached legal age) has provided written consent for publication of the Case Study.
Competing interests
Paolo Ventura and Matteo Marcacci – received grants for consulting and lectures from Alnylam Pharmaceuticals and Recordati–Rare Diseases
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Marcacci, M., Ricci, A., Cuoghi, C. et al. Challenges in diagnosis and management of acute hepatic porphyrias: from an uncommon pediatric onset to innovative treatments and perspectives. Orphanet J Rare Dis 17, 160 (2022). https://doi.org/10.1186/s13023-022-02314-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13023-022-02314-9