Abstract
Background
Information about the specific regulatory environment of orphan drugs is scarce and inconsistent. Uncertainties surrounding the postmarketing long-term safety of orphan drugs remain. This study aimed to evaluate the labelling changes of orphan drugs and to identify postmarketing safety-associated approval factors.
Methods
This retrospective cohort study includes all drugs with orphan drug designation approved by the Center for Drug Evaluation and Research of the US Food and Drug Administration between 1999 and 2018. Main outcomes are safety-related labelling changes up to 31 December 2019. We defined any safety-related labelling changes as postmarketing safety events (PMSE). Safety-related withdrawals, suspensions, and boxed warnings were further categorised as severe postmarketing safety events (SPSE). Outcome measurements include frequencies of PMSE, SPSE, and association between approval factors and the occurrence of safety events.
Results
Amongst the 214 drugs identified with orphan drug designation (25.7% biologics), 83.6% were approved through at least one expedited programme, and 29.4% were approved with boxed warnings. During a median follow-up of 6.74 years since approval, 69.2% and 14.5% of the analysed orphan drugs had PMSE and SPSE, respectively. Safety-related withdrawal (0%, 0/214), suspended marketing (0.46%, 1/214) and new boxed warnings are uncommon (3.7%, 8/214). The safety-related labelling changes were more frequent in the drugs approved with boxed warnings [Incidence rate ratio (IRR): 1.95 (1.02–3.73)] and approved for long-term use [IRR: 2.76 (1.52–5.00)].
Conclusions and Relevance
In this long-term postmarketing analysis, approximately 70% of FDA-approved orphan drugs had safety-related labelling changes although severe safety events were rare. While maintaining early access to orphan drugs, the drug regulatory body has taken timely regulatory action with postmarketing surveillance to ensure patient safety.
Similar content being viewed by others
Introduction
In the United States (US), orphan drug designation refers to a special status granted by the Food and Drug Administration (FDA) to drugs that are indicated for a disease that affects 200,000 or fewer persons in the US or drugs with no reasonable expectation that sales will offset the costs of development and marketing [1]. Sponsors may apply for orphan drug designation at any point during the drug development process before submitting the marketing authorisation application. Drugs with orphan designation may be subject to research, development, approval and regulatory benefits such as tax credits for qualified research expenses, and waiver of the Prescription Drug User Fee [2]. To accelerate patients’ access to orphan drugs, some orphan drugs may be approved via one of four FDA expedited programmes, namely, priority review, breakthrough therapy, fast-track designations, and accelerated approval pathway [3]. Under certain circumstances, Phase II safety trials may be used as pivotal trials; similarly, Phase II and III trials may be combined when the patient population is exceptionally low that large trials are not logistically feasible [4].
Since the enactment of the Orphan Drug Act in 1983, newly approved chemical agents and biologics with orphan drug designation rose from 12.7% during 1995–1997 to 38.1% in 2015–2017 of all FDA-approved drugs [5]. Meanwhile, the long-term safety of orphan drugs is still uncertain, partially due to short follow-up duration, small sample sized- or single armed- clinical trials and the accelerated approval process [6]. To ensure patients’ access to life-saving treatment, it is not societally desirable to keep a drug at the testing phase until all possible safety considerations are determined. Postmarketing surveillance with regulatory action provides complementary evidence to the safety reports at trial stage to enhance patient safety. However, most postmarketing safety studies of orphan drugs are focused on individual drugs [7, 8]. As such, evidence of orphan drug safety collectively remains scarce and inconsistent while heterogeneity across studies renders the synthesis of results infeasible. Other orphan drug safety studies may have included all novel therapeutics with limited insight on the regulatory environment of orphan drugs. This presents challenges for patients with rare diseases and clinicians in understanding the process behind orphan drug approval, many of whom may already be deterred by the inherent uncertainty of disease progression, and thus overestimate the risks alongside newly approved orphan drugs.
As the demand for treating rare diseases is immense and highly time-sensitive, the postmarketing safety of orphan drugs and the drug approval environment should be judiciously evaluated to inform treatment access and monitoring decisions. By extracting longitudinal data from the FDA orphan drug database, this study aimed to 1) describe the landscape of long-term postmarketing safety of FDA approved orphan drugs; and 2) assess the association between approval factors and the occurrence of postmarketing safety events to ensure that clinicians and patients have the appropriate information to evaluate the risks and benefits for their particular rare condition.
Methods
Orphan drug identification
We analysed all drugs with orphan drug designation approved by the FDA between 1999 and 2018 [9]. The new drug list was extracted from the New Molecular Entity and Original Biologic Approvals Annual Reports provided by the Center for Drug Evaluation and Research (CDER), FDA (hereinafter referred to as Reports) [10, 11]. The Compilation of CDER dataset was used to reconfirm the data from Reports [12]. The approval date and orphan drug designation status were then verified using the FDA’s Orphan Drug Product Designation Database [13].
Approval characteristics
Information on approved orphan drugs were extracted from drug labels, approval letters, approval reviews, and other approval documents uploaded onto Drugs@FDA. Extracted drug information included brand name, generic name, manufacturer, orphan drug designation date, approval date, approval status, product type [New Molecular Entity Application (NME) or New Biologic License Application (BLA)], therapeutic area, expedited programmes, approved with boxed warning, and approved for long-term use. Categories of approval information were determined based on a previous study [14]. Additional files 1 and 2 summarises data collection flow and details the data extraction variables.
The therapeutic category of each analysed drug was based on the corresponding Anatomical Therapeutic Classification (ATC, third level) according to the World Health Organization ATC Index 2020 [15]. For drugs with an ATC code, the authors MF and AYLC independently categorised these into the respective therapeutic areas based on the active ingredients and indications from the drug labels. Any discrepancies in categorisation were further confirmed by author VKCY, a registered pharmacist.
The FDA has four expedited approvals programmes (Table 1). In this study, we analysed orphan drugs as those approved through expedited programmes with ‘priority review’, ‘breakthrough therapy’ or ‘accelerated approval’ recorded in the drug approval documents. The fast-track designation, implemented only since 2004, was not assessed in the current analysis. ‘Approved with boxed warning’ was defined as the presence of a boxed warning on the initially approved label on Drugs@FDA. ‘Approved for long-term use’ was defined as chronic or repeat intermittent use for 6 months or longer, based on information in the ‘Indication and Usage’ and ‘Dosing and Administration’ sections of the initially approved label, or in information regarding the length of treatment found on the label. Keywords such as ‘cancer,’ ‘chronic,’ ‘long term use,’ and ‘repeated’ on the drug label were also used to categorise duration of use.
Outcomes
Following the product launch, updated safety events reported by manufacturers, health professionals, and consumers are continuously collected by the FDA via MedWatch: The FDA Safety Information and Adverse Event Reporting Program [16]. These reports are made available on the FDA Adverse Event Reporting System for assessment by clinical reviewers [16]. Depending on the evidence presented and the severity of reported adverse events, these may be developed into product labels to inform prescription practice. The primary outcome of the study is postmarketing safety events (PMSE) resulting in labelling changes. We defined PMSE as any safety-related label change after approval including boxed warnings, contraindications, warnings and precaution, adverse reactions, drug interactions, withdrawal, and suspended marketing. The secondary study outcome is severe postmarketing safety events (SPSE), a subgroup of PMSE that considered safety-related withdrawals, suspensions, and boxed warnings post-approval. Safety-related label changes from the date of drug approval to 31 December 2019 were extracted from FDA MedWatch and Drugs@FDA. For drugs with multiple approved indications, the follow-up began from the first approval date with orphan drug designation to the study end date.
Statistical analysis
Descriptive statistics were used to summarise the characteristics of included orphan drugs. Mean ± standard deviation (SD), median ± interquartile range (IQR), and frequencies with percentages were reported as appropriate. Negative binomial regression was applied to assess the association between approval factors and the cumulative number of PMSE within 5-years after approval. Maximum observational time, from approval to 5 years or to the study end date, was included as an offset variable in the regression. Kaplan–Meier estimates with log-rank tests were applied to compare the risk of SPSE over time amongst the following binary variables: (1) product type (NME versus BLA); (2) therapeutic area (antineoplastic versus non-neoplastic; (3) priority review; (4) accelerated approval; (5) breakthrough therapy; (6) approved for long-term use; (7) approved with boxed warning. Multivariable Cox regression was applied to assess the association between the seven factors mentioned above and the occurrence of SPSE starting from the respective drug approval date to the date of first SPSE or 31 December 2019, whichever was earlier. A two-sided P value of less than 0.05 was considered statistically significant. Schoenfeld residual-based test was used for testing the proportional hazard assumption. R version 3.6.0 (R Foundation for Statistical Computing, Vienna, Austria) was used for data manipulation and analysis. The programming and results were cross-checked for consistency by MF and AYLC.
Results
Characteristics of FDA-approved orphan drugs
We identified 214 drugs with orphan drug designation approved by the US FDA between 1 January 1999 and 31 December 2018. Amongst these, 25.7% (n = 55) were biologics, and the remaining were small molecule drugs (Table 2). The most common therapeutic areas were antineoplastic and immunomodulating agents (46.3%), followed by alimentary tract and metabolism (12.1%), and nervous system (7.5%). Around four-fifths (83.6%) of the reviewed drugs were approved via at least one of the considered expedited programmes. Over a quarter (29.4%) were approved with boxed warnings. Around twelve percent (n = 27) of the analysed orphan drugs had multiple indications, all of which had orphan drug designation at first approval.
Postmarketing safety events
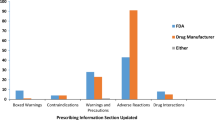
The number of FDA approved orphan drugs in general increased between 1999 and 2018. Figure 1 illustrates the timeline for orphan drugs approval and safety-related labelling changes. Of the approved orphan drugs, 69.2% (n = 148) were affected by at least one PMSE during a median follow-up time of 6.74 years. In total, there were 641 labelling changes related to postmarketing safety (boxed warning: 48; suspended marketing: 1; contraindications: 50; drug interactions: 68; warnings and precautions: 453; adverse reactions: 443, one label update could include multiple safety events). Of the analysed drugs 14.5% (n = 31) had SPSE with 49 labelling changes (safety-related withdrawals: 0; suspended marketing: 1; and boxed warnings: 48). The average time to first SPSE was 4.0 (SD: 3.9) years. New boxed warnings were added to eight drugs (Additional file 3) while the remaining 23 had reinforcements to the initial boxed warnings. Only one drug, Iclusig (ponatinib), had a temporary marketing suspension in 2013 because of the risk of life-threatening blood clots and severe narrowing of blood vessels. However, given the narrow population group, the benefits were considered to outweigh the risks and it was replaced on the market after two months.
Approval factors and the occurrence of safety events
Negative binomial regression show that ‘approved for long-term use’ [Incidence rate ratio (IRR): 2.76, 95% confidence interval 1.52–5.00] and ‘approved with boxed warning’ (IRR: 1.95, 95% CI 1.02–3.73) are two independent approval factors significantly associated with the frequency of PMSEs within 5 years since approval (Fig. 2). In the log-rank test, we observed a significantly increased proportion of SPSE among drugs with priority review during approval (p = 0.01), approved with boxed warning (p < 0.001), and approved for long-term use (p = 0.04) than those without (Additional file 4). We conducted a multivariable Cox proportional-hazards regression with all approval factors included. The Schoenfeld residuals test showed that the proportional hazard assumption was met for all the included variables and no significant violations were observed (Additional file 5). The Cox model confirmed similar findings that drugs ‘approved for long-term use' [Hazard ratio (HR): 2.68, 95% CI 1.27–5.65] and ‘approved with boxed warning’ (HR: 8.05, 95% CI 3.47–18.66) were independently significantly associated with the occurrence of SPSE (Fig. 3).
Association between drug approval factors and postmarketing safety events within 5 years of approval. Negative binomial regression was applied to assess the associations between approval factors and the cumulative number of PMSE within 5-years after approval. CI confidence interval, PMSE postmarketing safety events, yrs years
Discussion
Summary of findings
This study provides an overview of orphan drug safety through the first, most comprehensive longitudinal analysis of the FDA database on orphan drugs. Our analysis includes all FDA-approved orphan drugs since 1998 with up to 20 years of postmarketing surveillance. Of the 214 FDA-approved orphan drugs, 69.2% had labelling changes related to PMSE since designation and approval. In combination with available evidence from the drug regulatory agency and academic literature, this study reconfirms that timely regulatory action has been in place for orphan drugs with frequent safety-related labelling updates to inform prescription practice.
In our study, one of the approval factors associated with the frequency of PMSEs at 5-years after approval is ‘approved for long-term use’. This suggests a potential cumulative dose effect from the long-term use of a drug. However, this result could potentially be prone to survival bias, given that patient factors and disease trajectories commonly associated with chronic rare diseases differ from more rapidly progressive rare diseases. Patients with rapidly progressive rare diseases may not survive long enough to experience PMSE induced by the drug, and thus less safety reports may be generated. As such, when given sufficient sample sizes, longitudinal studies using electronic medical records may provide further insight regarding the safety of orphan drugs for short- and long-term use, where patient factors can be taken into consideration.
When studying orphan drugs safety, SPSE is a more pertinent consideration than PMSE due to the limited or even absent treatment options for life-threatening rare safety events. In our study, over 15% of FDA-approved orphan drugs had labelling changes related to SPSE, mostly as the reinforcement of initial boxed warnings issued on approval. Newly added boxed warnings only accounted for a small proportion overall. Drugs approved with boxed warnings had earlier labelling updates related to SPSE in multivariable analysis. This is further indication of enhanced label updates for drugs approved with boxed warnings, the evident interaction between pre- and postmarketing regulation and the importance of long-term safety surveillance.
Comparison with other studies
As mentioned previously, few studies had focused on postmarketing safety specific to orphan drugs. Onakpoya et al. assessed the safety of 74 orphan drugs approved by the European Medicines Agency (EMA) between 2002 and 2014 [17]. The study reported that 86.5% of identified orphan drugs had evidence of serious adverse events, a much higher proportion than our study. As with our study, orphan drugs approved for treating cancerous conditions had a higher proportion of adverse events. However, the definition of adverse events was unclear, and the study employed academic databases for evidence regarding orphan drug safety, where reporting and publication biases may exist. Meanwhile, the association between regulatory approval factors and postmarketing safety remain unexplored.
In a broader literature review, several studies that evaluated postmarketing drug safety potentially included a subgroup of orphan drugs, such as new molecular entities, new therapeutic biologics, and drugs that lack safety and efficacy data [14, 18,19,20,21,22,23,24,25,26,27,28]. One study on postmarketing safety of FDA-approved novel therapeutics showed that orphan status was not significantly associated with PMSE [14]. Studies using FDA and EMA databases found that novel therapeutics or biologics with accelerated approval or shorter time to obtain approval, respectively, experienced a higher rate of PMSE [14, 18, 19]. This finding was consistent with our multivariable analysis which focused only/?solely on orphan drugs. Furthermore, these studies focused predominantly on PMSE rather than SPSE.
Caution must be exercised when contextualising these findings from non-orphan drug specific studies as drugs with orphan drug designation might experience different review, surveillance and reporting procedures. Moreover, approval factors are not necessarily comparable among different drug approval agencies. Orphan drugs with identified risk factors for SPSE, namely ‘for long-term use’ and ‘approved with boxed warnings’, should be further examined using real-world data and multiple drug regulatory databases to inform safety monitoring processes.
Implications and future research directions
Findings from this study will inform multiple stakeholders about the frequency of safety-related labelling changes in orphan drugs detected by the FDA. This reinforces the role of postmarketing safety surveillance—to allow health professionals to be updated on any safety-related events for new orphan drugs alongside the predominant benefits to patients with rare diseases. Despite poor prognoses and limited treatment options, patients with rare diseases may be open towards drugs with more uncertainties than traditionally accepted. Decision-makers are therefore challenged to make trade-offs between conclusive safety evidence and timely life-saving treatment to address unmet patient needs. Quality safety data from structured surveillance programmes will assist regulators and payers to better mitigate uncertainties and balance the risks and benefits without further exposing patients to treatments with unproven benefits. Establishing orphan drugs or rare disease registries is imperative for extensive and continuous safety (and effectiveness) monitoring.
Future research should consider aggregating data from various drug surveillance databases to achieve power for more nuanced orphan drug safety assessment. Suggested databases include the safety and approval databases from the EMA, and drugs approved by the Center for Biologics Evaluation and Research of FDA. Other adverse event reporting systems such as the Yellow Card scheme in the United Kingdom, and the Canada Vigilance Program in the Canadian jurisdiction that collect and assess spontaneous reports of adverse drug reactions from patients and health professionals are also viable options for expanding the data [25].
Limitations
The study findings should be interpreted cautiously with the following caveats. Limited numbers of drugs were assessed in the study given the finite number of FDA-CDER-approved orphan drugs. Uncertainty remains regarding the association between postmarketing safety events and other approval factors that yielded insignificant findings in the current analysis. Underestimating long-term PMSE is likely, given that the reported safety events of orphan drugs are often based on a small population. This, along with the inherent differences between rare disease and orphan drug definitions employed by various drug regulatory authorities, could discount the generalisability of our findings. Furthermore, since the estimation of safety events is based on reports from the drug surveillance system, no comparison between placebo and intervention arms could be made and interpreting the results of our study should be taken cautiously.
It should also be noted that the study only examined newly approved chemical or biological agents with an orphan drug designation. Drugs initially approved for common disease conditions and later repurposed as orphan drugs were not considered. At the same time, drugs with orphan drug designation could be extended to indications of common diseases when adequate and high-quality clinical evidence becomes available, highlighting the importance of safety surveillance when drugs are used on a broader population. For drug developers and regulators there is an inherent trade-off between the demand for life-saving drugs with early treatment access and the need to gather conclusive evidence about the real-world effectiveness and long-term safety. As such, additional safety information discovered after a drug has been approved is both expected and appropriate.
Conclusions
Frequent postmarketing safety-labelling updates occur among FDA-approved drugs with orphan drug designation and expedites approval, particularly for drugs approved for long-term use or approved with boxed warning. Labelling changes related to severe safety events are uncommon and focus mainly on the reinforcement of initial boxed warning. Drug regulatory systems, collectively with research partners and sponsors, must strive to maintain timely medication safety surveillance and obtain more evidence to better inform clinicians and stakeholders about the risks and benefits of orphan drugs.
Availability of data and materials
The datasets used and analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- FDA:
-
Food and Drug Administration
- PMSEs:
-
Postmarketing Safety Events
- SPSEs:
-
Severe Postmarketing Safety Events
- IRR:
-
Incidence Rate Ratio
- 95% CI:
-
95% Confidence Interval
- NME:
-
New Molecular Entity Application
- BLA:
-
Biologics License Application
- EMA:
-
European Medicines Agency
- PDFUA:
-
Prescription Drug User Fee Act
- S.D.:
-
Standard Deviation
- IQR:
-
Interquartile Range
References
US Food and Drug Administration (FDA). Title 21 food and drugs. Chapter I food and drug administration department of health and human services. Subchapter D drugs for human use. Part 316 orphan drugs. United States: Federal Register; 2020. https://www.ecfr.gov/cgi-bin/retrieveECFR?gp=&SID=718f6fcbc20f2755bd1f5a980eb5eecd&mc=true&n=sp21.5.316.c&r=SUBPART&ty=HTML#se21.5.316_121.
Fonseca DA, Amaral I, Pinto AC, Cotrim MD. Orphan drugs: major development challenges at the clinical stage. Drug Discov Today. 2019;24(3):867–72.
Food and Drug Administration. Fast track, breakthrough therapy, accelerated approval, priority review, United States: Food and Drug Administration; 2018. https://www.fda.gov/patients/learn-about-drug-and-device-approvals/fast-track-breakthrough-therapy-accelerated-approval-priority-review.
Kepplinger EE. FDA’s expedited approval mechanisms for new drug products. Biotechnol Law Rep. 2015;34(1):15–37.
Zhang AD, Puthumana J, Downing NS, Shah ND, Krumholz HM, Ross JS. Assessment of clinical trials supporting US food and drug administration approval of novel therapeutic agents, 1995–2017. JAMA Netw Open. 2020;3(4):e203284.
Bell SA, TudurSmith C. A comparison of interventional clinical trials in rare versus non-rare diseases: an analysis of ClinicalTrials.gov. Orphanet J Rare Dis. 2014;9:170.
Motoo N, Hayashi Y, Shimizu A, Ura M, Nishikawa R. Safety and effectiveness of bevacizumab in Japanese patients with malignant glioma: a post-marketing surveillance study. Jpn J Clin Oncol. 2019;49(11):1016–23.
Kiyohara Y, Uhara H, Ito Y, Matsumoto N, Tsuchida T, Yamazaki N. Safety and efficacy of nivolumab in Japanese patients with malignant melanoma: An interim analysis of a postmarketing surveillance. J Dermatol. 2018;45(4):408–15.
U.S. Food & Drug Administration. Drugs@FDA: FDA-approved drugs United States; 2020. https://www.accessdata.fda.gov/scripts/cder/daf/.
Food and Drug Administration. New molecular entity (NME) drug and new biologic approvals. United States: Food and Drug Administration; 2017. https://wayback.archive-it.org/7993/20170404174205/https:/www.fda.gov/Drugs/DevelopmentApprovalProcess/HowDrugsareDevelopedandApproved/DrugandBiologicApprovalReports/NDAandBLAApprovalReports/ucm373420.htm.
Food and Drug Administration. New molecular entity (NME) drug and new biologic approvals. United States: Food and Drug Administration; 2019. https://www.fda.gov/drugs/nda-and-bla-approvals/new-molecular-entity-nme-drug-and-new-biologic-approvals.
U.S. Food & Drug Administration. Compilation of CDER new molecular entity (NME) drug and new biologic approvals [Internet]. United States: Food and Drug Administration; 2020. https://www.fda.gov/drugs/drug-approvals-and-databases/compilation-cder-new-molecular-entity-nme-drug-and-new-biologic-approvals.
Food and Drug Administration. Search orphan drug designations and approvals. United States: Food and Drug Administration; 2020. https://www.accessdata.fda.gov/scripts/opdlisting/oopd/.
Downing NS, Shah ND, Aminawung JA, Pease AM, Zeitoun JD, Krumholz HM, et al. Postmarket safety events among novel therapeutics approved by the US food and drug administration between 2001 and 2010. JAMA. 2017;317(18):1854–63.
WHO Collaborating Centre for Drug Statistics Methodology. ATC/DDD Index 2020 Norway: WHO collaborating centre for drug statistics methodology; 2019. https://www.whocc.no/atc_ddd_index/.
Kessler DA. Introducing MEDWatch. A new approach to reporting medication and device adverse effects and product problems. JAMA. 1993;269(21):2765–8.
Onakpoya IJ, Spencer EA, Thompson MJ, Heneghan CJ. Effectiveness, safety and costs of orphan drugs: an evidence-based review. BMJ Open. 2015;5(6):e007199.
Zeitoun JD, Lefevre JH, Downing NS, Bergeron H, Ross JS. Regulatory review time and post-market safety events for novel medicines approved by the EMA between 2001 and 2010: a cross-sectional study. Br J Clin Pharmacol. 2015;80(4):716–26.
Giezen TJ, Mantel-Teeuwisse AK, Straus SM, Schellekens H, Leufkens HG, Egberts AC. Safety-related regulatory actions for biologicals approved in the United States and the European Union. JAMA. 2008;300(16):1887–96.
Botelho SF, Martins MA, Vieira LB, Reis AM. Postmarketing safety events relating to new drugs approved in brazil between 2003 and 2013: a retrospective cohort study. J Clin Pharmacol. 2017;57(4):493–9.
Ikeda J, Kaneko M, Narukawa M. Analysis of factors related to the occurrence of important drug-specific postmarketing safety-related regulatory actions: a cohort study focused on first-in-class drugs. Pharmacoepidemiol Drug Saf. 2018;27(12):1393–401.
Ikeda J, Kaneko M, Narukawa M. Post-marketing safety-related regulatory actions on first-in-class drugs: a double-cohort study. J Clin Pharm Ther. 2020;45(3):496–502.
Lexchin J. Post-market safety warnings for drugs approved in Canada under the Notice of Compliance with conditions policy. Br J Clin Pharmacol. 2015;79(5):847–59.
Schick A, Miller KL, Lanthier M, Dal Pan G, Nardinelli C. Evaluation of pre-marketing factors to predict post-marketing boxed warnings and safety withdrawals. Drug Saf. 2017;40(6):497–503.
Tau N, Shochat T, Gafter-Gvili A, Tibau A, Amir E, Shepshelovich D. Association between data sources and US food and drug administration drug safety communications. JAMA Intern Med. 2019;179(11):1590–2.
Tamura N, Ishiguro C, Matsuda T. Post-approval appending of CSARs to drug package inserts: an analysis of the types of adverse reactions and time to addition. Pharmacoepidemiol Drug Saf. 2015;24(2):166–75.
Pinnow E, Amr S, Bentzen SM, Brajovic S, Hungerford L, St George DM, et al. Postmarket safety outcomes for new molecular entity (NME) drugs approved by the food and drug administration between 2002 and 2014. Clin Pharmacol Ther. 2018;104(2):390–400.
Bulatao I, Pinnow E, Day B, Cherkaoui S, Kalaria M, Brajovic S, et al. Postmarketing safety-related regulatory actions for new therapeutic biologics approved in the United States 2002–2014: similarities and differences with new molecular entities. Clin Pharmacol Ther. 2020;108(6):1243–53.
Acknowledgements
We thank Mr. Timothy MT Liu, Mr. Matthew HS Sun and Miss Candy WS Yuen for their important contributions at the pilot phase of this study. We thank Ms. Lisa Lam for proofreading the manuscript.
Funding
Enhanced Startup Fund for new academic staff, LKS Faculty of Medicine, University of Hong Kong; Internal Research Fund, Department of Medicine, University of Hong Kong. This is a general research grant provided by the University of Hong Kong. The funding body did not participate in any part of the study, whether design, collection, analysis, interpretation of data or in writing the manuscript.
Author information
Authors and Affiliations
Contributions
Study concept and design: XL, ICKW, MF. Data extraction, cleaning and analysis: MF, AYLC, VKCY. Data validation and cross-check: AYLC, MF, LKWL. Data interpretation: all authors. Drafting of the manuscript: XL, AYLC, MF. Critical revision of the manuscript of significant intellectual contribution: all authors. Study supervision: XL, ICKW. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was based on extraction of data from a series in the publicly accessible website of the U.S. Food and Drug Administration at https://www.fda.gov. No patient was involved in the study, therefore ethical approval was not applicable to this study.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1
. Data extraction flowchart
Additional file 2
. Extracted data list and data source
Additional file 3
. Orphan drugs with newly added boxed warning
Additional file 4
. Proportion of orphan drugs affected by severe postmarketing safety events
Additional file 5
. Schoenfeld residuals plots for proportional hazard assumption checking
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Fan, M., Chan, A.Y.L., Yan, V.K.C. et al. Postmarketing safety of orphan drugs: a longitudinal analysis of the US Food and Drug Administration database between 1999 and 2018. Orphanet J Rare Dis 17, 3 (2022). https://doi.org/10.1186/s13023-021-02166-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13023-021-02166-9