Abstract
Background
The number of market approvals of orphan medicinal products (OMPs) has been increasing steadily in the last 3 decades. While OMPs can offer a unique chance for patients suffering from rare diseases, they are usually very expensive. The growing number of approved OMPs increases their budget impact despite their low prevalence, making it pressing to find solutions to ethical challenges on how to fairly allocate scarce healthcare resources under this context. One potential solution could be to grant OMPs special status when considering them for reimbursement, meaning that they are subject to different, and less stringent criteria than other drugs. This study aims to provide a systematic analysis of moral reasons for and against such a special status for the reimbursement of OMPs in publicly funded healthcare systems from a multidisciplinary perspective.
Results
With a systematic review of reasons, we identified 39 reasons represented in 243 articles (scientific and grey literature) for and against special status for the reimbursement of OMPs, then categorized them into nine topics. Taking a multidisciplinary perspective, we found that most articles came from health policy (n = 103) and health economics (n = 49). More articles took the position for a special status of OMPs (n = 97) than those against it (n = 31) and there was a larger number of reasons identified in favour (29 reasons) than against (10 reasons) this special status.
Conclusion
Results suggest that OMP reimbursement issues should be assessed and analysed from a multidisciplinary perspective. Despite the higher occurrence of reasons and articles in favour of a special status, there is no clear-cut solution for this ethical challenge. The binary perspective of whether or not OMPs should be granted special status oversimplifies the issue: both OMPs and rare diseases are too heterogeneous in their characteristics for such a binary perspective. Thus, the scientific debate should focus less on the question of disease prevalence but rather on how the important variability of different OMPs concerning e.g. target population, cost-effectiveness, level of evidence or mechanism of action could be meaningfully addressed and implemented in Health Technology Assessments.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Background
Orphan medicinal products (OMPs) are highly specialized treatments for very small groups of patients. The definition of OMPs slightly varies between regulations. According to the European Commission Regulation [1], an orphan designation pertains exclusively to OMPs that are “intended for the diagnosis, prevention or treatment of a life-threatening or chronically debilitating condition affecting not more than five in 10,000 persons in the Community” (Article 2.1) or that fulfill the condition that “without incentives it is unlikely that the marketing of the medicinal product in the Community would generate sufficient return to justify the nece[s]sary investment” (Article 2.2). The sponsor of an orphan drug designation must additionally establish that there exist no satisfactory alternatives or that the new drug is better than the existing alternatives (Article 2.3) [1, 2].
Because developing drugs for rare diseases had not been lucrative for the pharmaceutical industry, the United States started incentivizing research and development of OMPs with the Orphan Drug Act in 1983 [3]. The European Union has followed this attempt with the Orphan Regulation in 1999 (Reg. no 141/2000) [4]. The incentives vary between jurisdictions but often include market exclusivity, tax exemptions, research funding, and free-of-charge research advice. Moreover, OMP incentives may be connected to more general incentive programs, such as accelerated market authorization procedures, the option for off-label use (meaning the use of a drug before its official approval for a specific therapy), and compassionate use programs [5]. Since the implementation of these incentive programs, the number of approved OMPs has been growing exponentially [6]. However, not all approved OMPs are targeted towards rare diseases: In light of the ongoing research progress in precision medicine, targeted therapies for common diseases might also fall into the definition of orphan drug regulations. Still, a US-based empirical study did not find targeted therapies to mainly cause the increase in OMP approvals [7].
Drugs are approved for marketing and orphan designation by medical agencies (such as the Federal Drug Administration in the United States and the European Medical Agency in the European Union) based on their efficacy and safety. In publicly funded healthcare systems, however, a separate country-specific process decides whether or not an approved drug is reimbursed. This process may include a Health Technology Assessment (HTA), which assesses the value of medical products as supported by clinical evidence and cost-effectiveness analyses.
OMPs share the characteristic of aiming to treat small populations with targeted therapies, which leads to higher costs and difficulties in obtaining clinical evidence [8]. As prices are often high and negatively correlate with disease prevalence [9], OMPs are often not cost-effective [10]. Recently approved curative OMPs have well exceeded previous pricing standards: onasemnogene abeparvovec, for instance, a gene replacement therapy attempting to cure Spinal Muscular Atrophy with a one-time treatment, costs more than 2 million US$. Still, the drug has been considered cost-effective with this price tag compared to alternatives if administered early [11] and has been approved for reimbursement in various countries [e.g., 12–14]. Consequently, many OMPs are reimbursed regardless of their high prices [e.g., 15, 16], thereby being granted special status. Special status, in this context, is defined as the application of differential criteria in reimbursement decision-making for OMPs compared to non-OMPs.
Because of the growing number of expensive OMPs, their reimbursement through public health insurances is increasingly manifesting itself as a moral dilemma for decision-makers: Basing OMP reimbursement on rules of exception is becoming unsustainable, as financing many expensive OMPs within a publicly funded healthcare system inevitably leads to cuts in other healthcare areas [17]. Not reimbursing any orphan drugs, however, is equally problematic given the immense need of patients who depend on them.
Theories of distributive justice provide some guidance in the ethical challenge of healthcare resource allocation [18, 19]. First, utilitarianism intends to maximise overall population-wide well-being [20] and is traditionally a guiding principle for health policy; the element of cost-effectiveness in the traditional reimbursement decision criteria is based on this utilitarian principle. Second, egalitarians represent the cause that everyone is treated equally, but some theorists acknowledge that certain compensation might be required for the disadvantaged to achieve equality [21]. Third, the libertarian position stresses individual freedom and calls for minimizing state interventions. Therefore, supporting the disadvantaged is only recognized when based on voluntary actions of individuals [22]. Fourth, communitarians contrast from libertarian positions with their emphasis on community interests. They call for policies that emphasize the needs and interests of communities over the interests and needs of individuals [23]. Finally, the “rule of rescue”, albeit it is not an established theory of distributive justice but rather a practice-oriented principle, calls for saving identifiable individuals no matter the cost if they are dying soon and have the chance to be rescued. It is thought to be a rule of exception and has been implemented in the Australian OMP reimbursement policy [24,25,26,27,28,29]. Moreover, because OMP reimbursement points to an ethical challenge without any clear-cut solution, many authors call for public debates to discuss its challenges and subsequent strategies that are legitimated via deliberative democratic processes [30, 31].
In light of the increasing number of high-priced OMP approvals and the unsolved ethical challenges concerning their reimbursement in publicly funded healthcare systems, this study aims to provide a systematic analysis of moral reasons for and against special status for the reimbursement of OMPs in publicly funded healthcare systems from an interdisciplinary perspective. To our knowledge, there is not yet a systematic review of the ethical considerations regarding OMP reimbursement available to date. The following research questions are addressed: RQ1—What reasons in favour or against the special status for reimbursing OMPs are discussed in the scientific and grey literature? RQ2—What reasons are dominating the scientific discourse? RQ3—What disciplines contribute to this debate and how heterogeneous is the field? RQ4—To what countries and regions worldwide does this debate refer to?
Methods
A systematic review of reasons [32] was the methodological basis for this study. This method allows for a systematic assessment of the moral reasons for and against special status for the reimbursement of OMPs as compared to non-orphan drugs in reimbursement decision-making. It aims to synthesize them rather than to assess their adequacy and quality [33]. Moral reasons were defined as arguments about what decision, from a moral perspective, ought to be the right one [34].
Search strategy
Scientific databases were systematically searched for relevant articles. They were selected to cover a variety of research fields relevant to the topic: new PubMed (medicine), EMBASE via Elsevier (biomedicine), CINAHL (nursing), Web of Science Core Collection (interdisciplinary), Philosopher’s Index with full text via EBSCOhost (philosophy), HeinOnline (law), Open Grey (grey literature), and Google Search was targeted for stakeholder position papers (grey literature). Additional file 1 provides detailed documentation of the search strategy, including database-specific search strings. No time restrictions were set for this study.
Article selection
Article duplicates were removed using the identifying function in Citavi 6.0, checking each duplicate manually before removal. BZ screened the articles' titles and abstracts and excluded articles that were not about reimbursement issues concerning rare diseases or OMPs. The remaining articles were assessed based on the full texts and were included if (1) there was at least one moral reason for or against special status for the reimbursement of OMPs; (2) the format was either a book chapter, research article, commentary, PhD thesis, editorial, conference paper (abstract collections were excluded), position paper or blog written by specialists and targeted to a specialised audience (articles targeted to a general audience, such as newspaper articles, were excluded); (3) full texts were in English or German language; (4) full texts were available. In Additional file 2, we report how many articles were excluded for what reasons. The first 556 (55%) full texts were assessed by two researchers (BZ and JE) independently and compared, discrepancies were discussed and inclusion decisions made based on agreement. The remaining full texts were assessed by BZ alone, but those that were difficult to judge were double-checked by JE. References of all articles were screened for additional articles, but reference screening was limited to the references that were cited in relevant text passages.
The analysis was limited to articles discussing OMP for rare diseases, thereby excluding those relevant to neglected diseases (diseases that are predominantly present in developing countries), distribution issues related to developing countries (unless they concern drugs with an orphan designation), issues related to pediatric drugs (unless they concerned drugs with an orphan designation) and articles only covering reimbursement for personalized treatment or other groups of treatment that might or might not fall under the orphan indication. We also did not consider articles that discussed pricing issues without considering reimbursement.
Data extraction and analysis
To address RQ1 and RQ2, we applied an inductive procedure, where we systematically identified and extracted moral reasons for or against special status for the reimbursement of OMPs; then we sorted and categorised them in several iterative steps. First, two researchers (BZ and JE) separately identified text passages that included a moral reason from three deliberately selected articles with a particular focus on reasons for and against special status for the reimbursement of OMPs. Text passages were compared and their discrepancies discussed. Every text passage identified was assigned a “narrow reason”, a code that summarized its core argument. If multiple arguments were combined in one text passage, multiple codes were subsequently applied to the same text passage. In two additional rounds, the same procedure was applied to another 42 randomly selected articles. Subsequently, these identified “narrow reasons” were preliminarily categorized and discussed based on the selected 45 articles. The remaining data extraction was done by BZ alone, following and refining this preliminary categorical system.
To address the heterogeneity of disciplines discussing moral reasons (RQ3) and the geographical distribution (RQ4), variables were extracted deductively using a predefined codebook (see Additional file 3) that was inspired by previous methodological considerations for systematic reviews of reasons [32]. Disciplines were assessed based on the content of the articles; if the content was not unambiguously assignable to one discipline, the first author's affiliation was considered; if it was a multidisciplinary affiliation the field of the journal was additionally considered. Geographical regions were assessed by first collecting, for each article, to what country or countries the article was referring to. From this extracted information, an exhaustive list of categories was built and applied to each article (see Additional file 3).
For data analysis, all reasons mentioned above were counted, including those citing reasons from other authors, because we were interested in the relative prevalence of the reasons. Data collection and analysis were supported by IBM SPSS Statistics 25 (for graphical representation and descriptive statistics) and MaxQDA 2020 (for coding and categorizing reasons).
Findings
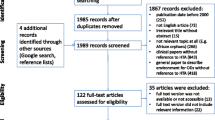
We identified 243 articles that met the inclusion criteria and contained at least one moral reason for or against special status for the reimbursement of OMPs. However, in 72 of all included articles (29.6%), moral reasons were merely mentioned in one paragraph or less. Figure 1 indicates the detailed results of the systematic article selection process. Reasons for exclusion on the full-text level are reported in Additional file 2. The raw data of the variables collected are available in Additional file 4.
Distribution over time
The earliest article included was published in 1995, but all other articles were released in the years 2001 or later. Figure 2 indicates that the number of articles addressing moral reasons regarding special status for the reimbursement of OMPs increased steadily over the years, reaching a maximum of 31 articles published per year in 2017.
Scientific disciplines
We identified a total of six scientific disciplines and added stakeholder statements from grey literature that were not assignable to any scientific discipline. Health policy was the most common discipline with a total of 103 articles (42.4%), followed by health economics (n = 49, 20.2%), philosophy and bioethics (n = 29, 11.9%), clinical medicine (n = 21, 8.6%), social sciences (n = 20, 8.2%) and law (n = 9 3.7%). Few articles included stakeholder's views, such as those from the pharmaceutical industry (n = 4, 1.6%), rare disease patients (n = 3, 1.2%), and others (n = 5, 2.0%). As Table 1 illustrates, the majority of articles from the fields of philosophy/bioethics, clinical medicine, and health economics were theoretical articles, commentaries or editorials. The health policy field applied the most variable methodology, dominated by reviews, empirical and theoretical work. In the social sciences, most articles included empirical work, especially surveys. Most stakeholder articles included policy guidelines.
Geographical regions
Most articles focused on European countries (n = 96, 39.5%), 20 on Canada (8.2%), 19 on the United States (7.8%), 10 on Asian countries (4.1%), 8 on Australia or New Zealand (3.3%), and 3 on South American countries (1.2%). Some 41 articles (16.9%) covered more than one geographical region, while 46 articles (18.9%) specified no geographical region.
Overall assessment regarding special status for the reimbursement of OMPs
As indicated in Table 2, more articles drew overall conclusions in favour of the special status for OMPs than ones that drew against it. The discipline we referred to as social sciences, mainly including empirical survey studies with parts of the general population or stakeholder groups, held the highest relative number of articles arguing against special status. By contrast, in the fields of clinical medicine and law, the majority of articles argued for special status.
Reasons for and against special status for the reimbursement of OMPs
We identified 29 reasons for, and 10 reasons against special status for the reimbursement of OMPs in the scientific and grey literature and categorized them into nine categories (Fig. 3 and Additional file 5). The following section presents the content of each category with various moral reasons in detail.
-
1)
Maximize population health
The utilitarian principle to maximize overall population health is the underlying moral standard for traditional cost-effectiveness measures. Relevant to this category, we identified three broad reasons against special status for reimbursement of OMPs. First, the most commonly mentioned reason against special status for the reimbursement of OMPs (n = 63, see also Additional file 5) was that it contradicted the principle to maximize population health [25,26,27, 35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94], leading to ineffective use of resources [92] and thereby emphasizing policy makers’ obligation to use resources effectively [91]. However, 19 of the articles that mentioned this reason were generally in favour of a special status [25, 41, 46, 48, 49, 56, 57, 60, 67, 70, 72,73,74, 85, 87, 89, 90, 93, 94] and either refuted this reason or judged other reasons as being more important. For instance, some authors argued that individual needs should also be considered, not only population needs [41, 72, 91, 95, 96].
Second, a related argument focused on the opportunity costs when reimbursing expensive OMPs, particularly that less money would be available to treat common disorders [35, 38, 39, 41, 45, 47, 48, 53, 62, 63, 66, 69, 73, 79, 81, 85, 88, 91,92,93, 97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112,113,114,115,116,117,118,119,120,121,122,123,124]. Some authors perceived this as problematic since few expensive OMP treatments would take away resources for cost-effective treatments for more patients. While some authors used this reason as an argument against special status, others more mildly called for more consideration during decision-making to weigh the benefits of OMPs against their opportunity costs. This second reason is also based on the principle of maximising population health gain but emphasizes the difference between rare and common disorders more explicitly than the first reason presented.
Finally, differing discussions were made concerning the budget impact of OMPs. While in some articles (n = 37), authors claimed that OMPs had a low budget impact and could therefore be reimbursed [29, 36, 42, 44, 46, 48, 49, 52,53,54, 66, 74, 77, 85, 88, 103, 104, 114, 115, 118,119,120, 123, 125,126,127,128,129,130,131,132,133,134,135,136,137,138], others (n = 27) rejected this reason based on the claim that all OMPs together had a significant budget impact that would increase further in the future, since more OMPs were expected to come onto the market [35, 47, 66, 69, 73, 78, 80, 92, 100, 104, 107, 108, 110, 112, 114, 118, 122, 139,140,141,142,143,144,145,146,147,148].
-
2)
Equality and equity
Reasons related to equality and equity were discussed in many articles (n = 194, 79.8%). It became evident that the expressions of equity and equality were confounded and sometimes used interchangeably in our data. Here, we use the term “equality” in an egalitarian tradition to treat every person equally. The term “equity” is related to the idea that some compensation is allowed for ethically relevant aspects that makes people unequal, thereby allowing for prioritizing those with special needs. In some articles, authors referred to horizontal and vertical equity to make this distinction [38, 45, 74, 81, 87, 121]. Opposing views in this category concluded, for instance, that special status for reimbursement of OMPs was incompatible with the egalitarian principle to treat each individual equally [35, 36, 39, 42, 47, 52, 62, 70, 75, 78, 81, 85,86,87,88, 98, 100, 107, 110, 114, 132, 137, 142, 145, 149,150,151,152,153,154,155,156].
On the other hand, an extensive body of literature argued that a special status was justified for reasons of equity [8, 35, 39, 46, 54, 66, 68, 73, 81, 85, 94, 107, 116, 117, 121, 122, 128, 136, 137, 152, 153, 157,158,159,160,161,162,163,164]. Reasons related to this equity principle included: First, that rare disease patients should have the same access to treatments as all other patients should and that this was only possible by providing OMPs even if they were expensive [5, 25, 37, 38, 40, 42,43,44, 51, 57, 60, 61, 63, 67, 70,71,72, 78,79,80,81, 87,88,89, 93, 95, 96, 100, 101, 103, 105, 106, 112, 115, 118, 126,127,128,129, 139, 140, 142, 151, 153, 154, 156, 157, 161, 165,166,167,168,169,170,171,172,173,174,175,176,177,178,179,180,181,182,183,184,185,186,187,188,189,190,191,192,193,194]. Any barriers to access should be “morally justifiable” [139, 183]. It was also stated that there was a general societal preference for equal access and opportunity [40, 61, 63, 79, 80, 106, 142, 151, 161, 175, 176, 182].
Second, some authors stated that all people should have equal opportunities for good health [27, 35, 47, 52, 56, 66, 68, 69, 73, 81, 88, 90, 92, 93, 95, 104, 105, 109, 124, 128, 137, 142, 146, 152, 156, 173, 175, 190, 195, 196], including a “fair chance to live an autonomous and fulfilling life” [124], which would particularly prioritize young patients, as often this is the case in patients with rare diseases [90, 124, 195, 196]. Counter-arguments included that it was not feasible to reach full equality of opportunity [69], that the focus on a fulfilling life would be unfair towards the elderly or severely disabled [47], and that this proposal would be best solved in a health care lottery, which was unacceptable for Juth [105] but proposed by others as being part of a potential solution [68].
A third and related reason for prioritizing OMPs for reasons of equity included that we should aim for equality in health outcome, instead of equality in access to care, which would prioritize those in worse health states, aiming at equal health and life-expectancy for everyone [35, 47, 70, 105, 173, 184]. Again, the main counter-argument concluded that this point was not specific to rare diseases and would therefore not justify special status for reimbursement of OMPs [105].
A fourth justification for equity included the reason that everyone had the same right to a decent minimum standard of care [47, 49, 52, 53, 56, 63, 73, 80, 81, 90, 105, 114, 115, 196]. This line of argument, also called sufficientariarism in the literature [35, 47, 105], was often referred to as a form of prioritarianism where those that are prioritized have more difficulties reaching this decent minimum standard of care. However, Gross [196] argued that first, access to primary health care for all should be ensured before considering reimbursement of OMPs. Others stated that sufficientariarism was inappropriate to judge for special status in OMPs because it was unclear what the “decent minimum” encompassed [90] and the concept would not necessarily prioritize OMPs [105].
Finally, prioritizing OMPs for reasons of equity was justified by the reason that rare disease patients were worse off compared to others [27, 28, 35, 38, 41, 46, 47, 69,70,71, 73, 74, 79, 87, 101, 105, 108, 109, 122, 152, 161, 196,197,198], which was also framed as a societal preference [27, 46, 73, 74, 79, 122, 161, 198]. The issue of how rare disease patients were worse off than others was discussed extensively, including (1) disease severity; (2) unmet needs; (3) lack of alternative treatments; (4) the high-cost treatments not allowing for self-payment; and (5) the general disadvantages of rare disease patients. In the following paragraphs, these five aspects will be reviewed in greater detail.
(1) Many authors called for granting special status as OMPs treated severe conditions [24,25,26,27, 35, 37, 38, 40, 42,43,44,45,46, 49, 51,52,53,54,55,56,57, 59, 63, 69, 70, 72,73,74,75, 78,79,80, 83, 86,87,88,89,90, 92, 93, 95, 102, 104, 106, 107, 109, 112, 114, 115, 117,118,119, 122,123,124, 129, 130, 132, 135, 139, 142, 144, 150, 156, 157, 161, 167, 176,177,178, 184, 192, 193, 199,200,201,202,203,204,205,206,207,208,209,210,211,212,213,214,215,216,217,218,219], where this disease severity was a factor of societal preference. Some authors generally referred to disease severity as an important criterion for reimbursement decisions, irrespective of whether a disease was rare or common [27, 46, 49, 57, 70, 83, 87, 89, 102, 117, 120, 139, 141, 202, 208, 218, 219]. As stated by Medic et al. [87], the extent that rarity contributes to perceived severity in decision-makers’ eyes is unclear. Consequently, several authors specified that disease severity was not an argument specific to OMPs [44, 75, 86, 88, 104, 112, 117, 142, 150, 205] and not all rare diseases were severe or life-threatening [52, 55, 63, 130], thereby concluding that disease severity was not a good criterion to justify special status. Moreover, disease severity would need a unified method of measurement to serve as an objective reimbursement criterion [208]. In the considered body of literature, diseases were considered severe if, for instance, they were “chronic, deliberating and associated with reduced life expectancy” [53] or were associated with severe pain [212]. (2) Another feature justifying special status for the reimbursement of OMPs were unmet needs. Authors argued that in the case of unmet needs, it was unfair not to cover those treatments [24, 36, 57, 69, 75, 77, 79, 87, 89, 99, 102, 114, 116, 122, 129, 130, 136, 178, 184, 189, 202, 203, 205, 208, 220,221,222,223,224]. However, Sandman and Hofmann [184] found the concept of unmet needs unsustainable in this context and recommended instead considering disease severity. (3) One extensively discussed reason (n = 68) related to unmet need was the lack of alternative treatment [8, 24, 37, 44,45,46, 51, 54, 56, 60, 62, 64, 70, 75, 77,78,79, 83, 84, 87, 96, 111, 112, 114,115,116, 122, 123, 128,129,130, 135, 139, 141, 142, 147, 150,151,152, 161,162,163, 167, 174, 177, 178, 184, 193, 199,200,201,202,203, 206, 208, 210,211,212,213,214,215, 217, 219, 223, 225,226,227,228]: Since OMPs often represented the only hope for patients suffering from rare diseases, which justified their special status [70]. Relatedly, some studies also found a societal preference for covering drugs for diseases without treatment alternatives [45, 51, 79, 115, 213, 217]. However, some other authors countered that supportive or palliative care was always an available alternative [75, 83, 111, 112, 142, 184]. These reasons of disease severity, unmet needs or the lack of alternative treatments were often mentioned in the same paragraph (n = 36), and were usually presented as features that needed to be fulfilled to justify special status [24, 37, 44,45,46, 51, 54, 56, 75, 78, 79, 83, 87, 112, 122, 123, 129, 135, 139, 142, 150, 161, 163, 177, 178, 193, 199,200,201, 206, 210,211,212,213, 217, 219]. A common counter-argument for disease severity, unmet needs and the lack of alternative treatment was that they were not specific to rare diseases and could also be a feature of certain common diseases [35, 36, 44, 75, 86, 88, 104, 105, 111, 112, 117, 142, 150, 205, 224, 225]. (4) An argument that specifically draws on the feature of rarity was that rare disease patients were historically disadvantaged due to the rarity of their diseases and therefore generally worse off [35, 43, 73, 88, 94, 97, 108, 117, 152, 165, 173, 175, 179, 210, 229,230,231]. These disadvantages include: the lack of drug development efforts before incentives were installed [97, 117, 173, 175, 229]; disadvantages in traditional cost-effectiveness procedures [43], as Quality-Adjusted Life Years (QALY) measures would disadvantage people with chronic disabilities [73, 210]; the lack of public awareness; and difficulties in collecting effectiveness evidence for OMPs [165]. (5) A final justification for why rare disease patients were worse off is that OMPs were too expensive to be paid by patients themselves and therefore should be reimbursed [46, 51, 61, 116, 118, 123, 133, 138, 147, 161, 163, 183, 199,200,201, 204, 208,209,210, 232,233,234]. It was also argued that rare disease patients already carried a substantial financial burden as compared to the general population [209, 210, 233], including indirect costs for transportation to specialized facilities or the inability of family caregivers to be in full-time employment [123].
-
3)
Personal responsibility
Several authors mentioned that society values the role of individual lifestyle choices, such as smoking, diet or physical activity [73, 74, 88, 124, 208]. In some societies, the willingness to cover expensive treatments for diseases that are negatively influenced by individual lifestyle choices seems to be lower [73, 74, 124, 217]. For example, this was noted by the National Institute for Clinical Excellence (NICE) Citizens Council report [88]. In line with this argument, the reason given for special status of the reimbursement of OMPs was that rare disease patients were sick out of bad luck and could not be held responsible for their situation [47, 137, 177, 231].
-
4)
Rule of rescue
A considerable body of literature (n = 48) discussed the controversial Rule of Rescue, which justifies the rescue of endangered lives of identifiable patients by moral intuition, no matter the cost [24,25,26, 29, 35, 38, 41, 42, 47,48,49, 52, 53, 58, 60, 63, 64, 67, 70, 71, 73, 74, 77, 77, 81,82,83, 91, 104, 108, 109, 114, 116, 123, 125, 139, 151, 153, 175, 181, 187, 196, 217, 230, 235,236,237,238]. Mass media coverage [151, 236,237,238], the moral instincts of physicians [236] as well as society in general [175, 217, 237] were said to support the Rule of Rescue. The benefit of reimbursement was argued to be particularly high because, to be covered under the Rule of Rescue, OMPs must be life-saving or at least be able to significantly improve the quality of life [108]. Authors reasoned that rare disease patients were easily identifiable due to the rarity of their conditions, which would make it morally difficult to deny available treatment, even if it was costly [70, 91, 104, 108, 139, 181], thereby deriving from this feature of identifiability a moral obligation to rescue for reasons of social relatedness [26, 91]. It was also argued that the rarity of the conditions would limit the budget impact when applying the Rule of Rescue [24, 83, 108, 123, 153].
Counter-arguments to using the Rule of Rescue for resource allocation decisions in the context of OMPs included that the concept was inappropriate as its consistent application on a population level was not possible [46, 61, 75, 83, 109, 111, 123, 143, 151, 231] and would even be discriminatory against those not in an immediate life-threatening situation [107]. The Rule of Rescue, some argued, was therefore unsustainable as a policy guiding principle [58, 82, 83, 108, 109]. Moreover, many aspects of the Rule of Rescue were said not to be specific to rare diseases [62, 66, 75, 83] and not all OMPs would meet the criteria of saving lives from immediate life-threatening illnesses [92, 196]. It was also criticized that the Rule of Rescue was based on emotion thus was incompatible with rational decision-making [40, 83, 111], failed to maximise population health [41, 109], and that its aim of saving lives was inappropriate since death was unavoidable for human beings [26, 83]. Finally, the aspect of identifiability was subject to broad criticism, as this reliance on identifiability was argued to be inequitable and unfair [40, 41, 66, 72, 81, 91, 92, 108, 109, 111, 114, 196, 230], morally irrelevant [91, 105] and “merely a matter of time and perspective” [111]. Finally, it was feared that relying on identifiability would be a bottomless pit concerning budget impact [105, 109].
-
5)
Duty
Several reasons connected to a sense of duty to serve those affected by rare diseases. First, it was argued that society had a duty to not abandon rare disease patients and to provide effective treatment if available [25, 56, 68, 69, 72, 79, 81, 88, 90, 107, 115, 122, 124, 127, 136, 137, 157, 168, 173, 188, 189, 196, 218, 239,240,241,242,243]. The principle of social justice would demand that everyone was treated with dignity and respect [56, 81, 127, 168, 189]. This would conform to the principle of social solidarity, which is the moral basis of publicly funded health care systems that reflect societal compassion to those affected by rare diseases [27, 48, 73, 88, 109, 122, 124, 126, 136, 208, 215, 229, 244]. It was also argued that receiving appropriate treatment was a human right, that included the right to healthcare [25, 44, 46, 66, 70, 73, 76, 93, 101, 114, 125, 128, 143, 147, 159, 178, 244,245,246,247], even if OMPs were expensive [66, 70, 93, 159, 244, 245].
A final duty-based reason was that it was every doctor's duty to provide treatment in the interest of patients, according to the Principle of Beneficence [25, 26, 43, 60, 93, 107, 137, 236], which was then countered by the argument that it was also a doctor's duty to avoid overly expensive treatments [43].
-
6)
Rarity
The influence of rarity as a property that directly justifies special status for the reimbursement of OMPs was debated controversially. Those in favour stated that rarity in itself was a factor that warranted special status [35, 45, 73, 74, 87, 92, 103, 109, 118, 122, 127, 135, 155, 157, 161, 206, 208, 214, 221, 245, 246, 248, 249] because rarity made evidence collection more challenging [73] and necessarily led to high prices [35, 74, 87, 103, 109, 122, 127, 135, 155, 208, 214, 248, 249]. By contrast, others argued that disease prevalence was a morally arbitrary distinction for reimbursement decision-making [35, 43, 62, 100, 105, 114, 117, 120, 130, 148, 152, 156, 182, 208, 219, 224, 246, 250].
A societal preference for prioritizing rarity was substantiated by notions that payers already made exceptions when reimbursing OMPs [35, 46, 87, 202, 215] and that laws incentivising OMP development demonstrated a societal willingness to pay [152, 173]. Part of the NICE Citizens' Council [88], as well as a European population survey on rare diseases [251], reported in favour of prioritizing rare disease patients in resource allocation.
Since 2010, several country-specific population surveys assessing the societal preference for rarity were published. In contrast to the previously mentioned reports, they mostly concluded that there was no societal preference for rarity alone [24, 26, 27, 35, 38, 39, 42,43,44,45,46, 63, 73, 75, 78,79,80, 87, 92, 93, 99, 111, 112, 114, 116, 132, 151, 157, 161, 167, 173, 182, 198, 205, 208, 212, 213, 217, 252,253,254], but did find general preferences for considering equity in healthcare resource allocation, including preferential reimbursement for severe diseases without available alternatives [24, 44, 63, 80, 116, 167, 182, 205, 212, 213, 254]. Similarly, it was shown that healthcare professionals were not generally prioritizing rarity [43, 182]. Nevertheless, population surveys face important methodological challenges, including framing effects, unstable societal preferences stemming from low public engagement with the issue, and the unwillingness of study participants to adhere to the frame that finite resource must be redistributed between rare and common diseases [99].
-
7)
Appropriateness of cost-effectiveness criteria
Some authors argued against special status, stating that OMPs could and should meet the same cost-effectiveness criteria as any other drug [38, 45, 53, 62, 73, 78, 82, 85, 90, 104, 105, 111, 114, 127, 134, 135, 142, 150, 194]. However, other authors mentioned that OMPs were unlikely to meet traditional cost-effectiveness criteria [24, 35, 36, 38, 41, 43, 45, 54, 57, 59, 62, 64, 65, 68, 72, 73, 75, 87, 89, 109, 116, 118, 120, 122, 128, 140, 143, 144, 151,152,153, 155,156,157,158, 162, 178, 196, 199,200,201, 206, 208, 220, 221, 224, 244, 252, 255,256,257,258], which would leave many rare disease patients without any treatments. Moreover, the standard cost-effectiveness analysis was argued to be inappropriate or at least not optimal for assessing the value of OMPs since it did not take equity or other societal values into account [27, 38, 54, 72, 75, 132, 152, 178, 192], thereby disrespecting societal preferences [41, 59, 72, 101, 135, 144, 145, 147, 208, 259,260,261,262,263] and the idea of a solidaristic health care system [145, 264]. Decision-makers should also take into account the difficulties in obtaining the necessary evidence [24, 36, 62, 64, 72, 122, 178, 208, 220, 221, 257]. In addition, it was argued that cost-effectiveness exceptions were also made in other situations, such as in rescuing mountaineers or transplant operations [46, 173].
In the middle ground between the arguments calling for the same cost-effectiveness criteria and those calling for reimbursing OMPs at any cost, several authors argued that OMPs, too, should meet certain cost-effectiveness standards, but that these should adapt to the particular situations of rare diseases and OMPs [47, 54, 72, 82, 87, 88, 92, 95, 98, 104, 109, 114, 120, 128, 129, 136, 141, 150, 151, 174, 202, 208, 217, 224, 244]. For instance, it was suggested to use additional criteria for assessing the reimbursement of OMPs [29, 45, 114], such as the budget impact of a drug [54, 87, 114, 120, 120, 141, 150, 174, 200, 202, 208, 224, 244].
-
8)
Treatment benefit
Another line of argument was that a special status was justified if OMPs provided a high benefit [24, 26, 43,44,45,46,47, 63, 72, 78, 79, 87, 88, 93, 104, 107, 109, 112, 114,115,116, 120, 125, 127,128,129,130,131,132, 136, 152, 153, 161, 167, 178, 184, 195, 202, 203, 208, 212, 214,215,216, 249, 260, 265, 266]. The nature of this benefit, however, was defined differently [208]: some authors referred to characteristics such as life-saving [47, 93, 109, 115, 127, 128, 132, 136, 152, 153] or curative treatments [78, 79, 104, 125, 129, 214, 215, 266]; others mentioned the ability to restore societal functioning [79, 109, 130, 195, 249, 265]. For some, an improved quality of life [26, 79, 88, 107, 120, 132, 136, 152, 161, 202, 266] or even just a stabilization [88, 125, 152, 212, 249] was enough of a benefit for OMPs to be covered. It was also argued that OMPs contributed to lowering the social and economic burdens that rare diseases imposed on society [54, 59, 79, 93, 94, 96, 101, 114, 115, 118, 122, 123, 131, 140, 168, 174, 195, 210, 211, 215, 216, 249, 261, 265, 266] and that these costs should be taken into account when assessing the effectiveness of OMPs. Some authors stated that, despite the difficulties in obtaining evidence combined with the additional development costs, it was crucial that these reimbursed OMPs had proven effectiveness and safety [36, 41, 49, 62, 68, 72, 78, 79, 88, 114, 116, 128, 129, 136, 141, 142, 174, 191, 193, 194, 202, 207, 208, 229, 241, 257], thereby calling for alternative ways of evidence creation (e.g. real-world evidence, dose–response studies, and expert opinions) [68, 79, 141, 193, 194, 229] or defining it under the manufacturer's obligation to deliver as much evidence as possible [36, 72].
-
9)
Investments in OMP research and development
Fig. 3 Some authors argued that reimbursing OMPs would have positive effects on future drug development efforts [29, 43, 52, 54, 60, 61, 75, 85, 87, 88, 96, 103, 114, 115, 118, 120, 122, 127, 128, 137, 142, 147, 169, 193, 198, 202, 205, 210, 215, 219, 220, 222, 242, 243, 248, 249, 265, 267], which would increase competition and lower prices of OMPs [60, 85, 118, 122, 147, 249, 265]. Consequently, the OMP drug development would have a positive effect for other diseases [43, 52, 54, 60, 61, 88, 115, 169, 202, 210, 242, 243], letting future generations profit from today's investments [198]. Therefore, according to some authors, the innovativeness of OMPs should be valued when considering their costs [54, 85, 114, 120, 122, 128, 141, 198, 202, 219, 220, 222].
Furthermore, some reasoned that OMPs should be reimbursed despite their high prices because taxpayers had already invested in their development [45, 46, 63, 88, 112, 114, 115, 127, 128, 142, 155, 161, 231, 239, 248, 249, 259, 268, 269] through the existing incentives. Consequently, not reimbursing them would be a waste of public resources [127, 248]. Moreover, it would be wrong if patients who had contributed to successful trials could not benefit further from treatments due to costs [239, 259]. Counter-arguments to this point included that these incentives were developed for the research and development of OMPs, not for their reimbursement [112] and that the lack of cost-effectiveness would question the existing incentives rather than obligating them for reimbursement [161].
Discussion
In this study, we reviewed moral reasons for and against special status for the reimbursement of OMPs from a multidisciplinary perspective. As indicated, the problem is manifold and not only discussed among ethicist, but also considered extensively in the fields of health policy and health economics. On the normative-theoretical level, there is no clear-cut solution to whether or not OMPs should receive special status for their reimbursement, as different moral theories come to different, sometimes opposite conclusions. This underlines the importance of analysing the problem from a broader, multidisciplinary perspective.
Currently, OMP reimbursement is often based on rules of exception in publicly-funded healthcare systems. This is problematic from a clinical perspective because it leaves the responsibility of whether and when to reimburse OMPs on clinicians and healthcare insurances raising problems related to equity and fairness. The issue is further complicated for clinicians by differing perspectives when considering the well-being of patients. From a public health perspective, it may be necessary to impose cost constraints on individual patients to not abandon others; from the perspective of an individual patient affected by a rare disease, however, it may be ethically challenging to deny an available treatment merely due to cost constraints. These two perspectives are also represented in the various reasons identified: while reasons that focused on the individual patient, such as the duty to care or the rule of rescue, all argue in favour of special status, several reasons that focused on the societal level argue against special status (for example those whose reasons were relevant to maximising population health). This demonstrates the dilemma clinicians and health insurances are facing when having to prescribe OMPs based on exceptions. It is, therefore, crucial to identify fair, consistent and equitable rules for when to prescribe OMPs. In publicly funded health care systems, this lies in the responsibility of HTA agencies. In the following, we will discuss the connection between economic and moral considerations as well as the heterogeneous characteristics of rare diseases and OMPs in light of their implications for HTA agencies and other bodies responsible for defining reimbursement rules.
Because traditional standards for reimbursement in publicly funded health care systems are based on cost-effectiveness evaluations, giving OMPs a special status in this process requires additional reasoning. This is reflected in the literature, as the review identified more articles and a higher number of reasons in favour of special status. Cost-effectiveness has a strong economic component. However, as utilitarianism demonstrates, optimizing the allocation of health care costs also has a moral component. Indeed, the relevance of economic criteria in health care resource allocation roots in utilitarianism. Most of the reasons detected against special status are based on or connected to utilitarianism, whereas many reasons in favour of special status aim to overcome economic considerations due to alternative moral concepts and theories. Consequently, health economics plays a crucial role even in the moral assessment of HTA decision-making and economic considerations should neither be considered the gold standard nor be neglected in HTA decision-making in the context of OMPs.
Many reasons based their arguments for or against special status on disease prevalence. The question of whether rarity justifies special status demonstrates a binary understanding of rarity: a disease is either rare or common. This is problematic for two reasons. First, it oversimplifies the issue at stake since disease prevalence is not the only factor considered in HTA assessments. Second, the focus on disease prevalence also reverts to the question of how to define rare diseases. They are highly heterogeneous by being, for instance, of differing severity, having a varying prevalence in different regions of the world, and composing varying budget implications for treatment options. This makes it difficult, if not impossible, to find a one-fits-all solution for OMP reimbursement. Rather, ways to address this heterogeneity rationally should be identified with all the stakeholders involved. Factors representing this heterogeneity from a moral perspective include, for instance, disease severity, availability of alternative treatment options, innovativeness of OMPs, or the budget impact. Moreover, considering disease prevalence as a continuous rather than a binary variable and using alternative evidence creation, such as real-world evidence or n-of-1 trials [270], might support efforts for a more fine-grained and heterogeneous, yet fair and consistent examination of OMP HTAs. Decision frameworks using more flexible and variable approaches, such as the EVIDEM framework [79, 164] or the Multi-Criteria Decision Analyses (MCDA) [50, 154, 256], represent promising alternatives implementing these factors but might need further refinement to optimize all factors included. The advantages of these frameworks include that they can be applied to all drugs, which avoids the issue of the special status of OMP reimbursement at least from a procedural perspective while allowing for a more fine-grained distinction between the two groups “OMPs” versus “non-orphan drugs”. Due to the increasing decision-making complexity, computer-assisted HTA could support parts of the decision-making process and should be evaluated in the future [271].
Future research on the reimbursement of OMPs should also focus on crowdfunding as alternative funding schemes from both empirical and normative perspectives, as the rationale of crowdfunding is closely linked to the controversially debated Rule of Rescue. Moreover, existing studies investigating the views of the general population on OMP reimbursement come with methodological shortcuts, as it is difficult to assess the preferences in healthcare resource allocation amid such a complex ethical challenge. Survey studies, for instance, might be subject to biases in the framing of questions or the choice of scenarios [272]. As the opinion of the majority of tax payers is commonly used to reason for or against special status of OMPs, this warrants further effort in identifying additional, reliable ways to empirically assess public preferences concerning OMP reimbursement.
Limitations
For this review, we followed the established methodology of systematic reviews of reasons. Yet, the study still faces several limitations. First, parts of the findings, including the reasons for or against special status for the reimbursement of OMPs, were assessed in an inductive, qualitative manner. However, a reliability test that is usually performed for quantitative content analyses to ensure consistency and transparency in data collection, was not feasible due to the variety of different reasons and the complexity of the analysis. This has already been acknowledged in studies applying a similar methodology, and we attempted to overcome this issue by working and discussing the collection of data in a research team, but it should be noted that any presented frequencies should be viewed descriptively and in relative importance to each other. It was also for this reason that we refrained from any statistical analysis. A second limitation is that, due to the complexity of the topic, it was challenging to identify all of the relevant articles from all relevant disciplines. We purposefully used a broad search term in a variety of databases and additionally screened the references of the included articles, but it might still be possible that relevant articles are missing. Third, the systematic screening of grey literature was challenging because grey literature databases did not retrieve any results, thus many grey literature reports were identified through individual online searches or reference screening. Moreover, the strong focus on OMP reimbursement might be the reason for the predominance of articles within the European context and relatively low representation of the United States. While the analysis was not limited to the European context, this analysis targets the focus on reimbursement, which is a typical feature of publicly funded health care systems. This might narrow down the reasons identified, and might be the reasons behind, for instance, the underrepresentation of libertarian arguments. Moreover, we did not assess the adequacy and quality of reasons but rather condensed them as they were found in the literature. We also did not collect data on how reasons within one article were interconnected and we counted reasons that were cited from other authors as distinct reason mentions. However, by portraying the reasons in context to the overall conclusion of articles, we still provide some context within which the reasons were mentioned.
Conclusion
On the normative-theoretical level, there is no clear-cut solution to whether or not OMPs should receive special status for their reimbursement. However, for reasons of fairness, it is crucial to identify consistent and equitable rules for when to prescribe OMPs. The heterogeneity of characteristics of both rare diseases and OMPs makes it difficult, if not impossible, to find a one-fits-all solution for OMP reimbursement. Therefore, the binary perspective of whether or not OMPs should be granted special status seems to oversimplify the issue. Yet, moral reasonings mostly focus on such a binary perspective. The authors suggest that it might help address the issue of OMP reimbursement if the scientific debate focused more on how the important variabilities of different OMPs concerning target population, cost-effectiveness, level of evidence, or mechanism of action could be meaningfully addressed and implemented in HTAs. We further suggest that computer-assisted decision aids might be helpful supporters to rationalize and simplify the work of HTA agencies. However, those rely on robust empirical evidence, and this review revealed a number of under-researched areas that need to be addressed in the future, including the views of patients and caregivers, robust empirical assessment of population surveys, or the normative and empirical assessment of crowdfunding OMPs.
Availability of data and materials
The datasets supporting the conclusions of this article are included within the article and its additional files.
Abbreviations
- HTA:
-
Health Technology Assessment
- MCDA:
-
Multi-criteria decision analysis
- OMP:
-
Orphan medicinal product
- RQ:
-
Research question
References
Regulation (EC) No 847/2000 of 27 April 2000; 2000 [cited 2021 Jan 19]. https://eur-lex.europa.eu/legal-content/EN/TXT/HTML/?uri=CELEX:32000R0847&from=EN.
Electronic Code of Federal Regulations (eCFR): Title 21 Part 316; 2013 [cited 2021 Jan 19]. https://www.ecfr.gov/cgi-bin/text-idx?SID=55067b2d8804816620c10025d0362bf0&mc=true&node=se21.5.316_120&rgn=div8.
Orphan Drug Act; 1983 [cited 2021 Jan 14]. https://www.govinfo.gov/content/pkg/STATUTE-96/pdf/STATUTE-96-Pg2049.pdf.
Regulation (EC) No 141/2000 of the European Parliament and of the Council of 16 December 1999 on orphan medicinal products; 1999 [cited 2021 Jan 19]. https://eur-lex.europa.eu/legal-content/EN/TXT/HTML/?uri=CELEX:32000R0141&from=EN.
Gammie T, Lu CY, Babar ZUD. Access to orphan drugs: a comprehensive review of legislations, regulations and policies in 35 countries. PLoS ONE. 2015;10(10):e0140002.
Giannuzzi V, Conte R, Landi A, Ottomano SA, Bonifazi D, Baiardi P, et al. Orphan medicinal products in Europe and United States to cover needs of patients with rare diseases: an increased common effort is to be foreseen. Orphanet J Rare Dis. 2017;12(1):64.
Mueller CM, Rao GR, Miller Needleman KI. Precision medicines’ impact on orphan drug designation. Clin Transl Sci. 2019;12:633–40.
Degtiar I. A review of international coverage and pricing strategies for personalized medicine and orphan drugs. Health Policy. 2017;121(12):1240–8.
Onakpoya IJ, Spencer EA, Thompson MJ, Heneghan CJ. Effectiveness, safety and costs of orphan drugs: an evidence-based review. BMJ Open. 2015;5(6):e007199.
Contesse MG, Valentine JE, Wall TE, Leffler MG. The case for the use of patient and caregiver perception of change assessments in rare disease clinical trials: a methodologic overview. Adv Ther. 2019;36(5):997–1010.
Dean R, Jensen I, Cyr P, Miller B, Maru B, Sproule DM, et al. An updated cost-utility model for onasemnogene abeparvovec (Zolgensma®) in spinal muscular atrophy type 1 patients and comparison with evaluation by the Institute for Clinical and Effectiveness Review (ICER). J Mark Access Health Policy. 2021;9(1):1889841.
National Institute for Clinical Excellence. NICE approves life-changing gene therapy for treating spinal muscular atrophy; 2021 [cited 2021 Apr 15]. https://www.nice.org.uk/news/article/nice-approves-life-changing-gene-therapy-for-treating-spinal-muscular-atrophy.
Bundesministerium für Soziales, Gesundheit, Pflege und Konsumentenschutz (BMSGPK). Anschober: Bundesgesundheitsagentur übernimmt Kosten für innovatives Medikament zur Behandlung spinaler Muskelatrophie bei Kleinkindern; 2020 [cited 2021 Apr 15]. https://www.ots.at/presseaussendung/OTS_20201017_OTS0035/anschober-bundesgesundheitsagentur-uebernimmt-kosten-fuer-innovatives-medikament-zur-behandlung-spinaler-muskelatrophie-bei-kleinkindern.
SMA Schweiz. Zolgensma—Vergütung der nicht zugelassenen Therapie im Ausnahmefall möglich; 2020 [cited 2021 Apr 15]. https://www.sma-schweiz.ch/avexis-innovativer-weg-fuer-zolgensma-zugang-in-der-schweiz/.
Franken M, Heintz E, Gerber-Grote A, Raftery J. Health economics as rhetoric: the limited impact of health economics on funding decisions in four European countries. Value Health. 2016;19(8):951–6.
Schuller Y, Hollak CE, Biegstraaten M. The quality of economic evaluations of ultra-orphan drugs in Europe–a systematic review. Orphanet J Rare Dis. 2015;10(1):92.
Cannizzo S, Lorenzoni V, Palla I, Pirri S, Trieste L, Triulzi I, et al. Rare diseases under different levels of economic analysis: current activities, challenges and perspectives. RMD Open. 2018;4(Supplement 1):e000794.
Lamont J, Favor C. Distributive justice. In: Gaus GF, Kukathas C, editors. Handbook of political theory. London: Sage; 2017. p. 223–38.
Lamont J, Favor C. Distributive Justice. In: Zalta EN, editor. The Stanford encyclopedia of philosophy. Winter 2017. Stanford: Metaphysics Research Lab, Stanford University; 2017.
Gandjour A, Lauterbach KW. Utilitarian theories reconsidered: common misconceptions, more recent developments, and health policy implications. Health Care Anal. 2003;11(3):229–44.
Rawls J. A theory of justice. Oxford: Oxford University Press; 1972.
van der Vossen B. Libertarianism. In: Zalta EN, editor. The Stanford encyclopedia of philosophy. Spring 2019. Stanford: Metaphysics Research Lab, Stanford University; 2019.
Bell D. Communitarianism. In: Zalta EN, editor. The Stanford encyclopedia of philosophy. Fall 2020. Stanford: Metaphysics Research Lab, Stanford University; 2020.
Chim L, Salkeld G, Kelly P, Lipworth W, Hughes D, Stockler MR. Community views on factors affecting medicines resource allocation: cross-sectional survey of 3080 adults in Australia. Aust Health Rev. 2019;43(3):254–60.
Taylor C, Jan S, Thompson K. Funding therapies for rare diseases: an ethical dilemma with a potential solution. Aust Health Rev. 2018;42(1):117–9.
Isaacs D. Ethical dilemmas about orphan drugs for orphan diseases. J Paediatr Child Health. 2014;50(4):249–50.
Richardson J, Iezzi A, Chen G, Maxwell A. Communal sharing and the provision of low-volume high-cost health services: results of a survey. Pharmacoecon Open. 2017;1(1):13–23.
Whitty JA, Littlejohns P. Social values and health priority setting in Australia: an analysis applied to the context of health technology assessment. Health Policy. 2015;119(2):127–36.
Kessabi S, de Abreu Lourenco R, Wonder M. Rescuing patients from the rule of efficiency: a need to debate the ‘rule of rescue.’ Pharmacoeconomics. 2003;21(9):681.
Landwehr C. Deciding how to decide: the case of health care rationing. Public Adm. 2009;87(3):586–603.
Daniels N, Sabin J. Setting limits fairly: can we learn to share medical resources? Oxford: Oxford University Press; 2002.
Strech D, Sofaer N. How to write a systematic review of reasons. J Med Ethics. 2012;38(2):121–6.
Sofaer N, Strech D. The need for systematic reviews of reasons. Bioethics. 2012;26(6):315–28.
Richardson HS. Moral reasoning. In: Zalta EN, editor. The Stanford encyclopedia of philosophy. Fall 2018. Stanford: Metaphysics Research Lab, Stanford University; 2018.
Wiss J. Healthcare priority setting and rare diseases: what matters when reimbursing orphan drugs. Linköping University Electronic Press; 2017.
Babar ZUD, Francis S. Identifying priority medicines policy issues for New Zealand: a general inductive study. BMJ Open. 2014;4(5):e004415.
Bilkey GA, Burns BL, Coles EP, Mahede T, Baynam G, Nowak KJ. Optimizing precision medicine for public health. Front Public Health. 2019;7:42.
Brenna E, Polistena B, Spandonaro F. The implementation of health technology assessment principles in public decisions concerning orphan drugs. Eur J Clin Pharmacol. 2020;76:755–64.
Burls A, Austin D, Moore D. Commissioning for rare diseases: view from the frontline. BMJ. 2005;331(7523):1019–21.
Clarke JTR. The price of care versus the cost of caring. In: Elstein D, Altarescu G, Beck M (eds) Fabry Disease; 2010. p. 489–97.
da Silva EN, Vieira Sousa TR. Economic evaluation in the context of rare diseases: is it possible? Cad Saude Publica. 2015;31(3):496–506.
Dear JW, Lilitkarntakul P, Webb DJ. Are rare diseases still orphans or happily adopted? The challenges of developing and using orphan medicinal products. Br J Clin Pharmacol. 2006;62(3):264–71.
Desser AS. Prioritizing treatment of rare diseases: a survey of preferences of Norwegian doctors. Soc Sci Med. 2013;94:56–62.
Desser AS, Gyrd-Hansen D, Olsen JA, Grepperud S, Kristiansen IS. Societal views on orphan drugs: cross sectional survey of Norwegians aged 40 to 67. Br Med J. 2010;341:c4715.
Drummond M, Towse A. Orphan drugs policies: a suitable case for treatment. Eur J Health Econ. 2014;15(4):335–40.
Drummond M, Wilson DA, Kanavos P, Ubel P, Rovira J. Assessing the economic challenges posed by orphan drugs. Int J Technol Assess Health Care. 2007;23(1):36–42.
Ehni H-J. Expensive cancer drugs and just health care. Best Pract Res Clin Gastroenterol. 2014;28(2):327–37.
Feldman BM, Berger K, Bohn R, Carcao M, Fischer K, Gringeri A, et al. Haemophilia prophylaxis: how can we justify the costs? Haemophilia. 2012;18(5):680–4.
Fishman JC, Skrepnek GH. Pharmacoeconomic analyses of treatments for rare disease. Pharm Policy Law. 2012;14(1):51–67.
Gilabert-Perramon A, Torrent-Farnell J, Catalan A, Prat A, Fontanet M, Puig-Peiró R, et al. Drug evaluation and decision making in catalonia: development and validation of a methodological framework based on multi-criteria decision analysis (MCDA) for orphan drugs. Int J Technol Assess Health Care. 2017;33(1):111–20.
Gonzato O. The new risk-sharing paradigm in rare cancers: patient perspective. J Cancer Policy. 2017;12:36–42.
Hughes D, Tunnage B, Yeo ST. Drugs for exceptionally rare diseases: do they deserve special status for funding? QJM Int J Med. 2005;98(11):829–36.
Hughes D. Rationing of drugs for rare diseases. Pharmacoeconomics. 2006;24(4):315–6.
Iskrov G, Miteva-Katrandzhieva T, Stefanov R. Health technology assessment and appraisal of therapies for rare diseases. In: DeLaPaz MP, Taruscio D, Groft SC, editors. Rare diseases epidemiology: update and overview. 2nd ed. Springer: Cham; 2017. p. 221–31.
Kesselman I, Elstein D, Israeli A, Chertkoff R, Zimran A. National health budgets for expensive orphan drugs: Gaucher disease in Israel as a model. Blood Cells Mol Dis. 2006;37(1):46–9.
Kinney J. Health disparities: exploring the ethics of orphan drugs. Am J Health Syst Pharm AJHP. 2014;71(9):692–3.
Korchagina D, Jaroslawski S, Jadot G, Toumi M. Orphan drugs in oncology. Recent Results Cancer Res. 2019;213:109–42.
London AJ. How should we model rare disease allocation decisions? Hastings Cent Rep. 2012;42(1):3.
López-Bastida J, Oliva-Moreno J. Cost of illness and economic evaluation in rare diseases. In: DeLaPaz MP, Taruscio D, Groft SC, editors. Rare diseases epidemiology: update and overview. 2nd ed. Springer: Cham; 2017. p. 273–82.
Mavroudis C, Jacobs JP. The elephant in the room: ethical issues associated with rare and expensive medical conditions. Cardiol Young. 2015;25(8):1621–5.
McCabe C. Balancing economic, ethical and equity concerns in orphan drugs and rare diseases. Eur J Hosp Pharm Pract. 2010;16(4):22–5.
McCabe C, Claxton K, Tsuchiya A. Orphan drugs and the NHS: should we value rarity? BMJ. 2005;331(7523):1016–9.
Mentzakis E, Stefanowska P, Hurley J. A discrete choice experiment investigating preferences for funding drugs used to treat orphan diseases: an exploratory study. Health Econ Policy Law. 2011;6(3):405–33.
Mincarone P, Leo CG, Sabina S, Sarria-Santamera A, Taruscio D, Guillermo Serrano-Aguilar P, et al. Reimbursed price of orphan drugs: current strategies and potential improvements. Public Health Genom. 2017;20(1):1–8.
Monahan AB. Fairness versus welfare in health insurance content regulation. U Ill L Rev. 2012;2012:139.
Ollendorf DA, Chapman RH, Pearson SD. Evaluating and valuing drugs for rare conditions: no easy answers. Value Health. 2018;21(5):547–52.
Panju AH, Bell CM. Policy alternatives for treatments for rare diseases. CMAJ. 2010;182(17):E787–92.
Picavet E, Cassiman D, Pinxten W, Simoens S. Ethical, legal and social implications of rare diseases and orphan drugs in Europe: meeting report of a Brocher symposium. Expert Rev Pharmacoecon Outcomes Res. 2013;13(5):571–3.
Pinxten W, Denier Y, Dooms M, Cassiman J-J, Dierickx K. A fair share for the orphans: ethical guidelines for a fair distribution of resources within the bounds of the 10-year-old European Orphan Drug Regulation. J Med Ethics. 2012;38(3):148–53.
Rodriguez-Monguio R, Spargo T, Seoane-Vazquez E. Ethical imperatives of timely access to orphan drugs: is possible to reconcile economic incentives and patients’ health needs? Orphanet J Rare Dis. 2017;12(1):1.
Roll K, Stargardt T, Schreyögg J. Authorization and reimbursement of orphan drugs in an international comparison. Gesundheitswesen. 2011;73(8–9):504–14.
Schlander M, Beck M. Expensive drugs for rare disorders: to treat or not to treat? The case of enzyme replacement therapy for mucopolysaccharidosis VI. Curr Med Res Opin. 2009;25(5):1285–93.
Schlander M, Garattini S, Holm S, Kolominsky-Rabas P, Nord E, Persson U, et al. Incremental cost per quality-adjusted life year gained? The need for alternative methods to evaluate medical interventions for ultra-rare disorders. J Comp Eff Res. 2014;3(4):399–422.
Schlander M, Garattini S, Kolominsky-Rabas P, Nord E, Persson U, Postma MJ, et al. Determining the value of medical technologies to treat ultra-rare disorders: a consensus statement. J Mark Access Health Policy. 2016;4:33039.
Simoens S, Cassiman D, Dooms M, Picavet E. Orphan drugs for rare diseases is it time to revisit their special market access status? Drugs. 2012;72(11):1437–43.
Simoens S, Picavet E, Dooms M, Cassiman D, Morel T. Cost-effectiveness assessment of orphan drugs: a scientific and political conundrum. Appl Health Econ Health Policy. 2013;11(1):1–3.
Simon F. Market access for biopharmaceuticals: new challenges. Health Aff (Millwood). 2006;25(5):1363–70.
van Egmond-Froehlich A, Schmitt K. Public guidance and price limitation for orphan drugs. Fair distribution and right to treatment of rare diseases. Monatsschrift Kinderheilkunde. 2018;166(9):785–97.
Wagner M, Khoury H, Willet J, Rindress D, Goetghebeur M. Can the EVIDEM framework tackle issues raised by evaluating treatments for rare diseases: analysis of issues and policies, and context-specific adaptation. Pharmacoeconomics. 2016;34(3):285–301.
Wiss J, Levin L-A, Andersson D, Tinghoeg G. Prioritizing rare diseases: psychological effects influencing medical decision making. Med Decis Mak. 2017;37(5):567–76.
Zelei T, Molnar MJ, Szegedi M, Kalo Z. Systematic review on the evaluation criteria of orphan medicines in Central and Eastern European countries. Orphanet J Rare Dis. 2016;11:1–11.
Kaebnick G. Two dreams. Hastings Cent Rep. 2012;42(1):2.
Cookson R, McCabe C, Tsuchiya A. Public healthcare resource allocation and the Rule of Rescue. J Med Ethics. 2008;34(7):540–4.
Devlin N, Parkin D. Does NICE have a cost-effectiveness threshold and what other factors influence its decisions? A binary choice analysis. Health Econ. 2004;13(5):437–52.
Hollis A. Drugs for rare diseases: paying for innovation. In: Beach CM, editor. Health services restructuring in Canada: new evidence and new directions. Montreal: McGill-Queen’s University Press; 2006. p. 155–77.
Leget C, Hoedemaekers R. Teaching medical students about fair distribution of healthcare resources. J Med Ethics J Inst Med Ethics. 2007;33(12):737–41.
Medic G, Korchagina D, Young KE, Toumi M, Postma MJ, Wille M, et al. Do payers value rarity? An analysis of the relationship between disease rarity and orphan drug prices in Europe. J Mark Access Health Policy. 2017;5(1):1299665.
National Institute for Clinical Excellence. Citizens Council report: Ultra Orphan drugs. London; 2004 [cited 2020 Nov 18]. https://www.ncbi.nlm.nih.gov/books/NBK401721/pdf/Bookshelf_NBK401721.pdf.
Wild C, Hintringer K, Nachtnebel A. Orphan drugs in oncology. In: Walter E, editor. Regulatory and economic aspects in oncology, vol. 213. Springer: Cham; 2019. p. 223–32.
Rai AK. Pharmacogenetic interventions, orphan drugs, and distributive justice: the role of cost-benefit analysis. Soc Philos Policy. 2002;19(2):246–70.
Sheehan M. Resources and the rule of rescue. J Appl Philos. 2007;24(4):352–66.
Coyle D, Cheung MC, Evans G. Opportunity cost of funding drugs for rare diseases: the cost-effectiveness of eculizumab in paroxysmal nocturnal hemoglobinuria. Med Decis Mak. 2014;34(8):1016–29.
Hyry HI, Roos JCP, Manuel J, Cox TM. The legal imperative for treating rare disorders. Orphanet J Rare Dis. 2013;8:135.
Roscamp JA, D’Cruz DP. The funding lottery for potentially life-threatening rare diseases It’s not fair, my disease is rare. Rheumatology. 2018;57(1):1–2.
Sheehan M. Orphan drugs and the NHS: fairness in health care entails more than cost effectiveness…McCabe C, Claxton K, Tsuchiya A. Orphan drugs and the NHS: should we value rarity? BMJ 2005;331:1016–9. (29 October). BMJ 2005;331(7525):1144–5.
BPI-Positionspapier Orphan Drugs: Compassionate Use - Wirtschaftliche Anreize - Verordnungsfähigkeit zur Lasten der GKV. Berlin; 2008.
Blankart CR, Stargardt T, Schreyögg J. Availability of and access to orphan drugs: an international comparison of pharmaceutical treatments for pulmonary arterial hypertension, Fabry disease, hereditary angioedema and chronic myeloid leukaemia. Pharmacoeconomics. 2011;29(1):63–82.
Denis A, Mergaert L, Fostier C, Cleemput I, Simoens S. Budget impact analysis of orphan drugs in Belgium: estimates from 2008 to 2013. J Med Econ. 2010;13(2):295–301.
Dragojlovic N, Rizzardo S, Bansback N, Mitton C, Marra C, Lynd LD. Challenges in measuring the societal value of orphan drugs: insights from a Canadian stated preference survey. Patient. 2015;8(1):93–101.
Dupont AG, van Wilder PB. Access to orphan drugs despite poor quality of clinical evidence. Br J Clin Pharmacol. 2011;71(4):488–96.
Encina G, Castillo-Laborde C, Lecaros JA, Dubois-Camacho K, Calderon JF, Aguilera X, et al. Rare diseases in Chile: challenges and recommendations in universal health coverage context. Orphanet J Rare Dis. 2019;14(1):1–8.
Goetghebeur M, Wagner M. Identifying value(s): a reflection on the ethical aspects of MCDA in healthcare decisionmaking. In: Marsh K, Goetghebeur M, Thokala P, Baltussen R, editors. Multi-criteria decision analysis to support healthcare decisions. Cham: Springer; 2017. p. 29–46.
Hasselmann O. Sind hohe Preise für “Orphan Drugs” ethisch zu rechtfertigen? Epileptologie. 2013;30:72–8.
Houlton S. Orphan medicines: the high cost of hope. Prescriber. 2018;29(1):23–7.
Juth N. For the sake of justice: should we prioritize rare diseases? Health Care Anal. 2017;25(1):1–20.
Kanavos P, Nicod E. What is wrong with orphan drug policies? Suggestions for ways forward. Value Health. 2012;15(8):1182–4.
Karpman D, Hoglund P. Orphan drug policies and use in pediatric nephrology. Pediatr Nephrol. 2017;32(1):1–6.
Kling S. Allocating treatment for rare allergic diseases—the rule of rescue. Curr Allergy Clin Immunol. 2013;26(2):94–6.
Largent EA, Pearson SD. Which orphans will find a home? The rule of rescue in resource allocation for rare diseases. Hastings Cent Rep. 2012;42(1):27–34.
Laupacis A. Economic evaluations in the Canadian common drug review. Pharmacoeconomics. 2006;24(11, SI):1157–62.
McCabe C, Tsuchiya A, Claxton K, Raftery J. Orphan drugs revisited. QJM. 2006;99(5):341–5.
McCabe C, Tsuchiya A, Claxton K, Raftery J. Assessing the economic challenges posed by orphan drugs: a comment on Drummond et al. Int J Technol Assess Health Care. 2007;23(3):397–401.
Newdick C. Accountability for rationing—theory into practice. J Law Med Ethics. 2005;33:660.
Paulden M, Stafinski T, Menon D, McCabe C. Value-based reimbursement decisions for orphan drugs: a scoping review and decision framework. Pharmacoeconomics. 2015;33(3):255–69.
Picavet E, Dooms M, Cassiman D, Simoens S. Orphan drugs for rare diseases: grounds for special status. Drug Dev Res. 2012;73(3):115–9.
Rizzardo S, Bansback N, Dragojlovic N, Douglas C, Li KH, Mitton C, et al. Evaluating Canadians’ Values For Drug Coverage Decision Making. Value Health. 2019;22(3):362–9.
Sandman L, Gustavsson E. The (Ir)relevance of group size in health care priority setting: a reply to juth. Health Care Anal. 2017;25(1):21–33.
Tambuyzer E. Rare diseases, orphan drugs and their regulation: questions and misconceptions. Nat Rev Drug Discov. 2010;9(12):921–9.
Clarke JTR, Amato D, Deber RB. Managing public payment for high-cost, high-benefit treatment: enzyme replacement therapy for Gaucher’s disease in Ontario. CMAJ. 2001;165(5):595–6.
Iskrov G, Stefanov R. Post-marketing access to orphan drugs: a critical analysis of health technology assessment and reimbursement decision-making considerations. ODRR. 2014;4:1–9.
Paulden M. Recent amendments to NICE’s value-based assessment of health technologies: implicitly inequitable? Expert Rev Pharmacoecon Outcomes Res. 2017;17(3):239–42.
Picavet E, Cassiman D, Simoens S. Do ultra-orphan medicinal products warrant ultra-high prices? A review. ODRR. 2013;3:23–31.
Rosselli D, Rueda J-D, Solano M. Ethical and economic considerations of rare diseases in ethnic minorities: the case of mucopolysaccharidosis VI in Colombia. J Med Ethics. 2012;38(11):699–700.
Schlander M, Holm S, Nord E, Richardson J, Garattini S, Kolominsky-Rabas P et al. Towards social cost value analysis: the need for new approaches for evaluating drugs for ultra-rare diseases (URDs); 2016. Position Paper No 31 [cited 2020 Nov 19]. http://www.innoval-hc.com/discussion-papers.html?file=files/publications/disc_papers/2015/IV-DP-31-Towards-SCVA-for-URDs-Oct-2015-March-v2-2016.pdf.
Banon Hernandez AM, Solves Almela JA. The debate on rare diseases a look at media response. Metode Sci Stud J. 2016;6:209–13.
Czech M, Baran-Kooiker A, Atikeler K, Demirtshyan M, Gaitova K, Holownia-Voloskova M, et al. A review of rare disease policies and orphan drug reimbursement systems in 12 Eurasian countries. Front Public Health. 2020;7:416.
Davies JE, Neidle S, Taylor DG. Developing and paying for medicines for orphan indications in oncology: utilitarian regulation vs equitable care? Br J Cancer. 2012;106(1):14–7.
Faeh A. A just distribution of health care in the case of orphan medicinal products: aligning the interests of European economic integration and national welfare policy. Eur J Soc Sec (March). 2012;14:21–40.
Goetghebeur M, Wagner M, Samaha D, O’Neil W, Badgley D, Castro-Jaramillo H, et al. Exploring values of health technology assessment agencies using reflective multicriteria and rare disease case. Int J Technol Assess Health Care. 2017;33(4):504–20.
Kleinhout-Vliek T, de Bont A, Boer B. The bare necessities? A realist review of necessity argumentations used in health care coverage decisions. Health Policy. 2017;121(7):731–44.
Lumry WR. Pharmacoeconomics of orphan disease treatment with a focus on hereditary angioedema. Immunol Allergy Clin N Am. 2017;37(3):617–28.
Moberly T. Rationing and access to orphan drugs. Pharm J. 2005;275(7374):569–70.
Weismann MF, Jorge I. The regulatory vision of universal healthcare in the United States: strategic, economic, and moral decision-making. U Pa J Bus L. 2018;21:647.
Erweitertes vips-Positionspapier zu einer Orphan-Drug-Strategie in der Schweiz: Handlungsfelder und Lösungsvorschläge; December 2011 [cited 2020 May 5]. https://docplayer.org/67943137-Erweitertes-vips-positionspapier-zu-einer-orphan-drug-strategie-in-der-schweiz-handlungsfelder-und-loesungsvorschlaege.html.
Drummond M. Challenges in the economic evaluation of orphan drugs. Eurohealth. 2008;14(2):16–7.
Gershon G. A report of the Ontario citizens’ council considerations for funding drugs for rare diseases. Toronto, Ontario: Ontario Ministry of Health; 2010 [cited 2020 Nov 17]. http://www.health.gov.on.ca/en/public/programs/drugs/councils/docs/report_201003.pdf.
Hunter D, Wilson J. Hyper-expensive treatments: Background paper. London; 2011 [cited 2020 Nov 17]. https://discovery.ucl.ac.uk/id/eprint/1325654/1/Hyper_expensive_treatments_background_paper.pdf.
Reinhardt U. Probing our moral values in health care: the pricing of specialty drugs. J Am Med Assoc. 2015;314(10):981–2.
Cote A, Keating B. What is wrong with orphan drug policies? Value Health. 2012;15(8):1185–91.
Iskrov G, Dermendzhiev S, Miteva-Katrandzhieva T, Stefanov R. Health economic data in reimbursement of new medical technologies: importance of the socio-economic burden as a decision-making criterion. Front Pharmacol. 2016;7:252.
Iskrov G, Miteva-Katrandzhieva T, Stefanov R. Multi-criteria Decision analysis for assessment and appraisal of Orphan Drugs. Front Public Health. 2016;4:214.
McCabe C, Edlin R, Round J. Economic considerations in the provision of treatments for rare diseases. In: DeLaPaz MP, Taruscio D, Groft SC, editors. Rare diseases epidemiology: update and overview. 2nd ed. Springer: Cham; 2017. p. 211–22.
Moore D, Ries M, Forget EL, Schiffmann R. Enzyme replacement therapy in orphan and ultra-orphan diseases—the limitations of standard economic metrics as exemplified by Fabry-Anderson disease. Pharmacoeconomics. 2007;25(3):201–8.
Carrera P, IJzerman MJ. Are current ICER thresholds outdated? Valuing medicines in the era of personalized healthcare. Expert Rev Pharmacoecon Outcomes Res. 2016;16(4):435–7.
Denis A, Simoens S, Fostier C, Mergaert L, Cleemput I. Policies for orphan diseases and orphan drugs; 2009 [cited 2020 Nov 16]. https://ec.europa.eu/health/ph_threats/non_com/docs/policies_orphan_en.pdf.
Niezen MGH, de Bont A, Busschbach JJV, Cohen JP, Stolk EA. Finding legitimacy for the role of budget impact in drug reimbursement decisions. Int J Technol Assess Health Care. 2009;25(1):49–55.
Simoens S, Dooms M. Market access of orphan drugs: one size fits all? Hospital Pharmacy Europe 2012 [cited 2020 Nov 19]; 62:59–63. https://hospitalpharmacyeurope.com/news/editors-pick/market-access-of-orphan-drugs-one-size-fits-all/.
Largent EA. The many vs. the few—reply. Hastings Cent Rep. 2012;42(5):8–9.
Bonanno PV, Bucsics A, Simoens S, Martin AP, Oortwijn W, Gulbinovic J, et al. Proposal for a regulation on health technology assessment in Europe—opinions of policy makers, payers and academics from the field of HTA. Expert Rev Pharmacoecon Outcomes Res. 2019;19(3):251–61.
Cohen JP, Felix A. Are payers treating orphan drugs differently? J Mark Access Health Policy. 2014;2:23513.
Douglas C, Wilcox E, Burgess M, Lynd LD. Why orphan drug coverage reimbursement decision-making needs patient and public involvement. Health Policy. 2015;119(5):588–96.
Hyry HI, Roos JCP, Cox TM. Orphan drugs: expensive yet necessary. QJM. 2015;108(4):269–72.
Rosenberg-Yunger ZRS, Daar AS, Thorsteinsdottir H, Martin DK. Priority setting for orphan drugs: an international comparison. Health Policy. 2011;100(1):25–34.
Angelis A, Kanavos P. Multiple criteria decision analysis (MCDA) for evaluating new medicines in health technology assessment and beyond: the advance value framework. Soc Sci Med. 2017;188:137–56.
Barak A, Shankar NJ. Orphan drugs: pricing, reimbursement and patient access. Int J Pharm Healthc Market. 2011;5(4):299–317.
Norheim OF, Baltussen R, Johri M, Chisholm D, Nord E, Brock D, et al. Guidance on priority setting in health care (GPS-Health): the inclusion of equity criteria not captured by cost-effectiveness analysis. Cost Effect Resour Alloc. 2014;12:18.
Annemans L, Aymé S, Le Cam Y, Facey K, Gunther P, Nicod E, et al. Recommendations from the European working group for value assessment and funding processes in rare diseases (ORPH-VAL). Orphanet J Rare Dis. 2017;12:1–15.
Dintsios CM, Gerber A. Some essential clarifications: IQWiG comments on two critiques of the efficiency frontier approach. Health Econ (United Kingdom). 2010;19(10):1139–41.
He J, Song P, Kang Q, Zhang X, Hu J, Yang Y, et al. Overview on social security system of rare diseases in China. Biosci Trends. 2019;13(4):314–23.
Loblova O, Csanadi M, Ozieranski P, Kalo Z, King L, McKee M. Patterns of alternative access: unpacking the Slovak extraordinary drug reimbursement regime 2012–2016. Health Policy. 2019;123(8):713–20.
Simoens S. Pricing and reimbursement of orphan drugs: the need for more transparency. Orphanet J Rare Dis. 2011;6:1–8.
Schwalm A, Danner M, Seidl A, Volz F, Dintsios CM, Gerber A. Wo steht die Kosten-Nutzen-Bewertung des IQWiG : Abgleich mit einem internationalen Referenzszenario? Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz. 2010;53(6):615–22.
Simoens S. How to assess the value of medicines? Front Pharmacol. 2010;1:115.
Tony M, Wagner M, Khoury H, Rindress D, Papastavros T, Oh P, et al. Bridging health technology assessment (HTA) with multicriteria decision analyses (MCDA): field testing of the EVIDEM framework for coverage decisions by a public payer in Canada. BMC Health Serv Res. 2011;11:329.
Al-Attar M. TRAPPED—an insight into two sisters’ struggle to access treatment for a rare genetic disease. Orphanet J Rare Dis. 2018;13(1):37.
Boon W, Moors EHM, Kuhlmann S, Smits RE. Demand articulation in intermediary organisations: the case of orphan drugs in the Netherlands. Technol Forecast Soc Change. 2008;75(5):644–71.
Bourke SM, Plumpton CO, Hughes D. Societal preferences for funding orphan drugs in the United Kingdom: an application of person trade-off and discrete choice experiment methods. Value Health. 2018;21(5):538–46.
Chowdhury MZI, Chowdhury MA. Canadian health care system: who should pay for all medically beneficial treatments? a burning issue. Int J Health Serv. 2018;48(2):289–301.
Doux J. Editorial: barriers and opportunities: a view across the developmental divide. J Investig Dermatol. 2015;135(9):2143–4.
Forestier-Zhang L, Watts L, Turner A, Teare H, Kaye J, Barrett J, et al. Health-related quality of life and a cost-utility simulation of adults in the UK with osteogenesis imperfecta, X-linked hypophosphatemia and fibrous dysplasia. Orphanet J Rare Dis. 2016;11(1):1–9.
Gong S, Jin S. Current progress in the management of rare diseases and orphan drugs in China. Intractable Rare Dis Res. 2012;1(2):45–52.
Guan X-D, Zhang J, Man C, Ni B, Shi L-W. How far have we come? Challenges to orphan drug access in China, 2011–2017. J Pharm Sci. 2019;108(6):2199–205.
Hyry HI, Stern AD, Cox TM, Roos JCP. Limits on use of health economic assessments for rare diseases. QJM Int J Med. 2014;107(3):241–5.
Iskrov G, Raycheva RD, Stefanov R. Insight into reimbursement decision-making criteria in Bulgaria: implications for orphan drugs. Folia Med (Plovdiv). 2013;55(3–4):80–6.
Kanters TA, Hakkaart L, Rutten-van Moelken MP, Redekop WK. Access to orphan drugs in western Europe: can more systematic policymaking really help to avoid different decisions about the same drug? Expert Rev Pharmacoecon Outcomes Res. 2015;15(4):557–9.
McCabe C, Stafinski T, Menon D. Is it time to revisit orphan drug policies? BMJ. 2010;341:c4777.
Menon D, Stafinski T. Ultra-orphan drugs: can we afford the price. Expert Opin Orphan Drugs. 2017;5(8):611–2.
Morel T, Simoens S. Coverage of orphan drugs. In: Ethgen O, Staginnus U, editors. Future of health economics. Abingdon: Routledge; 2017. p. 109–21.
Mrsić M, Nola M. Rare diseases in Croatia—lesson learned from Anderson-Fabry disease. Croat Med J. 2008;49(5):579–81.
Oral M, Ozcelikay G. Ethical overview of pharmaceutical industry policies in Turkey from various perspectives. Turk J Pharm Sci. 2017;14(3):264–73.
Picavet E, Cassiman D, Simoens S. Reimbursement of orphan drugs in Belgium: what (else) matters? Orphanet J Rare Dis. 2014;9:139.
Ramalle-Gomara E, Ruiz E, Quinones C, Andres S, Iruzubieta J, Gil-de-Gomez J. General knowledge and opinion of future health care and non-health care professionals on rare diseases. J Eval Clin Pract. 2015;21(2):198–201.
Rhee TG. Policymaking for orphan drugs and its challenges. AMA J Ethics. 2015;17(8):776–9.
Sandman L, Hofmann B. Why we don’t need “unmet needs”! on the concepts of unmet need and severity in health-care priority setting. Health Care Anal. 2019;27(1):26–44.
Tilles SA, Borish L. Author’s response. Ann Allergy Asthma Immunol. 2012;109(2):151.
Torrent-Farnell J, Comellas M, Poveda JL, Abaitua I, Gutierrez-Solana LG, Perez-Lopez J, et al. The view of experts on initiatives to be undertaken to promote equity in the access to orphan drugs and specialised care for rare diseases in Spain: a Delphi consensus. Health Policy. 2018;122(6):590–8.
Wells RJ. The many vs. the few. Hastings Cent Rep. 2012;42(5):7 (author reply 8-9).
Wong-Rieger D, Rieger F. Health policies for orphan diseases: international comparison of regulatory, reimbursement and health services policies. In: Bali RK, Bos L, Gibbons MC, Ibell SR, editors. Rare diseases in the age of health 2.0. Berlin: Springer; 2014. p. 267–77.
Breaking the Access Deadlock to Leave No One Behind; January 2018 [cited 2020 May 6]. http://download2.eurordis.org.s3.amazonaws.com/positionpapers/eurordis_access_position_paper_final_4122017.pdf.
Transparency and Health Technology Assessment cooperation as proposed by the Regulation are the only real antidote to secrecy and political games; March 2018 [cited 2020 May 6]. http://download2.eurordis.org.s3.amazonaws.com/positionpapers/Statement_final.pdf.
GKV Spitzenverband. Nutzen und Schaden auch bei Arzneimitteln gegen seltene Krankheiten vollständig prüfen. Berlin; 2016 [cited 2020 May 5]. https://www.gkv-spitzenverband.de/presse/pressemitteilungen_und_statements/pressemitteilung_339584.jsp.
Annemans L, Cleemput I, Hulstaert F, Simoens S. Valorising and creating access to innovative medicines in the European union. Front Pharmacol. 2011;2:57.
van Weely S, Leufkens HG. Priority Medicines for Europe and the World”A Public Health Approach to Innovation”: Update on 2004 Background Paper; 2013 Mar 12 [cited 2020 Nov 20]. https://www.who.int/medicines/areas/priority_medicines/BP6_19Rare.pdf.
Windeler J, Lange S. Nutzenbewertung in besonderen Situationen—Seltene Erkrankungen. Z Evid Fortbild Qual Gesundhwes. 2008;102(1):25–30.
Connolly MP, Panda S, Patris J, Hazenberg BPC. Estimating the fiscal impact of rare diseases using a public economic framework: a case study applied to hereditary transthyretin-mediated (hATTR) amyloidosis. Orphanet J Rare Dis. 2019;14(1):1–9.
Gross ML. Ethics, policy, and rare genetic disorders: the case of Gaucher disease in Israel. Theor Med Bioethics. 2002;23(2):151–70.
Stafinski T, Menon D, Davis C, McCabe C. Role of centralized review processes for making reimbursement decisions on new health technologies in Europe. Clinicoecon Outcomes Res. 2011;3:117–86.
de Solà Morales O. Funding orphan medicinal products beyond price: sustaining an ecosystem. Eur J Health Econ. 2019;20(9):1283–6.
Denis A, Mergaert L, Fostier C, Cleemput I, Simoens S. Issues surrounding orphan disease and orphan drug policies in Europe. Appl Health Econ Health Policy. 2010;8(5):343–50.
Denis A, Mergaert L, Fostier C, Cleemput I, Hulstaert F, Simoens S. Critical assessment of Belgian reimbursement dossiers of orphan drugs. Pharmacoeconomics. 2011;29(10):883–93.
Denis A, Mergaert L, Fostier C, Cleemput I, Simoens S. A comparative study of European rare disease and orphan drug markets. Health Policy. 2010;97(2–3):173–9.
Kanters TA, Van Der Ploeg AT, Kruijshaar ME, Rizopoulos D, Redekop WK, Rutten-van Moelken MP, et al. Cost-effectiveness of enzyme replacement therapy with alglucosidase alfa in adult patients with Pompe disease. Orphanet J Rare Dis. 2017;12(1):1–12.
Lasalvia P, Prieto-Pinto L, Moreno M, Castrillon J, Romano G, Garzon-Orjuela N, et al. International experiences in multicriteria decision analysis (MCDA) for evaluating orphan drugs: a scoping review. Expert Rev Pharmacoecon Outcomes Res. 2019;19(4):409–20.
Li X-Q, Peng X-X, Gong C-X. Access to orphan drugs is a challenge for sustainable management of cystinosis in China. Chin Med J. 2018;131(19):2388–9.
Linley WG, Hughes D. Societal views on NICE, cancer drugs fund and value-based pricing criteria for prioritising medicines: a cross-sectional survey of 4118 adults in Great Britain. Health Econ. 2013;22(8):948–64.
Sarnola K, Ahonen R, Martikainen JE, Timonen J. Policies and availability of orphan medicines in outpatient care in 24 European countries. Eur J Clin Pharmacol. 2018;74(7):895–902.
Towse A, Barnsley P. Approaches to identifying, measuring, and aggregating elements of value. Int J Technol Assess Health Care. 2013;29(4):360–4.
Wagner M, Samaha D, Casciano R, Brougham M, Abrishami P, Petrie C, et al. Moving towards accountability for reasonableness—a systematic exploration of the features of legitimate healthcare coverage decision-making processes using rare diseases and regenerative therapies as a case study. Int J Health Policy Manag. 2019;8(7):424–43.
Min R, Zhang X, Fang P, Wang B, Wang H. Health service security of patients with 8 certain rare diseases: evidence from China’s national system for health service utilization of patients with healthcare insurance. Orphanet J Rare Dis. 2019;14(1):204.
White W. A rare disease patient/caregiver perspective on fair pricing and access to gene-based therapies. Gene Ther. 2019;27:474–81.
Prevot J, Watters D. HTA’s and access to rare diseases therapies: the view from the PID community. Pharm Policy Law. 2011;13(3,4):177–81.
Magalhaes M. Can severity outweigh smaller numbers? A deliberative perspective from Canada. Value Health. 2018;21(5):532–7.
Chim L, Salkeld G, Kelly P, Lipworth W, Hughes D, Stockler MR. Societal perspective on access to publicly subsidised medicines: a cross sectional survey of 3080 adults in Australia. PLoS ONE. 2017;12(3):e0172971.
Hughes-Wilson W, Palma A, Schuurman A, Simoens S. Paying for the Orphan Drug System: break or bend? Is it time for a new evaluation system for payers in Europe to take account of new rare disease treatments? Orphanet J Rare Dis. 2012;7:1–8.
Jena A, Lakdawalla D. Value frameworks for rare diseases: should they be different?; 2017 Apr 12 [cited 2020 Nov 17]. https://www.healthaffairs.org/doi/10.1377/hblog20170412.059563/full/.
Medić B, Divac N, Stopić B, Vujović KS, Glišić A, Cerovac N, et al. The attitudes of medical students towards rare diseases: a cross-sectional study. Vojnosanit Pregl. 2016;73(8):703–13.
Nicod E, Annemans L, Bucsics A, Lee A, Upadhyaya S, Facey K. HTA programme response to the challenges of dealing with orphan medicinal products: process evaluation in selected European countries. Health Policy. 2019;123(2):140–51.
Wagner M, Khoury H, Bennetts L, Berto P, Ehreth J, Badia X, et al. Appraising the holistic value of Lenvatinib for radio-iodine refractory differentiated thyroid cancer: a multi-country study applying pragmatic MCDA. BMC Cancer. 2017;17(1):272.
Young A, Menon D, Street J, Al-Hertani W, Stafinski T. A checklist for managed access programmes for reimbursement co-designed by Canadian patients and caregivers. Health Expect. 2018;21(6):973–80.
Lowin J, Bergman A, Ray Chaudhuri K, Findley LJ, Roeder C, Schifflers M, et al. A cost-effectiveness analysis of levodopa/carbidopa intestinal gel compared to standard care in late stage Parkinson’s disease in the UK. J Med Econ. 2011;14(5):584–93.
Malinowski KP, Kawalec PL, Trabka W, Sowada C, Pilc A. Reimbursement of orphan drugs in Europe in relation to the type of authorization by the European medicines agency and the decision making based on health technology assessment. Front Pharmacol. 2018;9:1263.
Nicod E, Kanavos P. Scientific and social value judgments for orphan drugs in health technology assessment. Int J Technol Assess Health Care. 2016;32(4):218–32.
The Lancet Neurology. Treating rare disorders: time to act on unfair prices. Lancet Neurol. 2017;16(10):761.
Winquist E, Bell CM, Clarke JTR, Evans G, Martin J, Sabharwal M, et al. An evaluation framework for funding drugs for rare diseases. Value Health. 2012;15(6):982–6.
Hughes D. Orphan drugs revisited: author’s response [4]. QJM. 2006;99(5):350–1.
Sheldon T. Dutch doctors call for EU evaluation of cost effectiveness of high cost orphan drugs. BMJ Br Med J (Clin Res Ed). 2012;345:e5461–e5461.
The Lancet. Rare diseases need sustainable options. Lancet. 2020;395(10225):660.
Vogler S, Paris V, Ferrario A, Wirtz VJ, de Joncheere K, Schneider P, et al. How can pricing and reimbursement policies improve affordable access to medicines? Lessons learned from European Countries. Appl Health Econ Health Policy. 2017;15(3):307–21.
Farrugia A, O’Mahony B, Cassar J. Health technology assessment and haemophilia. Haemophilia. 2012;18(2):152–7.
Loewenstein G. The many vs. the few. Hastings Cent Rep. 2012;42(5):7–8 (author reply 8-9).
Menzel PT. The many vs. the few. Hastings Cent Rep. 2012;42(5):5–6 (author reply 8-9).
Teagarden JR, Unger TF, Hirsch G. Access and availability of orphan drugs in the United States: advances or cruel hoaxes? Expert Opin Orphan Drugs. 2014;2(11):1147–50.
Xin X-X, Guan X-D, Shi L-W. Catastrophic expenditure and impoverishment of patients affected by 7 rare diseases in China. Orphanet J Rare Dis. 2016;11(1):1–7.
Kesselheim AS, McGraw S, Thompson L, O’Keefe K, Gagne JJ. Development and use of new therapeutics for rare diseases: views from patients, caregivers, and advocates. Patient. 2015;8(1):75–84.
Allotey PA, Allotey-Reidpath CD, Reidpath DD. Health systems implications of rare genetic conditions in low- and middle-income countries: a case study approach. Crit Public Health. 2018;28(2):248–52.
Caulfield T, Toews M. Rare diseases and resource allocation policy: the role of Canadian legal and ethical norms. UBCL Rev. 2016;49:789.
Rachul C, Caulfield T. The media and access issues: content analysis of Canadian newspaper coverage of health policy decisions. Orphanet J Rare Dis. 2015;10:1–7.
Rachul C, Toews M, Caulfield T. Controversies with Kalydeco: newspaper coverage in Canada and the United States of the cystic fibrosis “wonder drug.” J Cyst Fibros. 2016;15(5):624–9.
Balfour-Lynn IM. Personalised medicine in cystic fibrosis is unaffordable. Paediatr Respir Rev. 2014;15(S1):2–5.
Bavisetty S, Grody WW, Yazdani S. Emergence of pediatric rare diseases: review of present policies and opportunities for improvement. Rare Dis. 2013;1:e23579.
Das AM, Lagler F, Beck M, Scarpa M, Lampe C. Lysosomal storage diseases: challenges in multiprofessional patient care with enzyme replacement therapy. Klin Padiatr. 2017;229(3):168–74.
Saint-Raymond A, Llinares J. Orphan medicines: a success with a challenging future. Expert Opin Orphan Drugs. 2013;1(3):185–7.
Zallen DT. The many vs. the few. Hastings Cent Rep. 2012;42(5):4–5 (author reply 8-9).
Schlander M. The use of cost-effectiveness by the National Institute for Health and Clinical Excellence (NICE): no(t yet an) exemplar of a deliberative process. J Med Ethics. 2008;34(7):534–9.
Cavalier GM. Pushing parentless pharmaceuticals: toward an international home for orphan drugs and a cure for zebra diseases. Law Pol’y Int’l Bus. 1995;27:447.
Perehudoff K, Toebes B, Hogerzeil H. A human rights-based approach to the reimbursement of expensive medicines. Bull World Health Organ. 2016;94(12):935.
Stafinski T, Menon D, Philippon DJ, McCabe C. Health technology funding decision-making processes around the world: the same, yet different. Pharmacoeconomics. 2011;29(6):475–95.
Taylor DW. Redressing the inequities in Canadian pharmacare. Healthc Manag Forum. 2015;28(2):50–4.
IPA position paper: Patient Access to Approved Therapies [cited 2020 May 6]. https://www.worldpompe.org/images/pdfs/ipa%20position%20paper%20-%20patient%20access.pdf.
Norheim OF. Ethical priority setting for universal health coverage: challenges in deciding upon fair distribution of health services. BMC Med. 2016;14:1–4.
Special Eurobarometer 361: European awareness of rare diseases; 2011 [cited 2020 Nov 16]. https://ec.europa.eu/health//sites/health/files/rare_diseases/docs/ebs_361_en.pdf.
Bourdoncle M, Juillard-Condat B, Taboulet F. Patient access to orphan drugs in France. Orphanet J Rare Dis. 2019;14(1):47.
McCabe C. The many vs. the few. Hastings Cent Rep. 2012;42(5):6–7 (author reply 8-9).
Whitty JA, Lancsar E, Rixon K, Golenko X, Ratcliffe J. A systematic review of stated preference studies reporting public preferences for healthcare priority setting. Patient. 2014;7(4):365–86.
Jessop E, Upadhyaya S. Ultra orphan drugs: the NHS model for managing extremely rare diseases. Expert Opin Orphan Drugs. 2014;2(12):1301–8.
Kolasa K, Zwolinski KM, Kalo Z, Hermanowski T. Potential impact of the implementation of multiple-criteria decision analysis (MCDA) on the Polish pricing and reimbursement process of orphan drugs. Orphanet J Rare Dis. 2016;11:1–12.
Lelgemann M, Francke R. Rare diseases in professional health care. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz. 2008;51(5):509–18.
Sassi F, Archard L, Le Grand J. Equity and the economic evaluation of healthcare. Health Technol Assess. 2001;5(3):1–138.
Boon W, Martins L, Koopmanschap M. Governance of conditional reimbursement practices in The Netherlands. Health Policy. 2015;119(2):180–5.
Polisena J, Burgess M, Mitton C, Lynd LD. Engaging the Canadian public on reimbursement decision-making for drugs for rare diseases: a national online survey. BMC Health Serv Res. 2017;17:1–9.
Schuller Y, Hollak CEM, Biegstraaten M. The quality of economic evaluations of ultra-orphan drugs in Europe—a systematic review. Orphanet J Rare Dis. 2015;10(1):1–12.
Winquist E, Coyle D, Clarke JTR, Evans G, Seager C, Chan W, et al. Application of a policy framework for the public funding of drugs for rare diseases. J Gen Intern Med. 2014;29(3):S774–9.
Drummond M, Tarricone R, Torbica A. Assessing the added value of health technologies: reconciling different perspectives. Value Health. 2013;16(1 Suppl):S7-13.
Lüthi U. Solidarity is what is needed. Krankenpfl Soins Infirm. 2015;108(5):1.
Lumry WR. Hereditary angioedema: the economics of treatment of an orphan disease. Front Med. 2018;5:22.
Salzman R, Cook F, Hunt T, Malech HL, Reilly P, Foss-Campbell B, et al. Addressing the value of gene therapy and enhancing patient access to transformative treatments. Mol Ther. 2018;26(12):2717–26.
Moors EHM, Faber J. Orphan drugs: unmet societal need for non-profitable privately supplied new products. Res Policy. 2007;36(3):336–54.
Herder M. When everyone is an orphan: against adopting a U.S.-styled orphan drug policy in Canada. Account Res. 2013;20(4):227–69.
Luzzatto L, Hyry HI, Schieppati A, Costa E, Simoens S, Schaefer F, et al. Outrageous prices of orphan drugs: a call for collaboration. Lancet. 2018;392(10149):791–4.
Abrahamyan L, Feldman BM, Tomlinson G, Faughnan ME, Johnson SR, Diamond IR, et al. Alternative designs for clinical trials in rare diseases. Am J Med Genet C Semin Med Genet. 2016;172(4):313–31.
Alami H, Lehoux P, Auclair Y, de Guise M, Gagnon M-P, Shaw J, et al. Artificial intelligence and health technology assessment: anticipating a new level of complexity. J Med Internet Res. 2020;22(7):e17707.
Dragojlovic N, Rizzardo S, Bansback N, Mitton C, Marra CA, Lynd LD. Challenges in measuring the societal value of orphan drugs: insights from a canadian stated preference survey. Patient. 2015;8(1):93–101.
Acknowledgements
We thank Yi Jiao Tian for language proofreading.
Funding
This study was funded by the Swiss Academies of Arts and Sciences’ Käthe-Zingg-Schwichtenberg fund (Grant Number KZS 33/19). The funding body did not influence the design of the study and collection, analysis, and interpretation of data or in writing the manuscript. MRB is supported by the University Research Priority Program “ITINERARE” of the University of Zurich, Switzerland.
Author information
Authors and Affiliations
Contributions
BZ designed the study, collected, analyzed and interpreted the data and wrote the manuscript. JE provided feedback on the methodology, collected data and critically revised the manuscript. MB supported BZ in designing the study, interpreted the data and critically revised the manuscript. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
MRB received a research fund from Nutricia and is a member of the clinical advisory boards of Hemoshear and Moderna. BMZ and JE declare no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1
. Database search strategy. Including database-specific search algorithms and search details.
Additional file 2
. Article selection: excluded articles. Specifies how many articles were excluded on the full text screening stage for what reasons.
Additional file 3
. Codebook. Includes variables and categories used for systematic data extraction.
Additional file 4
. Raw data. Includes all data extracted from included articles.
Additional file 5
. Specification of articles and moral reasons. Includes a table as a supplementary to Figure 3, specifies which articles contain what moral reasons.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Zimmermann, B.M., Eichinger, J. & Baumgartner, M.R. A systematic review of moral reasons on orphan drug reimbursement. Orphanet J Rare Dis 16, 292 (2021). https://doi.org/10.1186/s13023-021-01925-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13023-021-01925-y