Abstract
Background
Tuberous sclerosis complex (TSC) is a rare autosomal dominant genetic disorder characterized by the development of numerous benign tumors. Renal angiomyolipoma (RAML) occur in up to 80% of TSC patients, which is a leading cause of TSC-related death in adult patients. The aim of the study was to evaluate the efficacy and safety profiles of everolimus in Chinese patients of TSC associated with RAML(TSC-RAML).
Methods
In this 2-years, nonrandomized, open-label trial, 18 patients of TSC-RAML, with at least one RAML 3 cm or larger in its longest diameter, were enrolled to assess the efficacy and safety of everolimus therapy in Chinese patients. Everolimus was administered for the first 12 months only. The primary endpoint was a reduction of 50% or more relative in RAML volume to the baseline in the absence of new RAML ≥1 cm and no RAML-related bleeding of grade ≥ 2. The secondary endpoints included: safety, lung function and skin lesions response rate. Serial computed tomography of RAML, magnetic resonance imaging of brain lesions and pulmonary-function tests were performed. Adverse events were investigated using CTCAE v4.0. All analyses used a significance level of 0.05 and were generated in SPSS19.0 software.
Results
The proportion of patients who achieved ≥50% reduction from baseline in the sum of volumes of target lesions increased from 52.94% at 3 months, to 58.82% and 66.67% at months 6 and 12, respectively. During the period of everolimus therapy, among patients with lymphangioleiomyomatosis, the mean forced expiratory volume in 1 s (FEV1) increased by 276 ± 78 ml (P < 0.001), the forced vital capacity (FVC) increased by 433 ± 170 ml (P < 0.001), and the residual volume decreased by 408 ± 243 ml (P = 0.009), as compared with baseline values. The angiomyolipoma volume and the lung function approached, but did not completely return to, the baseline values. The skin lesions response rate was 37.5% after 12 months of therapy falling to 21.4% at 12 months after stopping everolimus. The most common adverse events were mucositis oral, irregular menstruation, abdominal pain, hypertriglyceridemia and headache. The most common grade 3 adverse events were irregular menstruation and mucositis oral. In addition, one patient died from RAML spontaneous haemorrhage during treatment with everolimus, even with reduction in RAML volume of 60.68% at 3 months. A second death was due to epithelioid RAML progression, with metastasis to multiple retroperitoneal lymph node, who died from severe infection one month after surgery.
Conclusions
Angiomyolipomas regressed somewhat during everolimus therapy but tended to increase in volume after the therapy was stopped. Everolimus was well tolerated and showed promising activity in Chinese patients with TSC-RAML, however, we should alert the life-threatening hemorrhage of large RAML in the early period and the lymph node metastasis of epithelioid RAML.
Trial registration
ChiCTR-OPC-14005488. Registered November 17, 2014.
Similar content being viewed by others
Background
Tuberous sclerosis complex (TSC) is an autosomal dominant syndrome affecting 1~ 2 million people worldwide [1]. It is characterized by prominent neurodevelopmental features and by tumors that develop in the brain, skin, heart, kidneys and lungs [1]. Renal angiomyolipoma (RAML) develop in approximately 80% of adults and adolescents with TSC [1, 2]. RAML associated with tuberous sclerosis complex (TSC-RAML) are characterized as multiple and commonly bilateral lesions that consist of blood vessels, smooth muscles, and adipose tissues [2]. TSC-RAML typically grow over time, presenting of arterial hypertension and risk of potentially life-threatening hemorrhage, which is the leading cause of TSC-associated death in adult patients [3]. To date, the main therapeutic options are embolisation, elective surgery, and emergency nephrectomy in cases of uncontrollable haemorrhage in China [4].
The majority of individuals with TSC have mutations in either the TSC1 or TSC2 genes, and subsequent somatic mutation results in constitutive activation of mammalian target of rapamycin (mTOR), a critical regulator of cell growth, proliferation, and angiogenesis [5,6,7]. Bissler JJ and colleagues demonstrated everolimus, a mammalian target of rapamycin (mTOR) inhibitor, could significant reduction in renal angiomyolipoma volume compared with placebo in Western populations [8]. The International tuberous sclerosis complex consensus conference held in 2012 recommended mTOR inhibitors as the first-line treatment for RAML when enlarged to 3 cm or more, even when asymptomatic [9]. Transcatheter arterial embolization and partial nephrectomy are recommended as second-line treatments [9]. However, we lack data of the efficacy and safety of everolimus in treatment of TSC-RAML in Chinese patients.
The objective of the present study was to evaluate the efficacy and safety of everolimus for TSC-RAML in Chinese patients.
Methods
Patients
This trial was a 2-years, nonrandomized, open-label trial, phase 2 study (ChiCTR-OPC-14005488, https://www.chictr.org.) conducted at Peking Union Medical College Hospital start from December 2014. From December 2014 to November 2015, patients were included if they met the following inclusion criteria: (1). Men or women (not pregnant) ≥18 years; (2). Clinical and/or genetic diagnosis of TSC; (3). CT or MRI shows one or more TSC-RAMLs with the longest diameter ≥ 3 cm; (4). Without RAML bleeding or embolism in the past 6 months. Patients were excluded from the study if they met the following exclusion criteria: (1). Age < 18 years; (2). Women who plan to be pregnant or have been pregnant or lactating; (3). CT or MRI shows the longest diameter of RAML < 3 cm; (4). Patients expected to undergo surgery or embolization therapy during the trial; (5). History of coronary heart disease, myocardial infarction or cerebral infarction related to atherosclerosis; (6). History of RAML bleeding or embolism in the past 6 months; (7). Impaired lung function defined as following: For patients without lymphangioleiomyomatosis (LAM): Known impaired lung function (e.g. FEV1 or DLco ≤70% of predicted); For patients with LAM: DLco ≤35%, or O2 saturations below normal at rest, or O2 saturation ≤ 88% on 6 min walking test with up to 6 l O2/min nasal oxygen; (8). Severe hematological disease or liver function abnormality (such as aminotransferase > 2.5 times of normal upper limit, serum bilirubin > 1.5 times of normal upper limit, hemoglobin < 9 g/dL, platelet < 80,000/mm3, or absolute neutrophil count < 1000/mm3); (9). Concomitant severe infection before or during the trial; (10). Previous organ transplantation; (11). History of other surgeries (involving entry into body cavity or suture) in the past 2 months; (12). Previous mTOR inhibitor treatment (such as sirolimus and everolimus); (13). Use of investigational drug within 30 days; (14). Poor control of hyperlipidemia: fasting serum cholesterol > 300 mg/dL (or > 7.75 mmol/L), fasting triglyceride > 2.5 times of normal upper limit; (15). Poor control of diabetes: fasting blood glucose > 1.5 times of normal upper limit; (16). Patients with bleeding tendency or using oral anti-vitamin K drugs (except low-dose warfarin); (17). History of HIV seropositivity; (18). Active hepatitis; (19). Patients who cannot participate in regular visits and follow-up; (20). Patients who are not suitable for MRI examination (such as excessive obesity, mental disorders, bullet fragment in body, stent and pacemaker); (21). Serum creatinine > 1.5 times of normal upper limit. This study protocol was approved by the Human Ethics Committee of Peking Union Medical College Hospital, before the first patient was enrolled.
Study design
This study includes two periods: the core period and the extended period. Everolimus was administered for the first 12 months only. The core period of the study lasts for 1 year, and then all possible patients will continue the next 1-year extended observation. The dose is initiated with 10 mg daily by oral, and then titrated to the target tough blood concentration of 5–15 ng/ml during the first 3 months and maintained throughout the observation, with dose modifications allowed on the basis of safety findings. Concomitant use of strong inhibitors or inducers of cytochrome P450 3A4 or p-glycoprotein (PgP) was to be avoided during the study; use of antiproliferative agents other than study drug was prohibited. All planed visits and assessments are listed in Additional file 1: Table S1. The primary efficacy endpoint was the proportion of patients with a confirmed angiomyolipoma response, defined as a reduction in angiomyolipoma volume (sum of volumes of all target angiomyolipomas > 1 cm identified at baseline) of 50% or more relative to baseline and absence of angiomyolipoma progression. In addition, RAML response requires satisfying the following criteria: (1) No new RAML ≥1.0 cm in longest diameter are identified; (2) The patient does not have any RAML-related bleeding of grade ≥ 2 as defined by the National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.0 (NCI-CTCAE v.4). Key secondary endpoints were time to angiomyolipoma progression and skin lesions response rate. Computed Tomography (CT) or magnetic resonance imaging(MRI), same modality used throughout the study for each patient, used to calculate RAMLs volume at baseline and repeated at 3, 6, 12, 18 and 24 months after the start of treatment. Skin lesions resulting from tuberous sclerosis complex include hypomelanotic macules, the shagreen patch, periungual or subungual fibromas, and facial angiofibromas, forehead plaques, or both, and were assessed at baseline and repeated at 3, 6, 12, 18 and 24 months after the start of treatment using the seven-point grading scale Physician’s Global Assessment of Clinical Condition [10, 11] (Additional file 2: Table S2). Patients with lymphangioleiomyomatosis underwent pulmonary-function testing at baseline, 12 months, and 24 months. Adverse events were monitored throughout the study and graded according to the Common Terminology Criteria for Adverse Events v4.0 via patient-reported or caregiver-reported responses as well as investigator assessment.
Statistical analysis
Data are expressed as the mean ± standard deviation (M ± SD) or n (%). Statistical significance was determined by paired or unpaired Student’s t-test in cases of standardized expression data. The SPSS software package (version 17.0) was used for all statistical analysis. P value< 0.05 was defined as statistically difference.
Results
Characteristics of the patients
Eighteen patients were enrolled in this trial. Their demographic details and disease characteristics are summarized in Table 1. The median age was 29 years, 9 patients were under 30 years of age. All 18 patients underwent genotyping by next-generation sequencing and confirmed all for TSC2 mutation (Additional file 3: Table S3). Six female patients were diagnosed for lymphangioleiomyomatosis (LAM) while one patient presence of subependymal giant cell astrocytoma. Two patients had undergone embolisation, and two patients had previously undergone partial nephrectomy while another two undergone unilateral nephrectomy. In addition, there were no large, at least 5 mm or larger in size, intra-renal aneurysm in all 18 patients. Three patients left the study during the first year: one had a unilateral renal hemorrhage and died at fourth fellow-up month, one died due to epithelioid RAML progression at eleven fellow-up month, and one did not comply with the protocol. Fifteen patients underwent the 12-month evaluation. One withdrew from the study after the 18-month visit to continue everolimus therapy off-label because of worry about tumor progress fast, leaving 14 patients at the 24-month assessment of angiomyolipoma.
Treatment efficacy
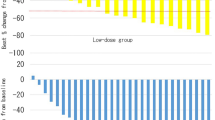
The proportion of patients who achieved ≥50% reduction from baseline in the sum of volumes of target lesions increased from 52.94% (9/17) at 3 months, to 58.82% (10/17) and 66.67% (10/15) at months 6 and 12, respectively. Primary-tumor shrinkage was most rapid during the initial 3 months of treatment, with evidence of a sustained response at subsequent time points during the core treatment phase. Median time to angiomyolipoma response for everolimus was 3.0 months. The mean renal angiomyolipoma volume at baseline was 1974 ± 2406 ml (Table 1). After 12 months of therapy, the mean volume decreased to 41.14 ± 26.54% of the baseline volume (P<0.002) (Table 2 and Fig. 1). At 6 and 12 months after stopping everolimus, the mean angiomyolipoma volume had increased to 60.67 ± 23.28% of the baseline volume (P = 0.006) and 77.62 ± 16.66% of the baseline volume (P = 0.014), respectively (Table 2 and Fig. 1). The response of a renal angiomyolipoma along with time of everolimus therapy, visualized on CT, is shown in Fig. 2. The current trial shows that everolimus are effective in reducing angiomyolipoma size in tuberous sclerosis in Chinese patients.
Renal Angiomyolipoma Volume in the Patients with the Tuberous Sclerosis Complex during the Study. Panel a shows the renal angiomyolipoma volume at each visit is expressed as a percentage of the baseline size. The dashed line represents 50% of the baseline value; data below the line indicate that the mean angiomyolipoma volume was reduced by 50% or more. Panel b shows the mean change (in milliliters) from the baseline values for renal angiomyolipoma volume. I bars indicate the standard errors
Target Renal Angiomyolipomas of a Patient with the Tuberous Sclerosis Complex. Panel a shows the target renal angiomyolipoma volume (in milliliters) at baseline. Panel b, c and d shows the target renal angiomyolipoma volume (in milliliters) after 3, 6 and 12 months of everolimus therapy. Panel e and f shows the target renal angiomyolipoma volume (in milliliters) at 6 and 12 months after stopping everolimus
Pulmonary functional data for 6 female patients with lymphangioleiomyomatosis are listed in Table 3. All six patients had never been smokers. At enrollment, spirometric measurements were normal in one patients, revealed moderate airflow obstruction (forced expiratory volume in 1 s [FEV1], 50 to 70% of the predicted value) in three patients, and indicated severe airflow obstruction (FEV1 < 50% of the predicted value) in two patients. During everolimus therapy, the mean FEV1 increased from the baseline mean by 276 ± 78 ml at 12 months (P < 0.001), while the mean FVC increased from the baseline mean by 433 ± 170 ml at 12 months (P < 0.001) (Fig. 3a and c). After 1 year of everolimus therapy, the FEV1 and FVC in these patients were significantly improved (Table 3). Twelve months after stopping everolimus, the mean FEV1 was 126 ± 48 ml greater than the mean baseline value (P = 0.004), while the mean FVC was 274 ± 142 ml greater than the mean baseline value (P = 0.008) (Fig. 3b and c). The mean percent of the predicted FEV1 value were significantly improved at 12 months (P < 0.001) and at 24 months (P = 0.008) (Table 3). The mean percent of the predicted FVC value were significantly improved at 12 months (P < 0.001) and at 24 months (P < 0.001) (Table 3). The mean residual volume fell by 408 ± 243 ml after 1 year of everolimus therapy, as compared with the baseline value (P = 0.009) (Table 3). The mean percent of the predicted residual volume were significantly improved at 12 months (P < 0.001) and at 24 months (P < 0.001) (Table 3). Neither the DLco nor total lung capacity changed significantly during the study (Table 3).
Pulmonary Function in Patients with Lymphangioleiomyomatosis. Panel a shows the forced expiratory volume in 1 s (FEV1) for each patient. Panel b shows the forced vital capacity (FVC) for each patient. Panel c shows the mean change (in milliliters) from the baseline values for FEV1 and for FVC. I bars indicate the standard errors. Panel d shows the residual volume for each patient
All patients had cortical tubers; 15 patients had subependymal nodules and one had subependymal giant-cell astrocytomas. There were no changes in the size of the subependymal nodules. We did not get the evaluable data of the patient with subependymal giant-cell astrocytomas as she died for RAML spontaneous haemorrhage in four months. Although this was not a study endpoint, a slight improvement in the frequency of seizures was reported by some parents.
Skin lesions associated with tuberous sclerosis were present at baseline in all 18 patients. Facial angiofibromas decreased in size and became paler and less rough after 12 months of therapy (Fig. 4). The skin lesions response rate was 37.5% (6 of 16) after 12 months of therapy falling to 21.4% (3 of 14) at 12 months after stopping everolimus.
Adverse events
Adverse events were consistent with the known everolimus safety profile. The most common adverse events were mucositis oral (100%), irregular menstruation (91.7%), abdominal pain (77.8%), hypertriglyceridemia (72.2%) and headache (66.7%) (Table 4). The most common grade 3 adverse events were irregular menstruation (25%) and mucositis oral (11.1%) (Table 4). Three patients with irregular menstruation was classified as grade 3 adverse events due to persistent amenorrhea for more than 6 months. In the trial, one patient died from RAML spontaneous haemorrhage during treatment with everolimus, even with reduction in RAML volume of 60.68% at 3 months. A second death was due to epithelioid RAML progression, who underwent left radical nephrectomy and immunohistochemical examination revealed features of epithelioid AML accompany with retroperitoneal multiple lymph node metastasis. The patient died from severe infection one month after surgery.
Discussion
In the current trial, we reported the efficacy and safety of everolimus in treatment of TSC-RAML in Chinese patients for the first time. Everolimus therapy in patients with the tuberous sclerosis complex was associated with a reduction in angiomyolipoma volume, improvements in skin lesions and pulmonary function. The renal and pulmonary benefits of treatment with everolimus tended to reverse after the drug was withdrawn, though the improvements were persistent in part patients.
In patients with the tuberous sclerosis complex, renal disease is a leading cause of death or disability in adult patients [3]. TSC is a rare autosomal dominant genetic disease caused by mutations in theTSC1 gene coding for hamartin and TSC2 gene coding tuberin [5, 6]. Finding that tuberin plays an important role in mTOR signaling pathway and further identification of tuberin hamartin complex as a main inhibitor of this pathway opened up new possibilities in disease-modifying therapy for TSC patients [12, 13]. Randomized clinical trials support the use of everolimus, an inhibitor of the mammalian target of rapamycin, in the treatment of subependymal giant cell astrocytomas (SEGA), RAML and seizure related to TSC in western populations [8, 14, 15]. However, its efficacy and safety in treatment of Chinese TSC patients is unknown. In the trial, tumor partial response by RECIST criteria was observed in 52.94%, 58.82% and 66.67% at months 3, 6 and 12 in Chinese adult patients, respectively. Bissler and colleagues investigated everolimus treatment for RAML in patients with tuberous sclerosis and found 44.2%, 55% and 64.5% patients achieved ≥50% reduction from baseline in the sum of volumes of target lesions at weeks 12, 24 and 96, respectively [8, 16]. Another subgroup analysis from EXIST-1 trial showed similar results that the proportions of patients in the everolimus arm with 50% reduction in the sum of target RAML were 56.5%, 78.3% and 80.0% after 12, 24 and 48 weeks, respectively [17]. In addition, Bissler and colleagues investigated another mTOR inhibitor, sirolimus, treatment for RAML in patients with tuberous sclerosis or sporadic LAM and found a mean reduction in RAML volume of 47% at 12 months [18]. The observation that angiomyolipoma size correlates with the risk of hemorrhage suggests that maintains or reduces angiomyolipoma size may reduce the risk of bleeding. However, the mean angiomyolipoma volume had increased ranging from a rapid return to baseline dimensions to a sustained reduction in size, after the withdrawal of everolimus. The current trial shows that everolimus are effective in reducing RAML size in tuberous sclerosis in Chinese patients. But long-term maintenance of everolimus therapy is necessary in TSC-RAML patients as it increased after stopping therapy. Everolimus administration to patients with TSC-RAML over the extension phase of the EXIST-2 trial supports a long-term benefit over approximately 4 years [19]. Therefore, it seems reasonable to assume that TSC-RAML patients should be kept under mTOR inhibition for life.
Six patients with lymphangioleiomyomatosis were evaluated for pulmonary outcomes. After 12 months of everolimus therapy, the FEV1 increased by 276 ml, and the FVC increased by 433 ml, while the mean residual volume fell by 408 ml. In addition, the FEV1, FVC and residual volume remained the most improved 12 months after everolimus was stopped, as compared with the baseline values. However, neither the DLco nor total lung capacity changed significantly during everolimus therapy. In addition, facial angiofibromas decreased in size and became paler and less rough after 12 months of therapy, which remained the most improved 12 months after stopping everolimus. There was no change in subependymal nodules size during the study. However, everolimus appears to have activity in the central nervous system, on the basis of a slight improvement in the frequency of seizures was reported by two patients. In all, treatment with everolimus for 1 year resulted in an improvement in lung function and skin lesions in adults with the TSC-RAML.
Adverse events were common, consistent with the known toxicities of everolimus and mostly of low grade. The most frequent adverse reactions we recorded were mucositis oral (100%), which was observed at the start of treatment. The most common grade 3 adverse events were irregular menstruation, occurred in five out of twelve female patients (25.0%). The frequency of mucositis oral and irregular menstruation was higher than the EXIST-2 and subgroup analysis of EXIST-1 study [1, 3], which may be due to the different race of patients. Although three cases of amenorrhoea in our study resolved without intervention, surveillance for this potential side effect is warranted in women patients of child-bearing potential and needed further investigated. In the current trial, one patient died from RAML spontaneous haemorrhage during treatment with everolimus, even with reduction in RAML volume of 60.68% at 3 months. As far as we know, it is the first case reported RAML spontaneous haemorrhage during treated with everolimus. The sum of RAML volume burben is over 4000 ml in this patient, which may the major reason for RAML spontaneous haemorrhage. A second death was due to epithelioid RAML progression, with metastasis to multiple retroperitoneal lymph node, and died from severe infection one month after surgery. Epithelioid RAML is considered to be a potentially malignant tumor, which is composed of a prominent epithelioid component, with spindle and giant cells, and contains none or a minimal amount of adipose tissue. Epithelioid RAML can occur in patients both with and without tuberous sclerosis. However, half of published cases have history of tuberous sclerosis, some show metastatic potential [20, 21]. In addition, the mTOR pathway was recently found to be activated in epithelioid angiomyolipoma [22], and Wolff N, et al. reported that mTOR inhibitors, such as sirolimus or temsirolimus, in two cases of epithelioid angiomyolipoma and showed a good short term response [23]. The two patients died during the clinical trial, reminds us that the risks and benefits of everolimus need specific and careful evaluation in the real world.
Several limitations in our study should be noted: (1) it is a single center based on a small sample without placebo-controlled trial; (2) none patient with TSC1 gene mutation was included; (3) all patients were adult, so clinical trials concerning the safety of everolimus for child patients needed in the future.
Conclusions
Collectively, the data suggest that everolimus therapy for 1 year not only resulted in a decrease in the size of angiomyolipomas, but also improved in lung function and skin lesions in adults Chinese patients with TSC-RAML. One year after the drug was discontinued, the angiomyolipoma size and the lung function approached, but did not completely return to, the baseline values. It seems reasonable to assume that TSC-RAML patients should be kept under mTOR inhibition for life. The most common adverse events were mucositis oral, irregular menstruation, abdominal pain, hypertriglyceridemia and headache. The two patient who died in the current trial reminds us that the risks and benefits of everolimus need specific and careful evaluation in the real world. In conclusion, this study shows that everolimus is a relatively safe and efficacious in treatment of TSC-RAML in Chinese adult patients.
Abbreviations
- CT:
-
Computed tomography
- DLco:
-
Denotes diffusing capacity of the lung for carbon monoxide
- FEV1:
-
Forced expiratory volume in 1 s
- FVC:
-
Forced vital capacity
- LAM:
-
Lymphangioleiomyomatosis
- MRI:
-
Magnetic resonance imaging
- mTOR:
-
Mammalian target of rapamycin
- RAML:
-
Renal angiomyolipoma
- SEGA:
-
Subependymal giant cell astrocytomas
- TSC:
-
Tuberous sclerosis complex
References
Northrup H, Krueger DA. Tuberous sclerosis complex diagnostic criteria update: recommendations of the 2012 Iinternational tuberous sclerosis complex consensus conference. Pediatr Neurol. 2013;49(4):243–54.
Cai Y, Li H, Zhang Y. Assessment of tuberous sclerosis complex associated with renal lesions by targeted next-generation sequencing in mainland China. Urology. 2017;101:170.e1–7.
Kapoor A, et al. Evolving strategies in the treatment of tuberous sclerosis complex-associated Angiomyolipomas (TSC-AML). Urology. 2016;89:19–26.
Ting WY, et al. Clinical analysis of tuberous sclerosis complex complicated with renal angiomyolipoma: a report of 22 cases. Zhonghua Yi Xue Za Zhi. 2013;93(26):2056–8.
Consortium ECTS. Identification and characterization of the tuberous sclerosis gene on chromosome 16. Cell. 1993;75(7):1305–15.
van Slegtenhorst M, et al. Identification of the tuberous sclerosis gene TSC1 on chromosome 9q34. Science. 1997;277(5327):805–8.
Laplante M, Sabatini DM. mTOR signaling in growth control and disease. Cell. 2012;149(2):274–93.
Bissler JJ, et al. Everolimus for angiomyolipoma associated with tuberous sclerosis complex or sporadic lymphangioleiomyomatosis (EXIST-2): a multicentre, randomised, double-blind, placebo-controlled trial. Lancet. 2013;381(9869):817–24.
Krueger DA, Northrup H. Tuberous sclerosis complex surveillance and management: recommendations of the 2012 international tuberous sclerosis complex consensus conference. Pediatr Neurol. 2013;49(4):255–65.
Duvic M, et al. Phase 2 and 3 clinical trial of oral bexarotene (Targretin capsules) for the treatment of refractory or persistent early-stage cutaneous T-cell lymphoma. Arch Dermatol. 2001;137(5):581–93.
Heald P, et al. Topical bexarotene therapy for patients with refractory or persistent early-stage cutaneous T-cell lymphoma: results of the phase III clinical trial. J Am Acad Dermatol. 2003;49(5):801–15.
Gao X, et al. Tsc tumour suppressor proteins antagonize amino-acid-TOR signalling. Nat Cell Biol. 2002;4(9):699–704.
Tee AR, et al. Tuberous sclerosis complex-1 and -2 gene products function together to inhibit mammalian target of rapamycin (mTOR)-mediated downstream signaling. Proc Natl Acad Sci U S A. 2002;99(21):13571–6.
French JA, et al. Adjunctive everolimus therapy for treatment-resistant focal-onset seizures associated with tuberous sclerosis (EXIST-3): a phase 3, randomised, double-blind, placebo-controlled study. Lancet. 2016;388(10056):2153–63.
Franz DN, et al. Efficacy and safety of everolimus for subependymal giant cell astrocytomas associated with tuberous sclerosis complex (EXIST-1): a multicentre, randomised, placebo-controlled phase 3 trial. Lancet. 2013;381(9861):125–32.
Bissler JJ, et al. Everolimus for renal angiomyolipoma in patients with tuberous sclerosis complex or sporadic lymphangioleiomyomatosis: extension of a randomized controlled trial. Nephrol Dial Transplant. 2016;31(1):111–9.
Kingswood JC, et al. The effect of everolimus on renal angiomyolipoma in patients with tuberous sclerosis complex being treated for subependymal giant cell astrocytoma: subgroup results from the randomized, placebo-controlled, phase 3 trial EXIST-1. Nephrol Dial Transplant. 2014;29(6):1203–10.
Bissler JJ, et al. Sirolimus for angiomyolipoma in tuberous sclerosis complex or lymphangioleiomyomatosis. N Engl J Med. 2008;358(2):140–51.
Bissler JJ, et al. Everolimus long-term use in patients with tuberous sclerosis complex: four-year update of the EXIST-2 study. PLoS One. 2017;12(8):e0180939.
Lopez-Beltran A, et al. 2004 WHO classification of the renal tumors of the adults. Eur Urol. 2006;49(5):798–805.
Lane BR, et al. Clinical correlates of renal angiomyolipoma subtypes in 209 patients: classic, fat poor, tuberous sclerosis associated and epithelioid. J Urol. 2008;180(3):836–43.
Pan CC, et al. Constant allelic alteration on chromosome 16p (TSC2 gene) in perivascular epithelioid cell tumour (PEComa): genetic evidence for the relationship of PEComa with angiomyolipoma. J Pathol. 2008;214(3):387–93.
Wolff N, et al. Sirolimus and temsirolimus for epithelioid angiomyolipoma. J Clin Oncol. 2010;28(5):e65–8.
Acknowledgements
We thank the personnel at the department of Dermatology, Peking Union Medical College Hospital and the personnel the department of Respiratory Medicine, Peking Union Medical College Hospital for their support.
Funding
The research was supported by Novartis Pharmaceuticals Corporation (I10288), the National Natural Science Foundation of China (81670611) and the the National Key Research and Development Program of China (2016YFC0901500).
Availability of data and materials
Data are available on request.
Author information
Authors and Affiliations
Contributions
YSZ had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: YSZ, HZL. Acquisition of data: YC, HG, WDW, HS, BS. Analysis and interpretation of data: YC, HG. Drafting of the manuscript: YC, HG, WDW. Critical revision of the manuscript for important intellectual content: YSZ, HZL. Statistical analysis: YC, HG, YSZ. Obtaining funding: YSZ. Administrative, technical, or material support: YSZ. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All patients provided written informed consent before entering the clinical trial. The study was done in accordance with the Declaration of Helsinki and local regulations This study protocol was approved by the Human Ethics Committee of Peking Union Medical College Hospital, before the first patient was enrolled.
Consent for publication
Not applicable.
Competing interests
Yi Cai received travel expenses from Novartis CO., LTD. The other authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Additional files
Additional file 1:
Visit Schedule and Assessments. (DOCX 73 kb)
Additional file 2:
Physician’s Global Assessment of Clinical Condition. (DOCX 98 kb)
Additional file 3:
Mutations detected by next-generation sequencing. (DOCX 92 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Cai, Y., Guo, H., Wang, W. et al. Assessing the outcomes of everolimus on renal angiomyolipoma associated with tuberous sclerosis complex in China: a two years trial. Orphanet J Rare Dis 13, 43 (2018). https://doi.org/10.1186/s13023-018-0781-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13023-018-0781-y