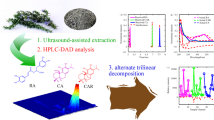
Abstract
Background
The processed roots of Polygonum multiflorum Thunb. (Heshouwu; processed HSW) are commonly used in anti-aging medicine. Few reports have combined chemical profiles with bioactivity to evaluate the quality of the processed HSW. This study aims to integrate chemometric fingerprints of antioxidant activities and high-performance liquid chromatography–diode array detection–chemiluminescence (HPLC–DAD–CL) to assess the quality of processed HSW.
Methods
An online HPLC–DAD–CL based on the three reactive oxygen species (ROS), superoxide anion, hydrogen peroxide, and peroxynitriteanion, was developed to screen the potential anti-aging constituents for a comprehensive quality evaluation of processed HSW. Additionally, antioxidant-activity-integrated fingerprints were constructed and hierarchical cluster analysis and principal component analysis were used to evaluate the variations among 14 batches of processed HSW samples purchased from drug stores in different habitats.
Results
Fourteen batches of processed HSW samples were highly similar and classified into two clusters using hierarchical cluster analysis. Twelve active compounds exhibited antioxidant activity on the ROS with different degrees of sensitivity that constituted specific fingerprints. Among them, protocatechuic acid, catechin, trans-2,3,5,4′-tetrahydroxy-stilbene-2-O-β-d-glucoside, 2,3,5, 4′-tetrahydroxy-stilbene-2-O-β-d-(2′′-galloyl)-glucoside, torachrysone-8-O-glucoside, and emodin-8-O-β-d-glucoside exerted relatively large influences on the differences between processed HSW samples.
Conclusion
Our study established the antioxidative activity-integrated fingerprint for processed HSW and achieved a screening of the potential anti-aging constituents using the online HPLC–DAD–CL method with H2O2, O •−2 , and ONOO−scavenging experiments.
Similar content being viewed by others
Background
The roots of Polygonum multiflorum Thunb. (Heshouwu; HSW) (Fig. 1) are often used in either raw or processed form to treat different diseases in Chinese medicine (CM). Raw HSW loosens the bowel and relieves constipation; HSW’s anti-aging property is mainly attributed to the processed form [1]. HSW also exhibits neuroprotective [2] and hairgrowth promoting activity [3, 4]. The characteristic constituent of HSW is 2,3,5,4′-tetrahydroxystilbene-2-O-β-d-glucoside, which is a potential natural inhibitor of advanced glycation end products [5] and exhibited anti-atherosclerosis [6] and anti-osteoporosis effects [7].
HSW contains numerous phenols, anthraquinones, and stilbene glycosides [8, 9]. Most studies have used various methods to focus on qualitative or quantitative analysis of HSW constituents, including high-performance liquid chromatography (HPLC) [10, 11], HPLC–electrospray ionization mass spectrometry (HPLC–ESI–MS) [12], and UHPLC (ultra-HPLC) with linear iontrap–Orbitrap tandem mass spectrometry (UHPLC–LTQ–Orbitrap MS) [13]. However, none of these studies have comprehensively evaluated the quality of HSW by combining chemical components with bioactivity. In addition, research has focused on studies of raw HSW rather than processed HSW. Different processing procedures used by manufacturers could lead to differences in the quality of processed HSW [14]. Therefore, it is necessary to analyze chemical and bioactivity information to comprehensively control the quality of processed HSW.
The fingerprint technique is the predominant tool to evaluate the quality of CM. However, the chromatographic fingerprint only contains the chemical message and is insufficient to demonstrate the overall quality of material [15, 16]. Therefore, activity-integrated fingerprints have been increasingly used to evaluate the quality of CM. High-performance liquid chromatography–diode array detection–chemiluminescence (HPLC–DAD–CL) is a sensitive method and often used in studies of activity-integrated fingerprints. Some studies have used a series of free radicals, such as hydrogen peroxide (H2O2), 2,2′-Azinobis-(3-ethylbenzothiazoline-6-sulfonic acid), superoxide anion (O •−2 ), and 2, 2-diphenyl-1-picrylhydrazyl, to screen bioactive constituents and evaluate the antioxidant activity of CM [15–17]. However, the bioactive constituents obtained using these methods by means of their scavenging activity on one free radical could be insufficient because of their different scavenging capacity on different free radicals. Therefore, it is necessary to develop a multi-free radical scavenging system to obtain chemical and bioactive information to evaluate the quality of CM.
Aging is a complex physiological process and the oxidative stress theory of aging has gained considerable support [18].Numerous studies indicate that reactive oxygen species (ROS), such as O •−2 , H2O2, and peroxynitriteanion (ONOO−), are involved in the aging process and cause oxidative damage [19–22]. Antioxidative activity may be one index of the anti-aging effect. The anti-aging effect of HSW has been studied in pharmacological experiments [4, 23], but the search for anti-aging constituents is time-consuming, particularly because the content of constituents from different habitats varies markedly. Therefore, the selection of characteristic chemical markers using the HPLC–DAD–CL method may be a faster way of comprehensively evaluating the quality of HSW.
This study aims to investigate the antioxidant profile of processed HSW by HPLC–DAD–CL combined with chemometrics to rapidly screen potential anti-aging constituents of processed HSW by scavenging three reactive species (O •−2 , H2O2, and ONOO−).
Methods
Materials and reagents
HPLC grade acetonitrile was obtained from Tedia (Tedia Company Inc., USA). Luminol (Fluka Chemie Buchs, Switzerland), hydrogen peroxide solution (30 % H2O2 in water), sodium nitrite (NaNO2), sodium carbonate (Na2CO3), sodium bicarbonate (NaHCO3), and hydrochloric acid (HCl) were all purchased from Nanjing Chemical Regent Corporation (Jiangsu, China). Pyrogallol was obtained from Zunyi Second Chemical Corporation (Guizhou, China). Ethylenediaminetetraacetic acid was supplied by Shanghai Chemical Reagent Corporation (Shanghai, China). Sodium hydroxide (NaOH) and manganese dioxide (MnO2) were purchased from Xilong Chemical Corporation (Guangzhou, China). The reagents used were all of analytical grade. The purified water used was prepared by a Millipore water purification system (Millipore, Bedford, MA, USA).
Preparation of samples
Fourteen batches of processed HSW samples were purchased from different drug stores. The habitats of samples were as follows: Guangdong (20110901, S1), Shanxi (20110702, S2), Hebei (20080526, S3), Guizhou (20080323, S4), Yunnan (20090327, S5), Anhui (20061228, S6), Guangdong (20090705, S7), Hubei (20110523, S8), Sichuan (20110419, S9), Sichuan (20091216, S10), Henan (20120615, S11), Guangxi (20120530, S12), Guizhou (20121129, S13), and Hunan (20120803, S14). All samples were authenticated by Professor Bo-Yang Yu based on their morphological features according to the Chinese Pharmacopoeia [24]. Their voucher specimens were deposited at the Department of Complex Prescription of CM, China Pharmaceutical University, Nanjing, China. Processed HSW samples were ground in a grinder producing a 60-mesh particle size powder. Each sample (1.0 g) was accurately weighed and extracted twice with 50 mL methanol for 30 min in an ultrasonic bath. Then, the extract was vacuum filtered each time. Extraction solutions were mixed together after cooling and evaporated under vacuum at 40 °C. The residue was diluted with methanol (10 mL).The sample solution was further filtered through a 0.22-µm membrane filter prior to injection into the HPLC–CL system.
Preparation of CL solutions
Carbonate buffers (pH 10.0 and 11.0; 0.1 M) were prepared by mixing appropriate volumes of 0.1 M Na2CO3 and 0.1 M NaHCO3 solution. A 1.8 × 10−2 M stock solution of luminol was prepared in a 0.1 M Na2CO3 solution and stored in a refrigerator for at least 3 days before dilution. A 1.0 × 10−2 M stock solution of pyrogallol was prepared in a 0.1 mM HCl solution and then stored in a dark bottle at 4 °C. Reagent solutions for the determination of H2O2, and O •−2 scavenging activity were prepared according to previous work conducted by our research group [15]. Peroxynitrite was prepared according to a previous method [25] and we used an online peroxynitrite scavenging detection method developed by our group [26]. Reagent solutions for the determination of peroxynitrite scavenging activity were as follows: a 9 × 10−6 M luminol solution (Solution I) was prepared with 0.1 M carbonate buffer solution (pH 9, NaHCO3: Na2CO3 = 9:1) and a 4.97 × 10−4 M peroxynitrite solution (Solution II) was prepared with a 0.1 M NaOH solution on an ice bath.
HPLC–DAD–CL analysis condition
The HPLC system used was a Shimadzu LC-2010C HT system consisting of a quaternary pump, an autosampler (Shimadzu Corporation, Japan), a thermostated column compartment (Shimadzu Corporation, Japan), and a DAD (SPD-M20A, Shimadzu Corporation, Japan). HPLC separation was achieved with a Venusil MP C18 column (250 × 4.6 mm, 5 μm, Agela Technologies, China) with a flow rate of 1.0 mL/min. The column temperature was set at 30 °C. The mobile phase was composed of A (acetonitrile) and B (0.1 % aqueous phosphoric acid, v/v). The gradient elution program was as follows: 0–6 min, isocratic gradient 6 %A; 6–10 min, linear gradient 6 %–10 %A; 10–30 min, linear gradient 10 %–20 %A; 30–70 min, linear gradient 20 %–50 %A; 70–75 min, linear gradient 50 %–60 %A; 75–85 min, isocratic gradient 60 %A.
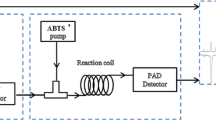
A BPCL ultraweak bioluminescence system (Academia Sinica Biophysics Institute, Beijing, China) was used to monitor the CL emission. The CL detector was equipped with a flat glass coil (80 µL) as detection cell and a photomultiplier operated at −800 V. The HPLC–DAD–CL detection system was interconnected with PEEK tubes (Mianyang Prochema Commercial Corporation, China). CL reagents, luminal solutions, H2O2, pyrogallol, and peroxynitrite were transported by peristaltic pumps at the same flow rate of 1.1 mL/min each. CL reagents were mixed with sample solution eluted from the HPLC detector then passed through the CL detector; the CL emission was detected by the BPCL system (Fig. 2). Resveratrol is a strong, natural antioxidant, therefore, the overall antioxidant activities for processed HSW were evaluated using resveratrol as a positive control (a similar method has been reported in the literature [15]). The activity of the antioxidant constituents in processed HSW was proportional to the intensity of negative peak and evaluated with the scavenging rate (%), as shown in the following Eq. (1):
where CL0 is the baseline intensity of CL (without sample) and CL1 is the inhibited CL intensity of every compound in the extracts.
HPLC–ESI–MS analysis condition
An Agilent 1100LC/MSD Trap XCT ESI (Agilent Technologies, MA, USA) was used to obtain information about the structure of the constituents in processed HSW. The mobile phase for HPLC–MS was the same as described for the HPLC analysis conditions but the composition of the B pump substituted 0.1 % aqueous phosphoric acid for 0.1 % aqueous formic acid. The ESI–MS spectra were acquired in negative ionization mode. Capillary voltage was 3300 V. Drying gas temperature was set at 350 °C with a flow rate of 9.0 L/min and nebulizing pressure was set at 275.8 kPa. Data were processed using the 6300 Series HPLC/MSD Trap and Data Analysis 3.4 (Agilent Technologies, MA, USA).
Data analysis of chromatographic profiles and ROS scavenging activity of processed HSW samples
The characteristic constituent in processed HSW samples was selected as the reference peak to calculate relative retention time (RRT) and relative peak area (RPA). The relative standard deviations (RSDs) of RRT and RPA for each common peak were calculated to estimate precision, repeatability, and stability. The method precision was evaluated using intraday and interday variation tests. The intraday variation test was evaluated using five replicate injections of the same sample and the interday variation test was evaluated over 3 days with five replicate injections each day. Method repeatability was analyzed for the six replicate samples. The stability of the sample solution was evaluated at different time points in one day (0, 2, 4, 8, 12, and 24 h).
We used a similarity evaluation system for chromatographic fingerprints of CM (Version 2004 A) to evaluate the similarity of the samples. Six samples from different places were prepared. The similarities between the chromatograms of the samples from different origins were examined by comparing the samples with the reference fingerprint that was generated by the median value of all the chromatograms.
Statistical analysis
Hierarchical cluster analysis (HCA) was performed by SPSS statistics software (SPSS for Windows 11.5, SPSS Inc., Chicago, USA) was used to sort the samples into different groups. The distance between the samples was used to assess the similarities among processed HSW samples. Samples with high similarity were clustered into homogenous groups. Principal components analysis (PCA) is an effective method to determine the main factors in large amounts of data by feature extraction and dimensionality reduction. PCA analysis of the fingerprint data of processed HSW was performed using SIMCA-P 12.0 software (Umetrics AB, Umea, Sweden).
Data were expressed as the mean ± standard deviation (SD). The statistical significance of differences between means was established by One-way ANOVA with Turkey post hoc tests. Significant differences and highly significant differences were classed as values of P < 0.05 and values of P < 0.01, P < 0.001, respectively.
Results and discussion
HPLC fingerprint method
Trans-2,3,5,4′-tetrahydroxy-stilbene-2-O-β-d-glucoside (peak 9) was selected as the reference peak. The RSDs of RRT and RPA for the precision of each common peak ranged from 0.51 to 2.33 % (n = 6) in the intraday variation test, respectively, and ranged from 0.83 to 3.97 % (n = 5) in the interday variation test, respectively. The RSDs for the repeatability of RRT and RPA of each common peak ranged from 0.9 to 4.1 % (n = 5), respectively. The RSDs of the stability test were below 2.8 % (n = 6) (Additional file1).
Identification of the main constituents in processed HSW by HPLC–ESI–MS
The structures of compounds in processed HSW were identified by comparing retention time and MS data with previous findings [13, 27–30]. The structures of these constituents were further confirmed using available reference standards, including gallic acid, protocatechuic acid, catechin, epicatechin, trans-2,3,5,4′-tetrahydroxy-stilbene-2-O-β-d-glucoside, emodin, physcion-8-O-β-d-glucoside, and emodin-8-O-β-D-glucoside (Additional file 2). The MS data are shown in Table 1.
Antioxidant activity of processed HSW samples
The standard potency equation of Ln(y) and the scavengingrate (%) (x) for resveratrol scavenging O •−2 , H2O2, and ONOO− in different concentrations were obtained. The equations for resveratrol scavenging the H2O2, O •−2 , and ONOO− were, respectively, as follows: Ln(y) = 1E − 05x3 − 0.0015x2 + 0.1083x − 6.775(R2 = 0.9997, P < 0.001), Ln(y) = 2E − 05x3 − 0.0032x2 + 0.2001x − 6.0478 (R2 = 0.9992, P < 0.05), Ln(y) = 1E − 05x3 − 0.0022x2 + 0.1373x − 6.3504(R2 = 0.9973, P < 0.001). If resveratrol (1 µg) was a potency unit, the relative total activities should be the sums of peak potency. The relative total activities of peaks in 14 batches of processed HSW were calculated according to Eq. (1). The results are shown in Tables 2, 3 and 4. Compared with S1 and S4 samples,the antioxidative activities of S10 and S11 in scavengin ROS were relatively low (P < 0.001) and the antioxidative activities of samples S2 and S12 exhibited insignificant differences (P = 1). The S2 and S12 samples had strong antioxidative activities, particularly in scavenging O •−2 and H2O2. The qualities and antioxidative effects of S7 in scavenging O •−2 , H2O2 and ONOO− were significantly different from S1, although the two samples were from Guangdong province (in scavenging O •−2 : P = 0.037; in scavenging H2O2 and scavenging ONOO−: P < 0.001). The same was true for samples S4 and S13. Compared with S4, the antioxidative effects of S13 were obviously weak in scavenging O •−2 , H2O2 and ONOO− (P < 0.001). The detailed comparison data was shown in Additional file 3. Different processing technology may have caused the variation of chemical compositions and differences in bioactivity. This indicates the importance of the standardization of processing technology.
In the reactive species scavenging test, stilbene glucosides, including cis-2,3,5,4′-tetrahydroxy-stilbene-2-O-β-d-glucoside (peak 8), trans-2,3,5,4′-tetrahydroxy-stilbene-2-O-β-d-glucoside (peak 9), and 2,3,5,4′-tetrahydroxy-stilbene-2-O-β-d-(2′′-galloyl)-glucoside (peak 11), were sensitive in scavenging three reactive species, and trans-2,3,5,4′-tetrahydroxy-stilbene-2-O-β-d-glucoside exhibited significant antioxidant activity because of its high content in processed HSW. Gallic acid (peak 1), protocatechuic acid (peak 3), catechin (peak 5), and torachrysone-8-O-glucoside (peak 12) exhibited scavenging activity on H2O2, O •−2 , or ONOO− (Fig. 3a–d). Compared with other components, epicatechin (peak 6), emodin-8-O-β-d-glucoside (peak 13), and emodin (peak 16) demonstrated higher selectivity in scavenging ONOO− than on other two reactive species, especially emodin. These constituents had different scavenging capacities on different ROS and could affect different aspects of the aging process, such as directly scavenging free radicals, decreasing the oxidation of nucleic acid and nitration of protein tyrosine residues, and inhibiting apoptosis. In addition, peak 4 had no antioxidant activity, and peaks 17 and 18 showed certain antioxidant activity in the ROS scavenging test despite their weak ultraviolet absorption or unavailable MS messages. The relationship between constituents of processed HSW and the corresponding free radicals are summarized in Fig. 4.
The HPLC fingerprints (a) of processed HSW samples from different habitats, H2O2 scavenging profiles (b) of processed HSW samples, O •−2 scavenging profiles (c) of processed HSW samples and ONOO−scavenging profiles (d) of processed HSW samples (refer to Table 1 for peak numbering)
Gallic acid, protocatechuic acid, epicatechin, catechin, trans(cis)-2,3,5,4′-tetrahydroxy-stilbene-2-O-β-d-glucoside, torachrysone-8-O-glucoside, emodin-8-O-β-d-glucoside, and emodinall exhibit pharmacological effects on the age-related pathological process [31–35]. In particular, trans(cis)-2,3,5,4′-tetrahydroxy-stilbene-2-O-β-d-glucoside, torachrysone-8-O-glucoside, and emodin-8-O-β-d-glucoside all significantly promote hair growth [4]. These constituents were selected as chemical markers for the quality control of processed HSW in this study. Among the constituents, cis-2,3,5,4′-tetrahydroxy-stilbene-2-O-β-d-glucoside, torachrysone-8-O-glucoside, and 2,3,5,4′-tetrahydroxy-stilbene-2-O-β-d-(2′′-galloyl)-glucoside were screened and proposed as chemical markers for the quality control of processed HSW. To the best of our knowledge, this is a novel research finding.
HPLC fingerprints and similarities of samples from different origins
Similarities of samples
The relationship among samples was ascertained by comparing the similarity of the samples chromatographic fingerprint series. The relationship between sets of chromatographic fingerprints was analyzed by comparing the similarity between the objects and the reference fingerprints. The similarity values of 14 batches of samples (S01–S14) were 0.989, 0.990, 0.990, 0.989, 0.982, 0.995, 0.995, 0.986, 0.970, 0.870, 0.905, 0.989, 0.945, and 0.996 (n = 6), respectively. Overall, the processed HSW samples were very similar. Compared with other samples, the similarity indexes of samples S10 and S11 were relatively lower. The different habitats and processing technology could have caused variation in the content or chemical compositions of processed HSW [14, 36].
Results of HCA
Peak 1, peak 3, peak 5, peak 6, peak 8, peak 9, peak 11, peak 12, peak 13, and peak 16 were the antioxidants in all processed HSW samples. Peak 17 and peak 18 were not examined for weak chemical messages. HCA analysis, was performed by the SPSS statistics software. The samples of processed HSW could be classified into two clusters despite high similarity: samples S10, S11, S5, S9, S6, S8, S14, S7, S13, and S3 were in Cluster 1; samples S2, S12, S1, and S4 were in Cluster 2. The HCA results were similar to the HPLC similarity index results (Fig. 5). The data indicated that these selected constituents were the chemical markers for the quality control of processed HSW.
Results of PCA
The PCA analysis produced a classification of samples similar to that produced by the HCA results (Fig. 6a). S1, S3, S5, S6, S7, S8, S9, S10, S11, S13, and S14 were classified into group 1; S2, S4, and S12 were classified into group 2. The results of the PCA loading plot indicated that protocatechuic acid, catechin, trans-2,3,5,4′-tetrahydroxy-stilbene-2-O-β-d-glucoside, 2,3,5, 4′-tetrahydroxy-stilbene-2-O-β-d-(2′′-galloyl)-glucoside, torachrysone-8-O-glucoside, and emodin-8-O-β-d-glucoside had relatively large influences on the difference between processed HSW samples (Fig. 6b). In addition to the common chemical markers used in the quality control of processed HSW, orachrysone-8-O-glucoside and 2,3,5,4′-tetrahydroxy-stilbene-2-O-β-d-(2′′-galloyl)-glucoside appeared to be chemical markers for the quality control of processed HSW.
Conclusion
Our study established the antioxidative activity-integrated fingerprint for processed HSW and achieved the potential anti-aging constituents using the online HPLC–DAD–CL method with H2O2, O •−2 , and ONOO−scavenging experiments.
Abbreviations
- HSW:
-
roots of Polygonum multiflorum Thunb.
- HPLC–DAD–CL:
-
high-performance liquid chromatography with diode array detector coupled with chemiluminescence detection
- CM:
-
Chinese medicine
- UHPLC-LTQ-Orbitrap MS:
-
UHPLC with linear ion trap-Orbitrap tandem mass spectrometry
- RSDs:
-
the relative standard deviations
- HCA:
-
hierarchical cluster analysis
References
Li SZ. Compendium of materia medica. Beijing: China Medical Science Press; 2011. p. 673–5.
Um MY, Choi WH, Aan JY, Kim SR, Ha TY. Protective effect of Polygonum multiflorum Thunb. on amyloid β-peptide 25-35 induced cognitive deficits in mice. J Ethnopharmacol. 2006;104:144–8.
Park HJ, Zhang NN, Park DK. Topical application of Polygonum multiflorum extract induces hair growth of resting hair follicles through upregulating Shh and β-catenin expression in C57BL/6 mice. J Ethnopharmacol. 2011;135:369–75.
Sun YN, Cui L, Li W, Yan XT, Yang SY, Kang JI l, Kang HK, Kim YH. Promotion effect of constituents from the root of Polygonum multiflorum on hair growth. Bioorg Med Chem Lett. 2013;23:4801–5.
Lv LS, Shao X, Wang LY, Huang DR, Ho CT, Sang SM. Stilbene glucoside from Polygonum Multiflorum Thunb.: a novel natural inhibitor of advanced glycation end product formation by trapping of methylglyoxal. J Agr Food Chem. 2010;58:2239–45.
Yao WJ, Fan WJ, Huang C, Zhong H, Chen XF, Zhang W. Proteomic analysis for anti-atherosclerotic effect of tetrahydroxy stilbene glucoside in rats. Biomed Pharmacother. 2013;67:140–5.
Zhang JK, Yang L, Meng GL, Fan J, Chen JZ, He QZ, Chen S, Fan JZ, Luo ZJ, Liu J. Protective effect of tetrahydroxystilbene glucoside against hydrogen peroxide-induced dysfunction and oxidative stress in osteoblastic MC3T3-E1 cells. Eur J Pharmacol. 2012;689:31–7.
Yao S, Li Y, Kong LY. Preparative isolation and purification of chemical constituents from the root of Polygonum multiflorum by high-speed counter-current chromatography. J Chromatogr A. 2006;1115:64–71.
Chen LL, Huang XJ, Li MM, Ou GM, Zhao BX, Chen MF, Zhang QW, Wang Y, Ye WC. Polygonflavanol A, a novel flavonostilbene glycoside from the roots of Polygonum Multiflorum. Phytochem Lett. 2012;5:756–60.
Han DQ, Zhao J, Xu J, Peng HS, Chen XJ, Li SP. Quality evaluation of Polygonum Multiflorumin China based on HPLC analysis of hydrophilic bioactive compounds and chemometrics. J Pharm Biomed Anal. 2013;72:223–30.
He DX, Chen B, Tian QQ, Yao SZ. Simultaneous determination of five anthraquinones in medicinal plants and pharmaceutical preparations by HPLC with fluorescence detection. J Pharm Biomed Anal. 2009;49:1123–7.
Zhu ZW, Li J, Gao XM, Amponsem E, Kang LY, Hu LM, Zhang BL, Chang YX. Simultaneous determination of stilbenes, phenolic acids, flavonoids and anthraquinones in Radix Polygonimultiflori by LC–MS/MS. J Pharm Biomed Anal. 2012;62:162–6.
Qiu XH, Zhang J, Huang ZH, Zhu DY, Xu W. Profiling of phenolic constituents in Polygonum multiflorum Thunb.by combination of ultra-high-pressure liquid chromatography with linear ion trap-Orbitrap mass spectrometry. J Chromatogr A. 2013;1292:121–31.
Liang ZT, Chen HB, Yu ZL, Zhao ZZ. Comparison of raw and processed radix Polygoni multiflori (Heshouwu) by high performance liquid chromatography and mass spectrometry. Chin Med-UK. 2010;5:29–37.
Ding XP, Qi J, Chang YX, Mu LL, Zhu DN, Yu BY. Quality control of flavonoids in Ginkgo biloba leaves by high-performance liquid chromatography with diode array detection and on-line radical scavenging activity detection. J Chromatogr A. 2009;1216:2204–10.
He WH, Liu X, Xu HG, Gong Y, Yuan F, Gao YX. On-line HPLC-ABTS screening and HPLC-DAD-MS/MS identification of free radical scavengers in Gardenia (Gardenia jasminoides Ellis) fruit extracts. Food Chem. 2010;123:521–8.
Zhang YT, Li Q, Xing H, Lu XF, Zhao LS, Qu KK, Bi KS. Evaluation of antioxidant activity of ten compounds in different tea samples by means of an on-line HPLC–DPPH assay. Food Res Int. 2013;53:847–56.
Sohal RS, Svensson I, Brunk UT. Hydrogen peroxide production by liver mitochondria in different species. Mech Ageing Dev. 1990;53:209–15.
Bergamini E, Cavallini G, Donati A, Gori Z. The role of macroautophagy in the ageing process, anti-ageing intervention and age-associated diseases. Int J Biochem Cell Biol. 2004;36:2392–404.
Moreira PI, Nunomura A, Nakamura M, Takeda A, Shenk JC, Aliev G, Smith MA, Perry G. Nucleic acid oxidation in Alzheimer disease. Free Radic Bio Med. 2008;44:1493–505.
Page MM, Robb EL, Salway KD, Stuart JA. Mitochondrial redox metabolism: aging, longevity and dietary effects. Mech Ageing Dev. 2010;131:242–52.
Poulsen HE, Specht E, Broedbaek K, Henriksen T, Ellervik C, Mandrup-Poulsen T, Tonnesen M, Nielsen PE, Andersen HU, Weimann A. RNA modifications by oxidation: a novel disease mechanism? Free Radic Bio Med. 2012;52:1353–61.
Zhou XX, Yang Q, Xie YH, Sun JY, Qiu PC, Cao W, Wang SW. Protective effect of tetrahydroxystilbene glucoside against d-galactose induced aging process in mice. Phytochem Lett. 2013;6:372–8.
Pharmacopoeia of the People’s Republic of China, Part I. The Pharmacopoeia Commission of PRC. Beijing: China Medical Science Press; 2010. p. 164–165.
Beckman JS, Chen J, Ischiropoulos H, Crow JP. Oxidative chemistry of peroxynitrite. New York: E-Publishing Inc.; 1994. p. 229–40.
Qi J, Chen YH, Wang Y, Chen X, Wang L, Hu YJ, Yu BY. Screening of peroxynitrite scavengers in Flos Lonicerae by using two new methods, an HPLC-DAD-CL technique and a peroxynitrite spiking test followed by HPLC-DAD analysis. Phytochem Anal. 2015; in press.
Ye M, Han J, Chen HB, Zheng JH, Guo D. Analysis of phenolic compounds in Rhubarbs using liquid chromatography coupled with electrospray ionization mass spectrometry. J Am Soc. 2007;18:82–91.
Chen WS, Liu WY, Yang GJ, Zhang WD, Chu ZY, Chen HS, Qiao CZ. Structural elucidation of a new tetrahydroxystilbene of Radix Polygoni multiflori preparata and study on its cardiovascular activity. Acta Pharm Sin. 2000;35:906–8.
Kim HK, Choi YH, Choi JS, Choi SU, Kim YS, Lee KR, Kim YK, Ryu SY. A new stilbene glucoside gallate from the roots of Polygonum multiflorum. Arch Pharm Res. 2008;31:1225–9.
Ashihara H, Deng WW, Mullen W, Crozier A. Distribution and biosynthesis of flavan-3-ols in Camellia sinensis seedlings and expression of genes encoding biosynthetic enzymes. Phytochemistry. 2010;71:559–66.
Wang WG, He YR, Lin P, Li YF, Sun RF, Gu W, Yu J. Zhao RH. In vitro effects of active components of Polygonum Multiflorum Radix on enzymes involved in the lipid metabolism. J Ethnopharmacol. 2014;153:763–70.
Haute GV, Eduardo C, Squizania E, Mesquita FC, Pedrazza L, Martha BA, Melo DA, Cassel E, Czepielewski RS, Bitencourt S, Goettert MI, Oliveira JR. Gallic acid reduces the effect of LPS on apoptosis and inhibits the formation of neutrophil extracellular traps. Toxicol In Vitro. 2015;30:309–17.
Song Y, Cui T, Xie N, Zhang XY, Qian ZB, Liu JY. Protocatechuic acid improves cognitive deficits and attenuates amyloid deposits, inflammatory response in aged AβPP/PS1 double transgenic mice. Int Immunopharmacol. 2014;20:276–81.
Prince PSM. (−) Epicatechin attenuates mitochondrial damage by enhancing mitochondrial multi-marker enzymes, adenosine triphosphate and lowering calcium in isoproterenol induced myocardial infarcted rats. Food Chem Toxicol. 2013;53:409–16.
Ma J, Zheng L, Deng T, Li CL, He YS, Li HJ, Ping Li. Stilbeneglucoside inhibits the glucuronidation of emodin in rats through the down-regulation of UDP-glucuronosyltransferases 1A8: application to a drug–drug interaction study in Radix Polygoni Multiflori. J Ethnopharmacol. 2013;147:335–40.
Zheng W, Zhang M, Zhang CF, Wang ZT. Study on quality evaluation for 36 Samples of Radix Polygonui Multiflori by HPLC-fingerprints. Chin Pharm J. 2006;41:257–60.
Authors’ contributions
HFC, YHC, JQ and BYY designed the study. HFC conducted the experiments. HFC,CHL, LW and XC wrote the manuscript. All authors read and approved the final manuscript.
Acknowledgements
This study was financially supported by National Natural Science Foundation of China (No. 81274004), 2011' Program for Excellent Scientific and Technological Innovation Team of Jiangsu Higher Education and the Priority Academic Program Development of Jiangsu Higher Education Institutions.
Competing interests
The authors declare that they have no competing interests.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Chen, H.F., Chen, Y.H., Liu, C.H. et al. Integrated chemometric fingerprints of antioxidant activities and HPLC–DAD–CL for assessing the quality of the processed roots of Polygonum multiflorum Thunb. (Heshouwu). Chin Med 11, 18 (2016). https://doi.org/10.1186/s13020-016-0087-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13020-016-0087-8