Abstract
Background
An inadequate donor left atrial cuff is a rare technical issue after graft procurement for lung transplantation. With regard to the shortage of suitable donor organs for lung transplantation, these organs should be surgically reconstructed to avoid the loss of an organ and a futile intervention in the critically ill recipient.
Case presentation
We report a case of a 62-year old patient who underwent bilateral sequential lung transplantation for chronic obstructive pulmonary disease. During isolated lung procurement, the right inferior pulmonary vein was circumferentially transsected and separated from the right superior pulmonary and middle lobe veins. Subsequently, a reconstruction of the left atrial cuff with an acellular biological patch was performed to complete the atrium anastomosis. The patient experienced an uneventful postoperative recovery and a follow-up ventilation/perfusion scan showed normal perfusion of the right lower lobe.
Conclusions
This case demonstrates that reconstruction of an inadequate left atrial cuff with a biological patch is feasible and allows for an adequate venous drainage and therefore normal transplant organ function.
Similar content being viewed by others
Background
In adequately selected patients with end-stage chronic pulmonary disease, lung transplantation offers a valuable treatment option [1]. A standard procedure involves a single anastomosis between the donor left atrial cuff and the clamped recipient left atrium. On rare occasions surgeons may encounter an inadequate donor left atrial cuff for a safe anastomosis, despite proximal placement of the Satinsky clamp on the recipient left atrium. This may either be due to anatomical abnormalities of the donor pulmonary vasculature, or due to technical problems during procurement or back-table preparation [2]. Especially when the heart is harvested beforehand, the pulmonary venous confluence may be left with only little or no surrounding atrial tissue [3]. Cases with a severely deficient left atrial cuff may even enforce a discontinuation of the transplant procedure. The implanting surgeons may therefore be confronted with the situation to repair the left atrial cuff in order to continue the transplantation.
Here we report the case and technique of a bilateral lung transplantation with inadequate donor left atrial cuff due to an accidentally transected lower lobe vein. This part is reconstructed with an acellular biological patch (bovine pericardium). For the review of clinical records and the publication of this case, an informed consent was obtained from the patient.
Case presentation
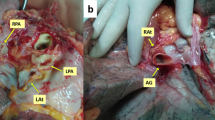
A 62 year old male patient with chronic obstructive pulmonary disease GOLD grade 4, centrilobular lung emphysema, normal alpha-1 antitrypsin levels and oxygen therapy during exertion was listed for bilateral lung transplantation. He had a smoking history of 80 pack years, but had stopped smoking 6 years before listing. A video-assisted lung volume reduction surgery 4 years prior to listing had resulted in a significant improvement in the patient’s exercise capacity and forced expiratory volume in one second (FEV1). The beneficial effect lasted for 2 years, until a further decrease in his pulmonary function occurred. Before listing, forced vital capacity (FVC) was 1.45L (38% predicted), FEV1 was 0.47L (16% predicted) and FEV1/FVC was 32%. An examination by right heart catheter revealed a mild precapillary pulmonary hypertension with a mean pulmonary artery pressure of 34 mmHg. One month after listing, a suitable donor organ from a 75-year old non-smoker was allocated. A chest computed tomography (CT) of the donor showed normal parenchymal structure and bronchoscopy revealed a clear bronchial tree. The graft procurement was performed according to a standard procedure [4]. No heart procurement was performed. During lung procurement, the atrial cuff around the orifice of the right inferior pulmonary vein was inadvertently cut too short leading to a retraction of the remaining vein into the pulmonary hilum (Fig. 1A).
Upon arrival at the implant site, the lung block was carefully inspected on the back table and the pulmonary veins were flushed with preservation fluid in a retrograde fashion. The right inferior pulmonary vein was circumferentially amputated, separated from the right superior pulmonary vein and retracted into the hilum. Since the inadequate size of the donor left atrial cuff was not noticed during procurement, no additional pericardial or atrial donor tissue was brought along for the reconstruction of the atrial cuff. While a reconstruction with allogeneic pericardial tissue would have been preferred, our reconstruction was therefore performed using an acellular, biological patch (Supple Peri-Guard®, Lamed, Germany). The patch was cut out centrally to match the pulmonary vein’s orifice and sutured to the remaining cuff using a 4–0 polypropylene running suture. The biological patch was then folded into a cone-shaped form to match the wider diameter of the recipient left atrium. In a second step, cone’s superior margin was sutured to the superior vein’s atrial cuff, thereby creating a neoatrial cuff (Figs. 1B and 2). The bilateral lung transplantation was then completed in a usual fashion [5] and the patient was transferred to the intensive care unit, where he was extubated on the 1st postoperative day. Following reconstruction, a high-dose prophylactic anticoagulation (anti-Factor Xa between 0.2 and 0.3 IU/ml) with unfractioned heparin was continued for 6 weeks. A ventilation/perfusion (V/Q) scan performed before discharge (day 29) showed a symmetrical perfusion of the donor lung (Fig. 3). Apart from an acute-on-chronic renal failure that improved upon conservative measurements and did not require renal replacement therapy, the patient made an uneventful recovery and was discharged home on the 30th postoperative day.
Discussion and conclusions
An inadequate donor left atrial cuff is a rare technical issue after graft procurement for lung transplantation. Our case demonstrates that reconstruction of an inadequate cuff by biological patch repair is feasible in such cases. Various degrees of left atrial cuff insufficiency and reconstruction techniques have previously been described by Oto et al. in 2006 [2]. In our case, the lower pulmonary vein was circumferentially amputated. A cone-shaped neoatrial cuff was created by suturing a centrally cut out biological patch to the orifice, thereby successfully avoiding a right lower lobectomy. While biological grafts are commonly used in the field of cardiovascular surgery, their application in lung transplantation has rarely been reported [2]. As an alternative, donor pulmonary artery remnant or a donor pericardial patch can be used to create additional length and diameter of the venous cuff, [2]. In our case however, the defect was too large to be reconstructed by the remnant donor pulmonary artery. Nevertheless, it can be valuable to retain residual donor pericardium, pulmonary artery or parts of the superior vena cava during the back table preparation in case of a reconstruction. Further options to overcome the challenge of an insufficient cuff include the use of the donor pericardium surrounding the pulmonary venous confluence as a “pericardial skirt” [3, 6] or on the left side, a direct implantation into the left atrial appendage [7]. After reconstruction, signs of congestion should be closely monitored. Intraoperatively, the implanted lung should be assessed for darker discoloration or increasing consolidation. A constrained oxygenation or rising pulmonary arterial pressure can be suggestive for a venous obstruction and should be investigated by transesophageal echocardiography to distinguish reduced pulmonary venous flow from signs of cardiac failure or fluid overload [2]. Postoperatively, a contrast-enhanced CT or V/Q-scan can confirm an open anastomosis. Due to the transient renal insufficiency, a V/Q scan was preferred to verify the patency of the right inferior pulmonary vein in our case. Considering the high flow rate at the atrial anastomosis and since bovine pericardial repair does not require permanent anticoagulation, sub-therapeutic anticoagulation was administered for 6 weeks.
In order to avoid potential technical errors during graft procurement, a standardized protocol for donor lung procurement should be followed. A recently published consensus statement by the International Society for Heart and Lung Transplantation (ISHLT) addresses the standardization in the procurement process and particularly the surgical technique [4]. The consensus statement furthermore emphasizes the importance of an early evaluation of the organ and prompt communication of the findings to the transplant center [4].
Considering the shortage of suitable donor organs for lung transplantation, it is important to consider every donor organ for transplantation, even when facing technical difficulties. This case exemplifies a way of overcoming the challenge of an insufficient left atrial cuff by reconstruction with an acellular biological patch. The technique allows the formation of a neo-atrial cuff which can then be safely anastomosed to the recipient left atrium. Postoperative assessment by V/Q scan confirmed an unrestricted patency of the venous drainage in the affected lobe. On a second note, this case emphasizes the importance of careful inspection and evaluation of the donor lung immediately after procurement and early communication with the recipient implant team.
Availability of data and materials
Not applicable.
Abbreviations
- FEV1:
-
Forced expiratory volume in 1 s
- FVC:
-
Forced vital capacity
- CT:
-
Computed tomography
- V/Q scan:
-
Ventilation/perfusion scan
References
Chambers DC, Cherikh WS, Harhay MO, Hayes D, Hsich E, Khush KK, et al. The international thoracic organ transplant registry of the international society for heart and lung transplantation: thirty-sixth adult lung and heart-lung transplantation report-2019; focus theme: donor and recipient size match. J Heart Lung Transpl. 2019;38:1042–55.
Oto T, Rabinov M, Negri J, Marasco S, Rowland M, Pick A, et al. Techniques of reconstruction for inadequate donor left atrial cuff in lung transplantation. Ann Thorac Surg. 2006;81:1199–204.
Casula RP, Stoica SC, Wallwork J, Dunning J. Pulmonary vein augmentation for single lung transplantation. Ann Thorac Surg. 2001;71:1373–4.
Copeland H, Hayanga JWA, Neyrinck A, MacDonald P, Dellgren G, Bertolotti A, et al. Donor heart and lung procurement: a consensus statement. J Heart Lung Transpl. 2020;39:501–17.
Inci I, Schuurmans MM, Boehler A, Weder W. Zurich University hospital lung transplantation programme: update 2012. Swiss Med Wkly. 2013;143:w13836.
Yarbrough WM, Bates MJ, Deuse T, Tang DG, Robbins RC, Reitz BA, et al. Alternative technique for salvage of donor lungs with insufficient atrial cuffs. Ann Thorac Surg. 2009;88:1374–6.
Massad MG, Sirois C, Tripathy S, Jaffe HA, Snow N, Geha AS. Pulmonary venous drainage into the left atrial appendage facilitates transplantation of the left lung with difficult exposure. Ann Thorac Surg. 2001;71:1046–7.
Acknowledgements
We thank Carol Chris De Simio-Hilton for her contribution in the form of medical art work.
Funding
No funding was received.
Author information
Authors and Affiliations
Contributions
Conceptualization by II. Writing and original draft preparation by RSW and CC. Review and editing by all authors. Supervision and administration by II and IO. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
For the review of clinical records and the publication of this case, a written informed consent was obtained from the patient.
Competing interests
Raphael S. Werner, Claudio Caviezel and Ilhan Inci have no competing interests. Isabelle Opitz has the following disclosures: Roche (speaker’s fee and institutional grant), Medtronic (institutional grant), AstraZeneca (Advisory Board), BMS (Advisory Board).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Werner, R.S., Caviezel, C., Opitz, I. et al. Donor neo-atrial cuff construction after accidental lower lobe vein transection. J Cardiothorac Surg 17, 251 (2022). https://doi.org/10.1186/s13019-022-02013-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13019-022-02013-3