Abstract
Background
Several studies have suggested that the addition of iPACK block (the popliteal artery and the posterior knee capsule have been given interspace local anesthetic infiltration) might get better analgesia than adductor canal block (ACB) only after total knee arthroplasty (TKA). This paper compiles all available evidence on the effect of two analgesia regimens (ACB and iPACK + ACB) involving all sides.
Methods
We searched in eight major databases for all clinical trials discussing the effect of two analgesia regimens after TKA. Statistical analyses were conducted by Stata and RevMan Software. In addition, we performed GOSH analysis, subgroup analysis, meta-regression analysis to study the source of heterogeneity. Publication bias was checked using Egger’s test. Trim-and-fill analysis was applied in terms of sensitivity analysis of the results.
Results
There are fourteen eligible studies for our meta-analysis. There are significant differences between the two groups in VAS score at rest and with activity, and the VAS scores were lower in the ACB + iPACK Group (VAS scores at rest: 95%CI [− 0.96, − 0.53], P < 0.00001. VAS scores with activity: 95%CI [− 0.79, − 0.43], P < 0.00001). A differential was discovered to support the ACB + iPACK Group when comparing the two groups on postoperative cumulative morphine consumption (95%CI: [− 0.52, − 0.14], P: 0.0007). The patients in the group of ACB + iPACK performed better in the postoperative range of knee movement (95%CI: [5.18, 10.21], P < 0.00001) and walking distance (95%CI: [0.15, 0.41], P < 0.00001). There were significant differences between the patients in the ACB + iPACK Group and ACB Group on the TUG test of POD1 and POD2. We found that patients' hospital stays in the ACB + iPACK Group were significantly shorter than in the ACB Group (95%CI: [− 0.78, − 0.16], P: 0.003). No difference was found between the patients in the ACB + iPACK Group and ACB Group on postoperative quadriceps muscle strength and the incidence of PONV.
Conclusion
The addition of iPACK lowers postoperative VAS scores, cumulative morphine consumption, and hospital stays. Meanwhile, the addition of iPACK improves postoperative patients’ activity performance without extra side effects. iPACK combined with ACB proves to be a suitable pain management technique after TKA.
Similar content being viewed by others
Introduction
Total knee arthroplasty (TKA) refers to a viable treatment asymptomatic osteoarthritis of the knee refractory to conservative measures. According to the estimated project by the year 2030, 3.48 million TKAs will have been conducted on a yearly basis [1]. However, relieving postoperative pains, following total knee arthroplasty is of vitally important to the postoperative recovery of the patients.
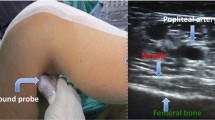
Currently, ultrasound-guided adductor canal blocks (ACBs) perform an adjunct with multimodal pain protocol on the patients with TKA effectively minimize postoperative pain and narcotic consumption [2]. Adductor canal block (ACB) is ever contributing an approach to femoral nerve block after TKA. ACB is usually conducted under ultrasound machines and local anesthetic is injected nearby the saphenous nerve in the adductor canal [3, 4]. However, ACB cannot lead to a relieved posterior knee pain [5, 6]. The recent ultrasound technique instructed local anesthetic infiltration of the interspace between the popliteal artery and the posterior knee capsule (iPACK block) has offered dramatic posterior knee analgesia. Accordingly, a number of clinic doctors chose to make additional iPACK block to ACB to control postoperative pain. The ultrasound transducer is laid for identifying the femoral condyle. After identifying the popliteal artery, the tip of needle is placed at the right middle part in the bone and the popliteal artery. Local anesthetic is injected at that spot [7]. But it is controversial that whether iPACK block should add to the analgesia regimen (ACB included) in the patients after TKA.
This research aims at a systematical review over the literature for ascertaining if iPACK block is able to take in extra analgesic advantage for present multimodal analgesia regimens after TKA. We compiled all available evidence on the effect of these two analgesia regimens (ACB and iPACK + ACB) involving postoperative pain score, postoperative muscle strength, postoperative rehabilitation training, and perioperative adverse effects.
Methods
Inclusion and exclusion criteria
This research provides a system-based review based meta-analysis oriented with randomized controlled trials (RCTs) for comparing the effects which two analgesia regimens (ACB and iPACK + ACB) can exert after TKA. The published contents abide by the PRISMA Statement [8].
The PICO framework formulated the review question. The research illustrated the discussion on (Population) adult patients who had TKA (Intervention vs. Comparator) in combination to the analgesia regimen iPACK + ACB versus with the analgesia regimen ACB and measure (Outcome) postoperative pain score, postoperative muscle strength, postoperative adverse effect and postoperative rehabilitation training.
Our primary outcomes include postoperative pain score at 8-h phase, 12-h phase, 24-h phase, 48-h phase and discharge (at rest and with activity), postoperative morphine consumption, postoperative quadriceps strength, postoperative range of knee movement (ROM), postoperative walk distance, Timed Up and Go (TUG) test, hospital stays, and the incidence of postoperative nausea and vomiting (PONV).
We used the visual analog scale (VAS) for the pain score. For postoperative morphine consumption, we collected postoperative consumption (mg) of each study on the first and second day after surgery and the total consumption. The hospital stays were calculated in hours. The postoperative walking distance was in meters, and the walking distance of postoperative on the first day, the second day, and accumulated in each study was collected. The incidence of PONV was based on the occurrence of nausea or vomiting symptoms. Our study separately collected the postoperative ROM on the first, second, and third day after the surgery. The postoperative ROM is based on the range of the extension and flexion of the knee. Similarly, our study collected the results of TUG tests of patients on the first day after the surgery, the second day after the surgery, and at discharge. The TUG test measures the time it takes a patient to rise from a chair, walk 3 m, and return to the same chair without physical assistance [9]. Our study collected patients' quadriceps muscle strength in each study on the first day and the second day after the surgery. And manual muscle testing scores assessed quadriceps strength, and the grading was recorded from 0 to 5 [10].
Search, selection, and data extraction
Our group investigated electronic databases, which contained the English database (PubMed, Embase, Cochrane Library, Web of Science, ClinicalTrials.gov) and the Chinese database (CNKI, WanFang Data, CQVIP). The following terms were used to search for relevant records: “iPACK,” “iPACK block,” “iPACK nerve block,” “adductor canal block,” “perioperative adductor canal block,” “adductor canal blockade,” “ACB,” “total knee replacement (TKA),” “Arthroplasties, Replacement, Knee,” “Arthroplasty, Knee Replacement,” “Knee Replacement Arthroplasties,” “Knee Replacement Arthroplasty,” “Replacement Arthroplasties, Knee,” “Knee Arthroplasty, Total,” “Arthroplasty, Total Knee,” “Total Knee Arthroplasty,” “Replacement, Total Knee,” “Total Knee Replacement,” “Knee Replacement, Total,” “Knee Arthroplasty,” “Arthroplasty, Knee,” “Arthroplasties, Knee Replacement,” “Replacement Arthroplasty, Knee,” “Arthroplasty, Replacement, Partial Knee,” “Unicompartmental Knee Arthroplasty,” “Arthroplasty, Unicompartmental Knee,” “Knee Arthroplasty, Unicompartmental,” “Unicondylar Knee Arthroplasty,” “Arthroplasty, Unicondylar Knee,” “Knee Arthroplasty, Unicondylar,” “Partial Knee Arthroplasty,” “Arthroplasty, Partial Knee,” “Knee Arthroplasty, Partial,” “Unicondylar Knee Replacement,” “Knee Replacement, Unicondylar,” “Partial Knee Replacement,” “Knee Replacement, Partial,” “Unicompartmental Knee Replacement,” “Knee Replacement, Unicompartmental.” We used the Boolean operator “OR” or “And” to connect these terms. Two experts used unified Microsoft Excel to collate the data independently. In case of inconsistencies, it was decided by the third expert.
Risk of bias (RoB) assessment
Coupled with crucial points in methodology (PH, GV, IP, and IT), the Cochrane Risk of Bias Tool was employed for rating the Risk of Bias [11]. The results of the RoB estimation were combined with findings illustrations, instead of integrating into statistical analysis. When a consensus was reached, disparities from the estimation were addressed.
Quality of evidence
Such approaches as Grading of Recommendations, Assessment, Development and Evaluation (GRADE) were employed for rating the quality featured by evidence on every outcome.
Statistical analysis
In virtue of RevMan and Stata Software, the statistical analysis was committed by an expertised statistician. Besides, evaluation was made on the pooled relative risks (RRs) based on 95% confidence intervals (CIs) over the total preliminary outcomes.
Statistical analyses were merely conducted on the condition of the availability least-wise two RCTs in each group. Because the research setting cannot make an exact match, a random effect model was implemented based on the DerSimonian–Laird estimation (11). I2 and chi2 tests, employed for qualifying statistical heterogeneity, aimed at P-values individually; P < 0.1 marked a dramatic heterogeneity [12]. When the heterogeneity was significant (I2 > 65%), we performed GOSH analysis and subgroup analysis to study the source of heterogeneity. In addition, publication bias was checked using Egger’s test. Trim-and-fill analysis was applied in terms of sensitivity analysis of the results.
Results
Identifications and characteristics of the researches
Figure 1 manifests the flowchart included in the meta-analysis of our group and elimination reasons. Finally, 14 studies were contained in the meta-analysis [13,14,15,16,17,18,19,20,21,22,23,24,25,26]. Besides, the study was featured by the summarization in Table 1. Table1 shows that these 14 clinical trial designs have many discrepancies: 1. Some experiments used general anesthesia [11, 13, 14, 16, 18, 20, 21, 24], while others adopted spinal anesthesia [12, 15, 17, 19, 22, 23]. 2. Some researchers performed nerve blocks before the surgery [11,12,13,14,15,16, 18, 20,21,22], while others performed nerve blocks after the surgery [15, 19, 23, 24]. 3. Some clinical trials used multimodal analgesia and placed postoperative analgesia pumps [12,13,14, 18, 21, 22], while others did not use it.
RoB, publication bias, and sensitivity analysis
Despite the scarcity of selective reporting, some aspects could not suit the criteria of low RoB, including random sequence generation, hidden allocation, blinding in addition to selective reporting. Three fourteenths of these researches were regarded as highly risky items. The summarization of RoB includes RCTs in Fig. 2. We performed statistical analyses, publication bias checking, and sensitivity analysis of all results. The results of the heterogeneity test, publication bias, and trim-and-fill analysis are summarized in Table 2.
ACB + iPACK versus ACB : VAS scores
Figure 3 shows the VAS scores at different postoperative phases (8 h, 12 h, 24 h, 48 h, and at discharge) in the two groups. There are significant differences between the two groups in VAS scores at rest and with activity. VAS scores at rest: SMD = − 0.75, 95%CI [− 0.96, − 0.53], I2: 94%, P < 0.00001. VAS scores with activity: SMD = − 0.61, 95%CI [− 0.79, − 0.43], I2: 76%, P < 0.00001. Both findings are obviously in favor of the group of ACB + iPACK. When we divided them into subgroups according to different phrases, there was an apparent difference between the two groups at 8 h, 12 h, 24 h, and 48 h after the surgery. However, there was no significant difference between the two groups in comparing VAS at discharge (Fig. 3). Table 2 shows some extent of publication bias on the results of VAS score at rest at 12 h and 48 h and VAS score with activity at 48 h. Sensitivity analysis was also conducted by trim-and-fill analysis, and all results are unchangeable. This analysis confirmed the stability of the results (Table 2).
Due to the significant heterogeneity of the results, we performed subgroup analysis, meta-regression, and GOSH analysis based on the 24-h VAS score at rest. The GOSH analysis showed that no matter which literature was excluded, the heterogeneity did not change significantly, and the overall effect did not change significantly before and after exclusion (Fig. 4). It shows that although the heterogeneity is significant, the results are stable.
GOSH analysis based on the 24-h VAS scores at rest (a: the heterogeneity and overall effect before exclusion; b: the heterogeneity and overall effect after excluding the study of Ping Mou [26], Sankineani [21], and Tak [19]; c: the heterogeneity and overall effect after excluding the study of Ping Mou [26]; d: the heterogeneity and overall effect after excluding the study of Sankineani [21]; and e: the heterogeneity and overall effect after excluding the study of Tak [19].)
At the same time, we divided the subgroups according to different aspects of the study design (Anesthesia Style/Nerve blocking Timing/Assisted Analgesia Mode/Area/Patients’ Age). Figure 5 shows the results of subgroup meta-analysis and meta-regression. When we did a subgroup meta-analysis, we found that these factors affect the analysis results to a certain extent. There are more obvious differences between the two groups in those studies on general anesthesia, nerve block before the operation, postoperative analgesia pump, patients older than 65 years old, and studies in Asia (Fig. 5). However, the meta-regression results show that the patients’ age is the primary source of significant heterogeneity (P = 0.042).
ACB + iPACK versus ACB : postoperative cumulative morphine consumption
Among 14 included studies, 5 studies reported on cumulative morphine consumption (Fig. 6). These studies evaluated the mean difference in postoperative cumulative morphine consumption in 219 patients under the treatment of ACB + iPACK versus 220 patients under the treatment of ACB. A differential was discovered to support ACB + iPACK Group (SMD: − 0.33, 95%CI: [− 0.52, − 0.14], P: 0.0007, I2: 0%,).
ACB + iPACK versus ACB : postoperative range of knee movement (ROM)
We included seven studies in the meta-analysis on the postoperative range of knee movement. Figure 7 shows an obvious difference between the two groups based on 1284 patients, which was obviously in favor of the group of ACB + iPACK (SMD: 7.69, 95%CI: [5.18, 10.21], P < 0.00001, I2:86%). At the same time, these studies are dissected into three subgroups by the differential time points after the surgery. There were statistically significant differences between the patients in the ACB + iPACK Group and ACB Group on ROM of POD1, POD2, and POD3 (Fig. 7).
ACB + iPACK versus ACB : TUG test
There is an obvious difference between the two groups based on 832 patients (Fig. 8). The finding was obviously in favor of the group of ACB + iPACK (SMD: − 3.63, 95%CI: [− 5.74, − 1.52], P: 0.0008, I2 = 43%). At the same time, these studies are dissected into three subgroups under the differential time points after the surgery. Four studies evaluated TUG one day after the surgery (Fig. 8). A differential was discovered to support ACB + iPACK Group (SMD: − 3.32, 95%CI: [− 5.57, − 1.06], P: 0.004, I2: 18%). Three explorations evaluated TUG on two days after the surgery (Fig. 8). There was a significant difference favoring the group of ACB + iPACK (SMD: − 6.47, 95% CI: [− 12.02, − 0.96], P: 0.02, I2: 58%,). The two groups presented no difference at discharge, according to two studies. (SMD: − 0.88, 95%CI: [− 4.20, 2.45], P: 0.61, I2: 0%) (Fig. 8).
ACB + iPACK versus ACB : postoperative walk distance
We included six studies in the meta-analysis on postoperative walk distance (Fig. 9). There is an obvious difference between the two groups based on 992 patients. The finding favors the group of ACB + iPACK (SMD: 0.28, 95%CI: [0.15, 0.41], P < 0.00001, I2: 2%) (Fig. 9). These studies are divided into three subgroups (one day/two days/cumulative walk distance after the surgery). Five studies evaluated the mean difference one day after surgery (Fig. 9). An obvious differential was found in favor of ACB + iPACK Group (SMD: 0.23 95%CI: [0.04, 0.41], P: 0.02, I2: 0%). Four studies assessed the mean difference on POD2, and no difference was found (SMD: 0.21 95%CI: [− 0.01, 0.43], P: 0.06, I2: 0%) (Fig. 9). Two studies assessed the mean difference in cumulative walk distance, and an apparent differential was found favoring the ACB + iPACK Group (SMD: 0.48, 95% CI: [0.15, 0.82], P: 0.004, I2 = 38%) (Fig. 9).
ACB + iPACK versus ACB : postoperative quadriceps muscle strength
We included four studies in the meta-analysis on postoperative quadriceps muscle strength (Fig. 10). No difference was found between the patients in ACB + iPACK Group and ACB Group, based on 640 patients (SMD: − 0.07, 95%CI: [− 0.17, 0.02], P: 0.13, I2: 0%). These researches are divided into two subgroups, and no obvious difference could be seen between the two groups in the subgroup meta-analysis.
ACB + iPACK versus ACB : hospital stays and the incidence of PONV
Among these 14 studies, six studies research patients' hospital stays (Fig. 11). There is an apparent difference between the two groups based on 444 patients supporting the ACB + iPACK Group (SMD: − 0.47, 95%CI: [− 0.78, − 0.16], P: 0.003, I2: 62%). There is no difference between the patients in ACB + iPACK Group and ACB Group on the incidence of PONV (OR: 0.62, 95%CI: [0.35, 1.09], P: 0.1, I2: 0%) (Fig. 12).
Discussion
The research expounds on the all-sides meta-analysis involving all clinical trials to investigate whether iPACK block added to ACB could improve analgesia outcomes after TKA. Despite that, a meta-analysis investigated the same question before [27]. However, it did not cover all clinical trials, and there are many other aspects this literature did not involve, which has left some questions open on these aspects. Furthermore, several different findings were found in our study. In our meta-analysis, the critical finding is that the addition of iPACK did reduce postoperative VAS scores no matter whether the patients were at rest or with activity. Furthermore, the supplement of iPACK could reduce postoperative cumulative morphine consumption. In all, we consider that the addition of iPACK can effectively reduce patients’ postoperative pain and reduce the use of postoperative morphine consumption.
ACB, which offers analgesia to the peripatellar and intra-articular aspects of the knee joint, cannot reduce posterior knee pains. As a novel technique, iPACK block mainly targets terminal branches of the sciatic nerve in the knee joint’s posterior capsule [28]. The point of injection is located in the position of the distal popliteal fossa at the level of the femoral condyle, in which the popliteal plexus is formed previous to the entry to the knee joint’s back [29, 30]. On the plane, the common peroneal nerve, which extends outward from the surface of the posterior capsule, makes an entire separation out of the tibial nerve. In addition, the functions made by the tibial nerve motor have already been primarily preserved as well [31, 32]. These studies are consistent with our results.
According to our results, the addition of iPACK did improve the activity performance in some aspects. The addition of iPACK increases the patients’ cumulative walk distance after the surgery and shorter the hospital stays of patients. Besides, the patients in the group of iPACK + ACB performed better in the TUG test and postoperative ROM. However, the two groups took on no difference in postoperative quadriceps muscle strength.
Postoperative ROM is a vital outcome evaluation index after TKA and reflects the related muscle strength of the knee [33]. TUG test and the postoperative walking distance directly reflect the mobility of lower limbs [34]. The patients in the group of iPACK + ACB performed better in these three aspects, indicating that the addition of iPACK can improve the activity performance of patients. The motor nerve of the quadriceps muscles is mainly the femoral nerve, and the iPACK block is mainly aimed at the terminal branch of the sciatic nerve in the posterior capsule of the knee joint. Thus, the addition of iPACK block does not affect the movement of the femoral nerve. Edmund Chan did a narrative review through 35 articles and mentioned that ACB and iPACK block would not increase the nerve block of quadriceps muscles, which is also consistent with our results [35].
Among our results, the heterogeneity of VAS score meta-analysis is considered significant, and we found this apparent heterogeneity does not originate from individual studies through performing GOSH analysis. At the same time, the GOSH analysis and sensitivity analysis manifest that although the heterogeneity is significant, the results are stable. That is, the addition of iPACK did reduce postoperative VAS scores no matter whether the patients were at rest or with activity. Meanwhile, the subgroup analysis shows that anesthesia style, nerve block timing, research area, patients’ age, and perioperative analgesia strategy affect the heterogeneity of the results to a certain extent. And meta-regression analysis shows that the patients’ age is the main origin of the significant heterogeneity. Besides, most results are stable and believable through performing sensitivity analysis. So iPACK block has a remarkable effect in relieving posterior knee pain with neither postoperative functional recovery nor adverse complications.
There are some limits in our study. In spite of several publications, exploring protocols and outcomes are featured by discrepancies (e.g., perioperative analgesia strategy), impeding statistical analysis and leading to considerable heterogeneity.
Conclusion
In conclusion, the study shows that iPACK integrated with ACB proves a hopefully bright technique that can upgrade pain management during the immediate postoperative period with no influence on motor activity. The addition of iPACK lowers postoperative VAS scores, cumulative morphine consumption, and hospital stays. Meanwhile, the addition of iPACK improves postoperative patients’ activity performance without extra side effects.
Data availability
All data included in this study are available upon request by contact with the corresponding author.
Abbreviations
- iPACK:
-
The popliteal artery and the posterior knee capsule have been given interspace local anesthetic infiltration
- ACB:
-
Adductor canal block
- TKA:
-
Total knee arthroplasty
- VAS:
-
Visual analog scale
- GOSH:
-
Graphical display of study heterogeneity
- POD1:
-
The first day after the surgery
- POD2:
-
The second day after the surgery
- POD3:
-
The third day after the surgery
- RCTs:
-
Randomized controlled trials
- TUG:
-
Timed Up and Go
- PONV:
-
Postoperative nausea and vomiting
- GRADE:
-
Grading of recommendations, assessment, development and evaluation
- RR:
-
Relative risk
- OR:
-
Odds ratio
- CI:
-
Confidence intervals
- RoB:
-
Risk of bias
- SMD:
-
Standard mean difference
- ROM:
-
Postoperative range of knee movement
References
Gemayel AC, Varacallo M. Total knee replacement techniques, in StatPearls. 2021, StatPearls Publishing Copyright © 2021, StatPearls Publishing LLC, Treasure Island (FL).
Deiter J, et al. Efficacy of adductor canal block protocol implementation in a multimodal pain management protocol for total knee arthroplasty. J Clin Orthop Trauma. 2020;11(1):118–21. https://doi.org/10.1016/j.jcot.2019.05.012.
Kandarian BS, et al. Updates on multimodal analgesia and regional anesthesia for total knee arthroplasty patients. Best Pract Res Clin Anaesthesiol. 2019;33(1):111–23. https://doi.org/10.1016/j.bpa.2019.02.004.
Bendtsen TF, et al. The optimal analgesic block for total knee arthroplasty. Reg Anesth Pain Med. 2016;41(6):711–9. https://doi.org/10.1097/AAP.0000000000000485.
Ilfeld BM, McCartney CJL. Searching for the optimal pain management technique after knee arthroplasty: analgesia is just the tip of the iceberg. Anesthesiology. 2017;126(5):768–70. https://doi.org/10.1097/ALN.0000000000001608.
Laoruengthana A, et al. Anterior vs posterior periarticular multimodal drug injections: a randomized, controlled trial in simultaneous bilateral total knee arthroplasty. J Arthroplasty. 2017;32(7):2100–4. https://doi.org/10.1016/j.arth.2017.02.033.
Thobhani S, et al. Novel regional techniques for total knee arthroplasty promote reduced hospital length of stay: an analysis of 106 patients. Ochsner J. 2017;17(3):233–8.
Moher D, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;3(3):e123–30.
Podsiadlo D, Richardson S. The timed “Up & Go”: a test of basic functional mobility for frail elderly persons. J Am Geriatr Soc. 1991;39(2):142–8. https://doi.org/10.1111/j.1532-5415.
Elkassabany NM, et al. The risk of falls after total knee arthroplasty with the use of a femoral nerve block versus an adductor canal block: a double-blinded randomized controlled study. Anesth Analg. 2016;122(5):1696–703. https://doi.org/10.1213/ANE.0000000000001237.
Higgins JP, et al. The Cochrane collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. https://doi.org/10.1136/bmj.d5928.
Julian PT. Higgins and sally green. Cochrane handbook for systematic reviews for interventions, Version 5.1.0. 2011. https://doi.org/10.1002/jrsm.38.
Li DH, et al. Efficacy of adductor canal block combined with additional analgesic methods for postoperative analgesia in total knee arthroplasty: a prospective, double-blind, randomized controlled study. J Arthroplasty. 2020;35(12):3554–62. https://doi.org/10.1016/j.arth.2020.06.060.
Ochroch J, et al. Analgesic efficacy of adding the IPACK block to a multimodal analgesia protocol for primary total knee arthroplasty. Reg Anesth Pain Med. 2020;45(10):799–804. https://doi.org/10.1136/rapm-2020-101558.
Ling H, Kang L, Ruiting W, Xuefeng W, Xiaoqing C. Application of ultrasound-guided adductor tube combined with IPack block in total knee arthroplasty in elderly patients. J Pract Med. 2020;36:950–3.
Patterson ME, et al. The effect of the IPACK block on pain after primary TKA: a double-blinded, prospective, randomized trial. J Arthroplasty. 2020;35(6):S173–7. https://doi.org/10.1016/j.arth.2020.01.014.
Ming L, et al. Effect of adductor tube combined with IPack block on multimodal analgesia after total knee arthroplasty. Chin J Anesthesiol. 2019;39:574–7. https://doi.org/10.3760/cma.j.issn.0254-1416.
Qiuru W, Baowei W, Jing Y, Pengde K. A randomized controlled trial of adductor block combined with popliteal artery and posterior capsular block of the knee joint for analgesia after total knee arthroplasty. Chin J Bone Jt. 2020;9:730–6. https://doi.org/10.3969/j.issn.2095-252X.
Tak R, et al. Continuous adductor canal block is superior to adductor canal block alone or adductor canal block combined with IPACK block (interspace between the popliteal artery and the posterior capsule of knee) in postoperative analgesia and ambulation following total knee arthroplasty: randomized control trial. Musculoskel Surg. 2020. https://doi.org/10.1007/s12306-020-00682-8.
Li S, Minglun W, Guixia M, Dengming M, Ping Z. Evaluation of the analgesic effect of ultrasound-guided IPack + adductor tube (ACB) combined block after total knee arthroplasty. Diabetes World. 2019;16:23–5. https://doi.org/10.3969/j.issn.1672-7851.
Sankineani SR, et al. Comparison of adductor canal block and IPACK block (interspace between the popliteal artery and the capsule of the posterior knee) with adductor canal block alone after total knee arthroplasty: a prospective control trial on pain and knee function in immediate postoperative period. Eur J Orthop Surg Traumatol. 2018;28(7):1391–5. https://doi.org/10.1007/s00590-018-2218-7.
Xingfeng Z, Hao C. Effect of ultrasound-guided adductor tube combined with IPack block combined with general anesthesia on analgesia after total knee arthroplasty. Zhejiang J Trauma Surg. 2020;25:1004–5. https://doi.org/10.3969/j.issn.1009-7147.
Yuquan L, Yanzhong C, Zhiwen Z, Yiling L, Weichang W. Effect of ultrasound-guided adductor tube combined with IPack block on anesthesia effect and prognosis of TKA patients. Med Innov China. 2020;17:66–70. https://doi.org/10.3969/j.issn.1674-4985.
Vichainarong C, et al. Analgesic efficacy of infiltration between the popliteal artery and capsule of the knee (iPACK) block added to local infiltration analgesia and continuous adductor canal block after total knee arthroplasty: a randomized clinical trial. Reg Anesth Pain Med. 2020;45(11):872–9. https://doi.org/10.1136/rapm-2020-101396.
Et T, et al. Comparison of iPACK and periarticular block with adductor block alone after total knee arthroplasty: a randomized clinical trial. J Anesth. 2022. https://doi.org/10.1007/s00540-022-03047-6.
Mou P, et al. Adductor canal block combined with IPACK block for postoperative analgesia and function recovery following total knee arthroplasty: a prospective, double-blind, randomized controlled study. J Arthroplasty. 2022;37(2):259–66. https://doi.org/10.1016/j.arth.2021.10.004.
Wang F, Ma W, Huang Z. Analgesia effects of IPACK block added to multimodal analgesia regiments after total knee replacement: a systematic review of the literature and meta-analysis of 5 randomized controlled trials. Medicine (Baltimore). 2021;100(22):e25884. https://doi.org/10.1097/MD.0000000000025884.
Gong WY, et al. Novel lateral approach for ultrasound-guided IPACK block. Anaesth Crit Care Pain Med. 2021;40(3):100863. https://doi.org/10.1016/j.accpm.2021.100863.
Tran J, et al. Evaluation of the iPACK block injectate spread: a cadaveric study. Reg Anesth Pain Med. 2019. https://doi.org/10.1136/rapm-2018-100355.
Niesen AD, et al. Interspace between popliteal artery and posterior capsule of the knee (IPACK) injectate spread: a cadaver study. J Ultrasound Med. 2019;38(3):741–5. https://doi.org/10.1002/jum.14761.
Kampitak W, et al. Optimal location of local anesthetic injection in the interspace between the popliteal artery and posterior capsule of the knee (iPACK) for posterior knee pain after total knee arthroplasty: an anatomical and clinical study. Korean J Anesthesiol. 2019;72(5):486–94. https://doi.org/10.4097/kja.19060.
Wang Q, et al. Efficacy of single-shot adductor canal block combined with posterior capsular infiltration on postoperative pain and functional outcome after total knee arthroplasty: a prospective, double-blind, randomized controlled study. J Arthroplasty. 2019;34(8):1650–5. https://doi.org/10.1016/j.arth.2019.03.076.
Mutsuzaki H, et al. Target range of motion for rehabilitation after total knee arthroplasty. J Rural Med. 2017;12(1):33–7. https://doi.org/10.2185/jrm.2923.
Bohannon RW. Reference values for the timed up and go test: a descriptive meta-analysis. J Geriatr Phys Ther. 2006;29(2):64–8. https://doi.org/10.1519/00139143-200608000-00004.
Chan E, et al. Infiltration between the popliteal artery and the capsule of the knee (IPACK) block in knee surgery: a narrative review. Reg Anesth Pain Med. 2021;46(9):784–805. https://doi.org/10.1136/rapm-2021-102681.
Acknowledgements
Not applicable
Author information
Authors and Affiliations
Contributions
NB contributed to the research conception and design; MH and GS contributed to data extraction; and JG and MH contributed to data analysis and interpretation. Each author contributed important intellectual content during manuscript drafting or revision and accepts personal accountability for the overall work and agrees to ensure that questions pertaining to the accuracy or integrity of any portion of the work are appropriately investigated and resolved. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Guo, J., Hou, M., Shi, G. et al. iPACK block (local anesthetic infiltration of the interspace between the popliteal artery and the posterior knee capsule) added to the adductor canal blocks versus the adductor canal blocks in the pain management after total knee arthroplasty: a systematic review and meta-analysis. J Orthop Surg Res 17, 387 (2022). https://doi.org/10.1186/s13018-022-03272-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13018-022-03272-5