Abstract
Background
Osteoarthritis (OA) is a complex joint disease and chronic pain source, affecting a patient’s quality of life and posing a financial burden. As the dog is considered a nearly ideal species for translation research of human OA and the most used model for research, exploring spontaneous dog OA under the One Health/One Medicine concept can improve both humans and dogs’ health and well-being.
Methods
In a clinical treatment experiment, forty (N=40) joints were selected and randomly assigned to a control group (CG), which received 0.9% NaCl or a treatment (HG), which received Hylan G-F 20. Evaluations were performed on treatment day (T0), 8, 15, 30, 90, and 180 days post-treatment. They consisted of four different Clinical Metrology Instruments (CMI), evaluation of weight distribution, joint range of motion, thigh girth, radiographic and digital thermography imaging, synovial fluid interleukin-1 (IL-1), and C-reactive protein concentrations. Results were compared with repeated measures ANOVA, with a Huynh-Feldt correction, Paired samples T-test, or Wilcoxon signed-ranks test, with p<0.05.
Results
Patients had a mean age of 6.5±2.4 years and a bodyweight of 26.6±5.2kg, and joints graded as mild (n=28, 70%), moderate (n=6, 15%), and severe OA (n=6, 15%). No differences were found between groups at T0. Symmetry index and deviation showed significant improvements in HG from 30 days (p<0.01) up to 180 days (p=0.01). Several CMI scores, particularly pain scores, improved from 90 to 180 days. Radiographic signs progressed in both groups. In both groups, increasing body weight and age corresponded to worse clinical presentation. IA hyaluronan administration produced increased lameness in six cases, which resolved spontaneously.
Conclusions
This study characterizes the response to treatment with Hylan G-F 20, which can produce significant functional and pain level improvements in patients with OA, even those with factors related to worse response to treatment.
Similar content being viewed by others
Background
Osteoarthritis (OA) is a highly prevalent disease worldwide, which affects all mammals and a leading cause of disability. It can negatively impact both the population’s physical and mental well-being, with substantial healthcare resources and costs associated with managing the disease [1, 2]. The dog is an ideal species to study human OA, with the advantages of being anatomically, biochemically, genomically, and molecularly similar to humans, with clinical progression and treatment similarities [3]. At the same time, they have a foreshortened lifespan but with human equivalent life and disease stages while sharing many environmental variations that influence human OA. The study of spontaneous canine OA and its treatment can add to the knowledge of the treatment of the human disease as well, under the One Medicine initiative [4, 5].
OA is an incurable condition, and its management focuses on alleviating symptoms, particularly pain. An additional goal is to improve overall joint function while slowing down disease progression [5, 6]. Hyaluronan, the high molecular glycosaminoglycan, is synthesized by chondrocytes and synovial fibroblasts [7]. It forms the backbone of proteoglycans aggregates interwoven with collagen to create hyaline cartilage’s unique structure [8]. Information from animal models shows that endogenous hyaluronan is cleaved by free radicals in OA. Its quantity and quality are affected in OA joints, more severely in clinically affected patients, supporting its exogenous administration [9]. Even though its mechanism of action is not entirely understood and clinical trials have provided contradictory results, hyaluronan treatment aims to reduce pain and improve function by supplementing synovial fluid viscosity and elasticity [10]. Additional anti-inflammatory, anti-nociceptive, and chondroprotective properties have been suggested, through the enhancement of cartilage synthesis, blunting response to IL-1, protection from the damage of oxygen free radicals, and protection of chondrocytes from apoptosis [7, 11].
Human reports show that intra-articular hyaluronan, given once weekly for 3 weeks, increased mobility and reduces pain and the need for nonsteroidal anti-inflammatory drugs to control pain [12]. A systematic review concluded that there is a lack of standardization regarding intra-articular hyaluronan administrations for hip OA, with no consensus on its efficacy [13]. Although it is not clear if any formulation has a superior disease-modifying effect [14], high molecular weight products seem to produce better results, particularly in patients with mild radiographic disease [15]. A recent report showed that both single or 1–3 weekly injections of Hylan G-F 20 at 1 year following the first injection for knee OA are efficacious and generally well tolerated for long-term use [16]. Many studies performed in canine experimental OA models have failed to demonstrate clear benefits of hyaluronan supplementation [17]. In a canine surgical model, IA hyaluronan provided clinically significant improvement in pain, function, lameness, and kinetics compared to pre-treatment and saline control, without preventing OA’s progression [18]. In a rabbit model, hyaluronan administration produced a more normal cartilage after immobilization [19]. In dogs with naturally occurring OA, treatment groups have significantly better results than a control group by the 6th week post-treatment [20].
Multiple agents influence OA catabolism, but interleukin 1 (IL-1) is commonly pointed out as the major pro-inflammatory cytokine [21]. C-reactive protein (CRP) is an acute-phase protein produced during inflammatory reactions or tissue injury from an early stage [22].
Radiographic examination is a staple in OA’s assessment, and the ventrodorsal (VD) hip extended view is the most commonly used projection. An additional useful projection is the ventrodorsal flexed view, also called frog-legged view (FL), specifically in the evaluation of the circumferential femoral head osteophyte (CFHO) and caudolateral curvilinear osteophyte (CCO), early radiographic signs related to the development of the clinical symptoms [23]. Digital thermal imaging relies on the between physiologic functions generated heat and its relation with skin temperature control, being reliable in assessing inflammatory arthritis pain and osteoarthritic subjects [24]. Functional evaluation is also paramount in determining response to treatment in OA, and stance analysis has been reported as a sensitive evaluation for detecting lameness in dogs [25]. It evaluates weight distribution since patients commonly bear less weight on a painful limb [26]. An additional functional evaluation includes determining activity levels and mobility impairments since they are associated with musculoskeletal pain [27]. Pedometers are capable of measuring ambulatory activity with acceptable accuracy [28]. Clinical examination of patients commonly includes evaluating muscle masses, muscular atrophy being a consistent finding in OA patients, and determining the joint range of motion (ROM, flexion, and extension), which can present restrictions [29].
Pain is a hallmark of OA, and canine studies offer valuable data that may translate to humans [30, 31]. For pain evaluation and its impact on patients’ lives, several clinical metrology instruments (CMI) have been developed. The Liverpool Osteoarthritis in Dogs (LOAD) and the Canine Brief Pain Inventory (CBPI) are the most commonly used [27], with the CBPI being divided into a pain severity score (PSS) and a pain interference score (PIS) [32]. The Canine Orthopedic Index (COI, divided into four scores: stiffness, gait, function, and quality of life (QOL) and the Hudson Visual Analogue Scale (HVAS), developed to assess the degree of lameness in dogs, are additional validated evaluation tools [33, 34]. Digital thermal imaging is a technique that has recently gained attention. It relies on heat generated during physiologic functions and its relation with skin temperature control [35]. It has been used to assess inflammatory arthritis pain and differentiate normal from osteoarthritis subjects [24].
This study aimed to describe the effect of a high molecular weight hyaluronan product (Hylan G-F 20) in OA management in a naturally occurring canine model. We hypothesize that a single administration will reduce clinical signs of OA compared with a control group.
Methods
The study protocol was approved by the ethical review committee of the Universidade de Évora (ORBEA, approval n° GD/32055/2018/P1, September 25, 2018) and complies with the ARRIVE guidelines. Written informed consent was obtained from the Institution responsible for the animals. Twenty patients with naturally occurring bilateral hip OA, constituting a convenience sample, were signaled from a population of active police working dogs, comprising forty (N=40) hips joints. The diagnosis was made based on history, physical, orthopedic, neurological, and radiographic examinations. Additional inclusion criteria included a bodyweight of ≥ 20kg, age ≥2 years, and they should not have received any medication or nutritional supplements for at least 6 weeks. Patients with other suspected or documented orthopedic or concomitant disease and not tolerant of data collection were excluded.
Treatment administration
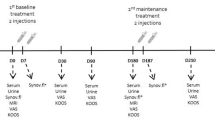
In a double-blinded study, patients were randomly assigned using the statistical analysis software to two groups, 10 dogs per group, and treated bilaterally: a control group (CG, n=20), which received an intra-articular (IA) administration of 2ml of 0.9%NaCl, and a treatment group (HG, n=20), which received a single IA administration of 2ml of Hylan G-F 20 (Synvisc®, Sanofi, Portugal). Radiographic examinations and IA administrations were conducted under light sedation, using a combination of medetomidine (0.01mg/kg) and buthorphanol (0.1mg/kg), both given intravenously simultaneously. The procedure for intra-articular administrations to the hip joint has been described before [36]. The patient was positioned in lateral recumbency, with the affected joint uppermost, to access the joint of interest. A window of 4 × 4cm surrounding the greater trochanter was clipped and aseptically prepared. An assistant then positioned the limb in a neutral and parallel to the table position. The joint space was accessed using a 21-gauge with 2.5″ length needle, introduced just dorsal to the greater trochanter and perpendicular to the limb’s long axis. Correct needle placement was confirmed by collecting synovial fluid (immediately collected and processed for future analysis), and the treatment or saline were administered. Patients were rested for three consecutive days following treatment, after which normal activity was resumed over 5 days. One and 3 days after the IA procedure, animals were examined by the assisting veterinarian for signs of exacerbated pain, persistent stiffness of gait, and changes in posture exhibited. Evaluations were conducted on days 0 (treatment day), 8, 15, 30, 90, and 180 by the same researcher. An outline of procedures and evaluations conducted in each evaluation moment is presented in Table 1.
Evaluation of weight-bearing distribution
The weight distribution evaluation was performed with a weight distribution platform (Companion Stance Analyser; LiteCure LLC®, Newark, DE, USA). According to the manufacturer’s guidelines, the equipment was placed in the center of a room, at least 1 m from the walls. It was calibrated at the beginning of each day and zeroed before each data collection. After this procedure, animals were placed with one foot in each quadrant of the platform, using gentle restraint when required. A left-right symmetry index (SI) was calculated with the following formula: SI=[(WBR-WBL)/((WBR+WBL) × 0.5)]×100 (WBR is the weight-bearing of the right limb, and WBL is the weight-bearing of the left limb). Negative values were made positive [37]. We additionally considered a deviation from normal 20% weight-bearing for a pelvic limb, calculated by subtracting WB to 20.
Digital thermography imaging
Digital thermography evaluation was conducted in a room with a controlled temperature, set at 21°C. Previous to collecting the images, animals were allowed to walk around the room for 30 min. They were then placed in an upright standing position, and a dorsoventral thermographic image was obtained, including the area from the last lumbar vertebra to the first coccygeal vertebra, at a distance of 60cm [38], FLIR ThermaCAM E25® model (FLIR Systems, Wilsonville, OR, USA). Images were analyzed using the free software Tools (FLIR Systems, Inc), with a rainbow color pallet. Boxes of equal size were placed on the hip joint’s anatomical area on both views to determine mean and maximal temperatures.
Radiographic evaluation
In the VD radiographic projection [23], seven radiographic signs were assessed: irregular wear on the femoral head, making it misshapen and with a loss of its rounded appearance; a flattened or shallow acetabulum, with irregular outline; CCO; new bone formation on the acetabulum and femoral head and neck; a worn away angle formed at the cranial effective acetabular rim; subchondral bone sclerosis along the cranial acetabular edge; and CFHO.
Clinical and laboratorial findings
Thigh girth was determined with a Gullick II measuring tape, at a distance of 70% thigh length, measured from the greater trochanter’s tip, with an extended leg [39]. Hip joint ROM was obtained with a goniometer at extension and flexion with a flexed stifle [40]. Pedometers were worn around the patient’s neck, attached to an adjustable lightweight collar [41]. Pedometers were worn for 1 week before the first evaluation moment to set a baseline value. For each of the following evaluations, animals worn the pedometer for a week before that evaluation moment. A mean daily count was calculated by dividing the registered number of steps by the number of considered days. In each evaluation moment, trainers completed a copy of HVAS, CBPI, COI, and LOAD after receiving the published instructions for each of them. They were completed sequentially by the same handler, in a quiet room, with as much time as needed to answer all items. From the synovial sample collected, IL 1β and CRP concentrations were determined with the DuoSet Ancillary Canine IL-1β Reagent kit (R&D Systems, UK), read with a FLUOstar OPTIMA (BMG Labtech), and Fuji Dri-Chem Slides VC-CRP PS (FUJIFILM Europe GmbH), read with a DRIChem NX500i (FUJIFILM Europe GmbH), respectively.
Data analysis
Normality was assessed with a Shapiro-Wilk test. Results were compared between groups in each of the evaluation moments. To evaluate the effect of different parameters on patients’ clinical evolution, results were compared by sex, body weight, age, and different radiographic findings with repeated measures ANOVA, with a Huynh-Feldt correction, paired samples T-test, or Wilcoxon signed-ranks test. A Kaplan-Meier test was performed to evaluate the time to return to baseline values of SI and CMI scores, compared with the Breslow test. All results were analyzed with IBM SPSS Statistics version 20, and a significance level of p<0.05 was set.
Results
This study sample comprised 40 joints of active police working dogs, with a mean age of 6.5±2.4 years, a mean bodyweight of 26.6±5.2kg, and of both sexes (13 males and 7 females). Dogs were of breeds commonly employed in police forces, similarly distributed between CG and TH: German Shepherd Dogs (n=6, 3 in CG and 3 in TH), Labrador Retriever (n=6, 3 in CG and 3 in TH), Belgian Malinois Shepherd Dogs (n=5, 3 in CG and 2 in TH), and Dutch Shepherd Dog (n=3, 2 in CG and 1 in TH). At the initial evaluation, joints were graded with the OFA hip grading scheme as mild (n=28, 70%), moderate (n=6, 15%), and severe (n=6, 15%). No differences were found between groups at the initial evaluation. Increased lameness was observed in 6 joints HG, which spontaneously resolved within 48–72h.
Clinical and CMI results
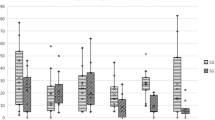
Values recorded for different evaluations in each group at T0 are presented in Table 2. Comparing results between groups with repeated measures ANOVA with a Huynh-Feldt correction, significant differences between groups were found concerning deviation (F(5, 160)=3.7, p=0.004), SI (F(3.6, 114.4)=3.6, p=0.011), mean temperature on a DV view (F(3.9, 103.1)=4.8, p=0.001), maximal temperature on a DV view (F(3.9, 101.7)=4.4, p=0.003), mean temperature on a Lt view (F(5, 140)=36.3, p<0.001), maximal temperature on a Lt view (F(4.8, 133.3)=86.7, p<0.001), joint flexion (F(4.2, 130.5)=18.4, p<0.001), and IL-1 synovial concentration (F(2.4, 85.8)=5.3, p=0.004). Significant differences were also observed with different CMI, specifically PSS (F(5, 140)=2.8, p=0.021), PIS (F(2.7, 75.1)=3.4, p=0.026), Function (F(5, 140)=2.6, p=0.026), Gait (F(5, 140)=2.3, p=0.044), and COI (F(5, 140=2.2, p<0.05). The evolution of SI in CG and HG is presented in Fig. 1. Results of the Kaplan-Meier test are presented in Table 3. Kaplan-Meier curves for stiffness score and PIS are presented in Figs. 2 and 3, respectively.
Radiographic evaluations
The frequency of different radiographic findings at the initial and final evaluations is presented in Table 4. Cases without CFHO on a VD view in the CG, on the first assessment, had a better joint extension at the 8-day evaluation (p<0.01) and better HVAS (p=0.02), PSS (p=0.01), and PIS scores (p=0.03). At 15 days, they had a higher mean thermographic evaluation on a Lt view (p=0.02), better PSS (p=0.02), and PIS scores (p<0.05). The higher mean thermographic evaluation on a Lt view was also observed at 30 days (p=0.01). At 90 days, these joints had better HVAS scores (p=0.02). At the final evaluation, they had higher maximal thermographic evaluation on a Lt view (p=0.04) and better PSS (p=0.05) and PIS scores (p<0.03). In the HG, cases without CFHO had higher thigh girth (p=0.03). At 8 days, they had higher body weight (p<0.01), lower deviation (p<0.01), lower mean and maximal thermographic evaluation on a DV (p=0.02 and p=0.04, respectively) and mean on a Lt view (p<0.02), and higher thigh girth (p=0.01). At 15 days, these joints had lower deviation (p=0.03), lower mean, and maximal thermographic evaluation on a DV (p=0.03 for both) and maximal on a Lt view (p<0.05). At 30 days, they had a higher thigh girth (p<0.01). At the 90-day evaluation moment, they had better deviation (p=0.02), a finding again observed at 180 days (p<0.05).
Evaluations by sex
In the CG, female dogs had significantly lower body weight in all evaluation moments (p=0.01). At the initial evaluation, females had higher values in all thermographic evaluations (p<0.01) and lower PIS scores (p=0.04). At 8 days, the same was true regarding thermographic evaluation (p<0.01), except maximal value on a Lt view and higher joint extension values (p<0.01). At 15 days, females still showed higher joint extension (p=0.04) and lower PIS scores (p=0.03). At the 30 days’ evaluation, females showed higher thermographic maximal values on an LT view max (p<0.01). At 90 days, female dogs had lower thigh girth (p=0.03) and better PSS and PIS scores (p=0.01). In the final evaluation moment, female dogs had higher extension values (p=0.02) and better HVAS (p=0.02), PSS (p<0.01), PIS (p<0.01), stiffness (p=0.02), function (p=0.02), gait (p<0.01), QOL (p=0.02), and COI (p=0.01) scores. In the HG, at the initial evaluation, females had lower pedometer counts (p=0.02), better deviation (p=0.02) and SI (p<0.05), higher mean and maximal values on a Lt view (p=0.02 and p<0.01, respectively), and lower thigh girth (p<0.01). At the 8-day evaluation moment, females had higher mean and maximal thermographic values on the DV view and mean value on a Lt view (p<0.01 for all) and lower thigh girth (p<0.01). At 15 days, females had lower pedometer counts (p=0.04), still had mean and maximal thermographic values on the DV, and mean value on a Lt view (p<0.01 for all), lower thigh girth (p<0.01), and worse joint extension (p=0.02). At 30 days, females still had mean and maximal thermographic values on the DV and mean value on a Lt view (p<0.01, p=0.01, and p<0.05, respectively), in addition to lower thigh girth (p<0.01). At the 90 days’ evaluation moment, females had worse SI (p=0.03), higher maximal thermography evaluation on a lateral (p<0.05), and synovial fluid CRP (p=0.02). At the final evaluation moment, females had better joint flexion (p=0.04) and serum higher CRP (p=0.02). They also had lower body weight throughout the study (p<0,01).
Evaluations by bodyweight
Comparing animals with a weight cut-off set at the sample’s mean value at 8 days, lighter subjects had higher thermographic mean and maximal values on a DV (p=0.03 and p=0.02, respectively), higher thigh girth (p=0.01), and worse stiffness (p=0.03), function (p<0.01), gait (p=0.03), and COI scores (p<0.01). At 15 days, lighter cases showed lower thigh girth (p=0.04) and worse HVAS (p<0.05), stiffness, function, gait QOL, and COI scores (p<0.01). Lighter animals had lower PCR concentrations at 30 days (p=0.04) and better HVAS scores (p=0.02). The same animals had lower thigh girth (p<0.01) and IL-1 levels (p=0.02) at 90 days. In the final evaluation point, lighter animals showed higher mean thermographic values on a DV view (p<0.01) and higher joint flexion (p=0.02) and extension (p<0.01). In HG, animals below the threshold had a higher mean value on a Lt view (p=0.03), lower thigh girth (p<0.01), and worse joint extension (p<0.05) on the initial evaluation. At 8 days, they had lower pedometer counts (p<0.01), worse deviation (p=0.03), higher mean temperature values on a Lt view (p<0.01), lower thigh girth (p<0.01), and worse function score (p=0.02). After 15 days, lighter subjects had lower pedometer counts (p=0.04), higher mean and maximal temperature values on the DV view (p=0.01 for both), as mean value on a Lt view (p=0.02), lower thigh girth (p<0.01), worse joint extension (p=0.02), and function score (p<0.01). At 30 days, these cases had lower thigh girth (p<0.01), worse joint extension (p<0.01), function, and QOL scores (p=0.03). At the 90-day evaluation, animals below the cut-off had worse flexion (p=0.03), higher synovial CRP concentration (p=0.04), and worse function score (p=0.03). At the final evaluation moment, lighter subjects had worse deviation (p<0.01), higher mean and maximal temperature values on the Lt view (p=0.01), and worse function score (p=0.02).
Evaluations by age
Considering cases above or below the mean age of the sample, in the CG, younger subjects had higher maximal values on the thermographic Lt view (p=0.04) and better LOAD (p=0.02), stiffness (p<0.01), function (p<0.01), gait (p<0.01), and COI (p<0.01) scores. At 8 days, they showed lower SI (p<0.01), higher maximal values on the thermographic Lt view (p=0.02), and better LOAD (p=0.04), stiffness (p<0.01), function (p<0.01), gait (p<0.01), QOL (p<0.01), and COI (p<0.01) scores. The same was also true at the 15-day evaluation, with these cases presenting better LOAD (p<0.01), stiffness (p<0.01), function (p<0.01), gait (p<0.01), QOL (p<0.01), and COI (p<0.01) scores. At the 30-day evaluation, younger subjects had lower mean and maximal values on the thermographic DV (p<0.01 and p=0.02, respectively) and Lt view (p=0.02, for the mean value), better joint flexion (p=0.01), and better LOAD (p<0.01), stiffness (p<0.01), function (p<0.01), gait (p<0.01), QOL (p<0.01), and COI (p<0.01) scores. At 90 days, the same cases had better LOAD (p=0.04), stiffness (p<0.01), function (p<0.01), gait (p<0.01), QOL (p<0.01), and COI (p<0.01) scores. At the final evaluation, younger subjects had better deviation and SI (p=0.03 and p<0.01, respectively), and stiffness (p<0.01), function (p<0.01), gait (p<0.01), QOL (p<0.01), and COI (p<0.01) scores. In the HG, younger subjects had higher pedometer counts (p<0.01), lower mean and maximal values on the thermographic DV (p<0.01 for both) and Lt view (p<0.02, for the mean value), higher thigh girth (p=0.04), and worse HVAS (p=0.02), PSS, PIS, LOAD, stiffness, and function scores (p<0.01 for all) at the initial evaluation. The same was observed at 8 days for mean and maximal values on the thermographic DV (p<0.01 for both), lower joint flexion (p<0.05), and worse HVAS, PSS, PIS, LOAD, stiffness, function, and QOL scores (p<0.01 for all). At 15 days, they had thigh girth (p=0.03) and worse HVAS, PSS, PIS, LOAD, stiffness, and gait scores (p<0.01 for all). After 30 days, these joints had lower mean and maximal values on the thermographic DV (p<0.01 for both) and Lt view (p<0.01 for both), better joint flexion (p=0.01), and better HVAS, PSS, PIS, stiffness, function, and QOL scores (p<0.01 for all). At the 90-day evaluation, again, they had lower mean and maximal values on the thermographic on the Lt view (p<0.01 for both), and better HVAS, PSS, PIS, LOAD, stiffness, function, QOL, and COI scores (p<0.01 for all). At the final evaluation, they had deviation and SI (p<0.05 and p=0.03, respectively), and better HVAS, PSS, PIS, stiffness, gait, and QOL scores (p<0.01 for all).
Discussion
Osteoarthritis is the most commonly diagnosed joint disease in human and veterinary medicine, with limited treatment options. In addition to the anatomical and biochemical similarities between dogs and humans, they also share an environment and lifestyle. For those reasons, the study of animal OA could be beneficial for both species [2, 5]. To our knowledge, this is the first study to describe the effect of a single injection of high-density hyaluronan (G-F 20) on several clinical, imaging, and laboratorial signs in a naturally occurring canine model, with a long follow-up period. Dog OA, particularly naturally occurring OA, resembles closely human OA regarding anatomy, disease heterogeneity, and progression [42].
Many studies performed in canine experimental OA models have failed to demonstrate clear benefits of hyaluronan supplementation [17]. IA hyaluronan provided clinically significant improvement in animals with stifle OA in pain, function, lameness, and kinetics compared to pre-treatment and saline control in a canine surgical model. Maximum benefits were noted at 4–8 weeks and gradually tapered down by a 6-month evaluation time point [18]. In dogs with naturally occurring OA, treatment groups have significantly better results than a control group by the 6th week post-treatment but accompanied by exercise restrictions, leading to improvements in the control group [20]. In this study, we have observed significant improvements in the HG with several evaluation modalities, which, in some cases, lasted up to the last evaluation moment, at 180 days post-treatment. These include functional improvements measured by the evaluation of weight-bearing, to improvements in other dimensions of OA, as measured with the CMIs, but particularly with the two scores of the CBPI. In addition to group improvements in HG, individual CMI scores also improved in most animals from the first evaluation post-treatment, but particularly after 15 days. This improvement is observable with the Kaplan-Meier test results for SI, with results in HG taking significantly longer to return to baseline values. It was also noticeable with different CMI scores and dimensions. Although clear anatomical similarities exist, some care must be taken when extrapolating dogs to humans. The dog, being a quadruped, supports 60% of body weight in the thoracic limbs and 40% in the pelvic limbs, which differs from the biped posture of humans, which can affect OA’s progression [43, 44].
A proposed direct analgesic effect for hyaluronan has been suggested in animal models by action over the opioid receptor [45]. An additional proposed mechanism of action for hyaluronan is producing endogenous hyaluronan production by the exogenous administration, based on in vitro and in vivo studies [46]. This last mechanism may be supported because the product is rapidly cleared from the joint, and maximal clinical improvement does not occur for several weeks, between 60 and 90 days, while persisting for much longer [47]. Our results partly support these findings, with the difference that significant improvements were reached sooner and lasted longer. Although we did not measure the amount and the persistence of the exogenous hyaluronan within the joint, the visual examination of SF in HG at the 8-day evaluation showed a clear SF, with increased viscosity that of Hylan G-F 20.
OA is a low-grade inflammatory disease, and IL-1 is the most important pro-inflammatory cytokine responsible for the catabolism in OA, affecting the disease’s progression [48], and the histopathology and pathogenesis of dog OA closely resembles that of human OA [5]. IA hyaluronan inhibits degenerative cartilage changes in animal models due mainly to its pro-inflammatory cytokines and degradative enzymes [49]. Low molecular weight hyaluronan seems to be most effective in reducing the release of cytokines [50]. Although a decrease in IL-1 levels was recorded in both groups, at 8 days, its concentration in CG was significantly lower. At this moment, this is probably due to the removal of synovial fluid at treatment day, followed by the injection of 0.9% NaCl, similar to the effect of a joint lavage, which may be more effective than the administration of hyaluronan in reducing IL-1 levels. Still, IL-1 concentration levels remained lower than those at the initial evaluation in both groups. As this study was a clinical treatment experiment, no joint histological samples were collected, which would help evaluate differences between Hylan G-F 20 and 0.9% NaCl injection. The reduction of IL-1 may reduce inflammatory levels, which are reflected in the temperature values recorded during the thermographic evaluations. Measurements made on the Lt view, in particular, recorded variations throughout the entire follow-up period, with lower levels being recorded in CG.
Pain is the most relevant clinical sign of OA, and its evaluation is paramount to determine OA treatment efficacy so that data may be translated to human medicine [30]. There is strong evidence that humans and animals’ type of pain is analogous, as they share neurophysiologic similarities [51]. However, painful experiences in OA are complex, involving several dimensions [52]. While extremely useful in a clinical setting, CMIs can be susceptible to the caregiver placebo effect, associated with the variability in emotional and cognitive components of pain perception. On the other hand, the animal itself will not show a significant placebo effect, and the ability to perform daily activities will likely reflect a lower level of pain [53, 54]. We used several CMIs, to try to capture multiple dimensions of OA. As a whole, individual CMI scores in CG tended to worsen through time, while HG scores tended to improve. Still, some animals in CG showed improvements. While some patients with OA may spontaneously improve, a more plausible explanation is related to removing cytokine-loaded SF at treatment day, followed by the injection of 0.9% NaCl, similar to the effect of a joint lavage. Placebo saline injections have shown functional improvements that can last up to a 6-month follow-up [55].
Radiographic evaluation is a staple of OA monitoring. CCO and CFHO represent early radiographic signs that predict the development of hip OA clinical signs [23]. Previous reports have described that hyaluronan could not prevent OA progression based on radiographic assessment [18]. However, it decreased signs of pain and improved joint function after the onset of OA [56]. Our results support these findings. In CG, several radiographic findings progressed throughout the follow-up period, as expected in the disease’s natural evolution. This was also observed in HG, even though some radiographic findings did only change at 180 days. Still, despite the evolution of radiographic findings, patients in HG showed better clinical, functional, and pain findings than CG. Also, in the 8–30 days’ evaluation period, no significant differences were observed in HG between animals with and without CCO and CFHO at the initial evaluation.
OA risk factors are well characterized and include having a higher bodyweight or being of older age [2]. To assess these factors’ influence in response to treatment, we applied different cut-off values for weight. In both groups, increasing body weight generally corresponded to worse CMI. In HG, heavier patients had SI evaluation and deviation, even though the group still had better results than CG throughout the study. Previous reports indicated that larger dogs achieved improvements of 30% or more at 12 weeks [47]. We described improvements earlier, even in heavier patients. Male dogs also scored worse in considered CMI, but this may be related to the fact that male dogs were significantly heavier than females in all considered moments.
Regarding age, similar results were observed. Considering animals above the sample’s mean age, these patients scoring worse on almost all CMI scores had lower pedometer counts and higher thermography values. Since OA is a chronic, progressive disease, it was not unexpected to see older patients record worse evaluations, which may be linked with the disease’s progression at its clinical signs. While expected, the difference in treatment results is quite pronounced, more than the effect of increased body weight.
IA hyaluronan administration has been described as producing mild heat, swelling, and/or erythema post-injection, which resolved spontaneously within a week [18]. These adverse effects are well tolerated and usually restricted to the injected joint [57, 58]. Similarly, we observed increased lameness in six cases, reflecting on the 8-day SI and deviation evaluations, when significantly worse scores were kept at HG. This spontaneously resolved by the 15-day evaluation. No additional medication was administered to the animals during the follow-up period. Considering the obtained results, Hylan G-F 20 may be a good therapeutic option for managing canine hip OA. Its administration was able to reduce pain levels and improve joint function compared to a control group. Due to the close resemblance of canine and human OA, it is possible that the same recommendation can be made for human hip OA. Still, as some differences in weight bearing exist between the two species, futures studies should enroll a greater number of animals and assess if similar results are observed in humans.
Conclusions
This study describes the effect of a single injection high molecular weight hyaluronan product in a naturally occurring canine model, with a long follow-up period. It provides important information for the characterization of the response to treatment, showing that Hylan G-F 20 can produce significant functional and pain level improvements in patients with OA, even those with factors related to worse response to treatment. For that reason, Hylan G-F 20 can be considered as a good therapeutic option for OA management, even in more advanced cases.
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Change history
28 April 2023
This article has been retracted. Please see the Retraction Notice for more detail: https://doi.org/10.1186/s13018-023-03806-5
Abbreviations
- CBPI:
-
Canine Brief Pain Inventory
- CMI:
-
Clinical Metrology instruments
- COI:
-
Canine Orthopedic Index
- CRP:
-
C-reactive protein
- HVAS:
-
Hudson Visual Analogue Scale
- IL-1:
-
Interleukin 1
- LOAD:
-
Liverpool Osteoarthritis in Dogs
- OA:
-
Osteoarthritis
- PIS:
-
Pain Interference Score
- PSS:
-
Pain Severity Score
- QOL:
-
Quality of life
References
Vina ER, Kwoh CK. Epidemiology of osteoarthritis. Curr Opin Rheumatol. 2018;30(2):160–7. Available from: https://journals.lww.com/00002281-201803000-00005. https://doi.org/10.1097/BOR.0000000000000479.
Anderson KL, O'Neill DG, Brodbelt DC, Church DB, Meeson RL, Sargan D, et al. Prevalence, duration and risk factors for appendicular osteoarthritis in a UK dog population under primary veterinary care. Sci Rep. 2018;8:5641 Available from: https://www.nature.com/articles/s41598-018-23940-z.
Shahid M, Manchi G, Slunsky P, Naseer O, Fatima A, Leo B, et al. A systemic review of existing serological possibilities to diagnose canine osteoarthritis with a particular focus on extracellular matrix proteoglycans and protein. Pol J Vet Sci. 2017;20(1):189–201. Available from: https://journals.pan.pl/dlibra/publication/121051/edition/105452/content. https://doi.org/10.1515/pjvs-2017-0024.
Kol A, Arzi B, Athanasiou KA, Farmer DL, Nolta JA, Rebhun RB, et al. Companion animals: translational scientist’s new best friends. Sci Transl Med. 2015;7:308ps21 Available from: https://stm.sciencemag.org/lookup/doi/10.1126/scitranslmed.aaa9116.
Meeson RL, Todhunter RJ, Blunn G, Nuki G, Pitsillides AA. Spontaneous dog osteoarthritis — a One Medicine vision. Nat Rev Rheumatol [Internet]. 2019; Available from: https://www.nature.com/articles/s41584-019-0202-1
Minnema L, Wheeler J, Enomoto M, Pitake S, Mishra SK, Lascelles BDX. Correlation of artemin and GFRα3 with osteoarthritis pain: Early evidence from naturally occurring osteoarthritis-associated chronic pain in dogs. Front Neurosci. 2020:14 Available from: https://www.frontiersin.org/article/10.3389/fnins.2020.00077/full.
Strauss EJ, Hart JA, Miller MD, Altman RD, Rosen JE. Hyaluronic acid viscosupplementation and osteoarthritis. Am J Sports Med. 2009;37(8):1636–44. Available from: https://ajs.sagepub.com/. https://doi.org/10.1177/0363546508326984.
Greenberg DD, Stoker A, Kane S, Cockrell M, Cook JL. Biochemical effects of two different hyaluronic acid products in a co-culture model of osteoarthritis. Osteoarthr Cartil. 2006;14(8):814–22. https://doi.org/10.1016/j.joca.2006.02.006.
Haapala J, Arokoski JPA, Rönkkö S, Ågren U, Kosma V-M, Lohmander LS, et al. Decline after immobilisation and recovery after remobilisation of synovial fluid IL1, TIMP, and chondroitin sulphate levels in young beagle dogs. Ann Rheum Dis. 2001;60(1):55–60. https://doi.org/10.1136/ard.60.1.55.
Gigante A, Callegari L. The role of intra-articular hyaluronan (Sinovial®) in the treatment of osteoarthritis. Rheumatol Int. 2011;31(4):427–44. Available from: https://link.springer.com/10.1007/s00296-010-1660-6.
Colen S, van den Bekerom MP, Bellemans J, Mulier M. Comparison of intra-articular injections of Hyaluronic Acid and Corticosteroid in the treatment of Osteoarthritis of the hip in comparison with intra-articular injections of Bupivacaine. Design of a prospective, randomized, controlled study with blinding. BMC Musculoskelet Disord. 2010;11:264 Available from: https://bmcmusculoskeletdisord.biomedcentral.com/articles/10.1186/1471-2474-11-264.
Frizziero A, Maffulli N, Masiero S, Frizziero L. Six-months pain relief and functional recovery after intra-articular injections with hyaluronic acid (mw 500-730 KDa) in trapeziometacarpal osteoarthritis. Muscles Ligaments Tendons J. 2014;4:256–61 Available from: https://www.ncbi.nlm.nih.gov/pubmed/25332944.
Oliva F. Viscosupplementation with intra-articular hyaluronic acid for hip disorders. A systematic review and meta-analysis. Muscles Ligaments Tendons J [Internet]. 2016; Available from: https://www.mltj.org/common/php/portiere.php?ID=a895c49383d73e94194d55f4257ccd18
Frizziero A, Vittadini F, Oliva F, Abatangelo G, Bacciu S, Bernardi A, et al. I.S.Mu.L.T. Hyaluronic acid injections in musculoskeletal disorders guidelines. Muscle Ligaments Tendons J. 2019;08:364 Available from: https://www.mltj.online/i-s-mu-l-t-hyaluronic-acid-injections-in-musculoskeletal-disorders-guidelines/.
Cheng OT, Souzdalnitski D, Vrooman B, Cheng J. Evidence-based knee injections for the management of arthritis. Pain Med. 2012;13:740–53 Available from: https://academic.oup.com/painmedicine/article-lookup/doi/10.1111/j.1526-4637.2012.01394.x.
De Lucia O, Jerosch J, Yoon S, Sayre T, Ngai W, Filippou G. One-year efficacy and safety of single or one to three weekly injections of hylan G-F 20 for knee osteoarthritis: a systematic literature review and meta-analysis. Clin Rheumatol. 2020; Available from: https://link.springer.com/10.1007/s10067-020-05477-7
Sanderson RO, Beata C, Flipo R-M, Genevois J-P, Macias C, Tacke S, et al. Systematic review of the management of canine osteoarthritis. Vet Rec. 2009;164:418–24 Available from: https://www.ncbi.nlm.nih.gov/pubmed/19346540.
Pashuck TD, Kuroki K, Cook CR, Stoker AM, Cook JL. Hyaluronic acid versus saline intra-articular injections for amelioration of chronic knee osteoarthritis: a canine model. J Orthop Res. 2016;34(10):1772–9. Available from: https://doi.wiley.com/10.1002/jor.23191.
Fu LLK, Maffulli N, Chan KM. Intra-articular hyaluronic acid following knee immobilisation for 6 weeks in rabbits. Clin Rheumatol. 2001;20(2):98–103. Available from: https://link.springer.com/10.1007/s100670170078.
Hellstrom L, Carlsson C, Boucher J, Michanek P. Intra-articular injections with high molecular weight sodium hyaluronate as a therapy for canine arthritis. Vet Rec. 2003;153(3):89–90. https://doi.org/10.1136/vr.153.3.89.
Fujita Y, Hara Y, Nezu Y, Schulz KS, Tagawa M. Proinflammatory cytokine activities, matrix metalloproteinase-3 activity, and sulfated glycosaminoglycan content in synovial fluid of dogs with naturally acquired cranial cruciate ligament rupture. Vet Surg. 2006;35(4):369–76. https://doi.org/10.1111/j.1532-950X.2006.00159.x.
Bennett D, Eckersall PD, Waterston M, Marchetti V, Rota A, Mcculloch E, et al. The effect of robenacoxib on the concentration of C-reactive protein in synovial fluid from dogs with osteoarthritis. BMC Vet Res. 2013;9(1):42. https://doi.org/10.1186/1746-6148-9-42.
Puckler K, Tellhelm B, Kirberger R. The hip joint and pelvis. In: Kirberger R, McEvoy F, editors. BSAVA Man Canine Feline Musculoskelet Imaging. Gloucester: Wiley; 2016. p. 212–31.
Fokam D, Lehmann C. Clinical assessment of arthritic knee pain by infrared thermography. J Basic Clin Physiol Pharmacol. 2019;30 Available from: https://www.degruyter.com/view/j/jbcpp.2019.30.issue-3/jbcpp-2017-0218/jbcpp-2017-0218.xml.
Clough W, Canapp S. Assessing clinical relevance of weight distribution as measured on a stance analyzer through comparison with lameness determined on a pressure sensitive walkway and clinical diagnosis. Vet Comp Orthop Traumatol. 2018;31:A1–25 Available from: https://www.thieme-connect.de/DOI/DOI?10.1055/s-0038-1668246.
Gordon-Evans WJ. Gait analysis. In: Tobias K, Johnson S, editors. Vet Surgery Small Animal. 1st ed. St. Louis: Elsevier Saunders; 2012. p. 1190–6.
Lascelles BDX, Brown DC, Maixner W, Mogil JS. Spontaneous painful disease in companion animals can facilitate the development of chronic pain therapies for humans. Osteoarthr Cartil. 2018;26(2):175–83. Available from: https://linkinghub.elsevier.com/retrieve/pii/S1063458417313298. https://doi.org/10.1016/j.joca.2017.11.011.
Tudor-Locke C, Williams JE, Reis JP, Pluto D. Utility of pedometers for assessing physical activity. Sport Med. 2002;32(12):795–808. Available from: https://link.springer.com/10.2165/00007256-200232120-00004.
Hyytiäinen HK, Mölsä SH, Junnila JT, Laitinen-Vapaavuori OM, Hielm-Björkman AK. Ranking of physiotherapeutic evaluation methods as outcome measures of stifle functionality in dogs. Acta Vet Scand. 2013;55:29 Available from: https://actavetscand.biomedcentral.com/articles/10.1186/1751-0147-55-29.
Robertson-Plouch C, Stille JR, Liu P, Smith C, Brown D, Warner M, et al. A randomized clinical efficacy study targeting mPGES1 or EP4 in dogs with spontaneous osteoarthritis. Sci Transl Med. 2019;11:eaaw9993 Available from: https://stm.sciencemag.org/lookup/doi/10.1126/scitranslmed.aaw9993.
Strasser T, Peham C, Bockstahler BA, Turmezei TD, Treece GM, Gee AH, et al. Identification of quantitative trait loci for osteoarthritis of hip joints in dogs. Am J Vet Res. 2016;52(3):369–77. Available from: https://doi.org/10.1016/j.tvjl.2014.09.022.
Upchurch DA, Renberg WC, Roush JK, Milliken GA, Weiss ML. Effects of administration of adipose-derived stromal vascular fraction and platelet-rich plasma to dogs with osteoarthritis of the hip joints. Am J Vet Res. 2016;77:940–51 Available from: https://avmajournals.avma.org/doi/10.2460/ajvr.77.9.940.
Brown DC. The Canine Orthopedic Index. Step 2: Psychometric testing. Vet Surg. 2014;43:241–6 Available from: https://doi.wiley.com/10.1111/j.1532-950X.2014.12141.x.
Hudson JT, Slater MR, Taylor L, Scott HM, Kerwin SC. Assessing repeatability and validity of a visual analogue scale questionnaire for use in assessing pain and lameness in dogs. Am J Vet Res. 2004;65(12):1634–43. Available from: https://avmajournals.avma.org/doi/abs/10.2460/ajvr.2004.65.1634.
Hildebrandt C, Zeilberger K, John Ring EF, Raschner C. The Application of Medical Infrared Thermography in Sports Medicine. An Int Perspect Top Sport Med Sport Inj [Internet]. InTech; 2012. Available from: https://www.intechopen.com/books/an-international-perspective-on-topics-in-sports-medicine-and-sports-injury/the-application-of-medical-infrared-thermography-in-sports-medicine
Alves JC, Santos A, Jorge P, Lavrador C, Carreira LM. A pilot study on the efficacy of a single intra-articular administration of triamcinolone acetonide, hyaluronan, and a combination of both for clinical management of osteoarthritis in police working dogs. Front Vet Sci. 2020:7 Available from: https://www.frontiersin.org/articles/10.3389/fvets.2020.512523/full.
Walton MB, Cowderoy E, Lascelles D, Innes JF. Evaluation of construct and criterion validity for the ‘Liverpool Osteoarthritis in Dogs’ (LOAD) clinical metrology instrument and comparison to two other instruments. Wade C, editor. PLoS One. 2013;8:e58125 Available from: https://dx.plos.org/10.1371/journal.pone.0058125.
Vainionpää M, Raekallio M, Tuhkalainen E, Hänninen H, Alhopuro N, Savolainen M, et al. Comparison of three thermal cameras with canine hip area thermographic images. J Vet Med Sci. 2012;74:1539–44 Available from: https://www.ncbi.nlm.nih.gov/pubmed/22785576.
McCarthy DA, Millis DL, Levine D, Weigel JP. Variables affecting thigh girth measurement and observer reliability in dogs. Front Vet Sci. 2018:5 Available from: https://www.frontiersin.org/article/10.3389/fvets.2018.00203/full.
Levine, D., Millis DL. Canine rehabilitation and physical therapy. 2014.
Chan CB, Spierenburg M, Ihle SL, Tudor-Locke C. Use of pedometers to measure physical activity in dogs. J Am Vet Med Assoc. 2005;226:2010–5 Available from: https://www.ncbi.nlm.nih.gov/pubmed/15989183.
Gregory MH, Capito N, Kuroki K, Stoker AM, Cook JL, Sherman SL. A review of translational animal models for knee osteoarthritis. Arthritis. 2012:1–14. Available from: https://www.hindawi.com/journals/arthritis/2012/764621/. https://doi.org/10.1155/2012/764621.
Page AE, Allan C, Jasty M, Harrigan TP, Bragdon CR, Harris WH. Determination of loading parameters in the canine hip in vivo. J Biomech. 1993;26(4-5):571–9. Available from: https://linkinghub.elsevier.com/retrieve/pii/002192909390018A. https://doi.org/10.1016/0021-9290(93)90018-A.
Clough W, Canapp S, Taboada L, Dycus D, Leasure C. Sensitivity and specificity of a weight distribution platform for the detection of objective lameness and orthopaedic disease. Vet Comp Orthop Traumatol. 2018;31(06):391–5. Available from: https://www.thieme-connect.de/DOI/DOI?10.1055/s-0038-1667063.
Zavan B, Ferroni L, Giorgi C, Calò G, Brun P, Cortivo R, et al. Hyaluronic Acid Induces Activation of the κ-Opioid Receptor. PLoS One. 2013;8:1–8.
Tamer TM. Hyaluronan and synovial joint: Function, distribution and healing. Interdiscip Toxicol. 2013;6(3):111–25. https://doi.org/10.2478/intox-2013-0019.
Carapeba GOL, Cavaleti P, Nicácio GM, Brinholi RB, Giuffrida R, Cassu RN. Intra-articular hyaluronic acid compared to traditional conservative treatment in dogs with osteoarthritis associated with hip dysplasia. Evid-Based Complement Altern Med. 2016:1–10 Available from: https://www.hindawi.com/journals/ecam/2016/2076921/.
Loeser RF, Goldring SR, Scanzello CR, Goldring MB. Osteoarthritis: a disease of the joint as an organ. Arthritis Rheum. 2012;64(6):1697–707. Available from: https://doi.wiley.com/10.1002/art.34453.
Comer JS, Kincaid SA, Baird AN, Kammermann JR, Hanson RR, Ogawa Y. Immunolocalization of stromelysin, tumor necrosis factor (TNF) alpha, and TNF receptors in atrophied canine articular cartilage treated with hyaluronic acid and transforming growth factor beta. Am J Vet Res. 1996;57:1488–96 Available from: https://www.ncbi.nlm.nih.gov/pubmed/8896690.
Neuenschwander HM, Moreira JJ, Vendruscolo CP, Fülber J, Seidel SRT, Michelacci YM, et al. Hyaluronic acid has chondroprotective and joint-preserving effects on LPS-induced synovitis in horses. J Vet Sci. 2019:20 Available from: https://synapse.koreamed.org/DOIx.php?id=10.4142/jvs.2019.20.e67.
Klinck MP, Mogil JS, Moreau M, Lascelles BDX, Flecknell PA, Poitte T, et al. Translational pain assessment. Pain. 2017;158(9):1633–46. Available from: https://insights.ovid.com/crossref?an=00006396-201709000-00004. https://doi.org/10.1097/j.pain.0000000000000978.
Eitner A, Hofmann GO, Schaible H-G. Mechanisms of osteoarthritic pain. Studies in humans and experimental models. Front Mol Neurosci. 2017:10 Available from: https://journal.frontiersin.org/article/10.3389/fnmol.2017.00349/full.
Conzemius MG, Evans RB. Caregiver placebo effect for dogs with lameness from osteoarthritis. J Am Vet Med Assoc. 2012;241:1314–9 Available from: https://www.ncbi.nlm.nih.gov/pubmed/23113523.
Piel MJ, Kroin JS, Van Wijnen AJ, Kc R, Im HJ. Pain assessment in animal models of osteoarthritis. Gene. 2014;537:184–8 Available from: https://doi.org/10.1016/j.gene.2013.11.091.
Previtali D, Merli G, Di Laura FG, Candrian C, Zaffagnini S, Filardo G. The long-lasting effects of “placebo injections” in knee osteoarthritis: a meta-analysis. Cartilage. 2020:194760352090659 Available from: https://journals.sagepub.com/doi/10.1177/1947603520906597.
Abantagelo G, Botti P, Bue M, Gei G, Samson JC, Cortivo R, et al. Intraarticular sodium hyaluronate injections in the Pond-Nuki experimental model of osteoarthritis in dogs. Clin Orthop Relat Res. 1989:278–85 Available from: https://content.wkhealth.com/linkback/openurl?sid=WKPTLP:landingpage&an=00003086-198904000-00038.
Waddell DD. Viscosupplementation with hyaluronans for osteoarthritis of the knee clinical efficacy and economic implications. Drugs Aging. 2007;24(8):629–42. https://doi.org/10.2165/00002512-200724080-00002.
Niemelä TM, Tulamo R-M, Carmona JU, López C. Evaluation of the effect of experimentally induced cartilage defect and intra-articular hyaluronan on synovial fluid biomarkers in intercarpal joints of horses. Acta Vet Scand. 2019;61:24 Available from: https://actavetscand.biomedcentral.com/articles/10.1186/s13028-019-0460-6.
Acknowledgements
The authors would like to thank Manuel Pereira for the assistance in the statistical analysis of the data, FujiFilm Europe GmbH for providing the CRP tests, Concessus and Companion, LiteCure LLC for providing Stance Analyser used in this study, and Specman, Lda, for providing the digital thermography camera.
Funding
The authors of this paper do not have any financial or personal relationship with other persons or organizations that could inappropriately influence or bias this paper’s content.
Author information
Authors and Affiliations
Contributions
JCA designed the protocol, conducted treatments, and prepared the manuscript. PJ and AS selected patients and conducted treatments. CL and LMC revised the protocol and prepared the manuscript. All authors have read and approved the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This project was approved by the ethical review committee of the University of Évora (Órgão Responsável pelo Bem-estar dos Animais da Universidade de Évora, approval n° GD/32055/2018/P1, September 25, 2018) and complies with ARRIVE guidelines. All methods were carried out in accordance with relevant guidelines and regulations. Written, informed consent was obtained from the Institution responsible for the animals (Guarda Nacional Republicana, Portuguese Gendarmerie) through dispatch of the Doctrine and Training Commander n°327/16, dated September 16, 2016.
Consent for publication
Not applicable.
Competing interests
FujiFilm Europe GmbH provided the CRP tests used in this study, the Stance Analyser was provided by Companion, LiteCure LLC®, and the digital thermography camera was provided by Specman, Lda®.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article has been retracted. Please see the retraction notice for more detail: https://doi.org/10.1186/s13018-023-03806-5
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Alves, J.C., dos Santos, A.M.M.P., Jorge, P. et al. RETRACTED ARTICLE: Effect of a single intra-articular high molecular weight hyaluronan in a naturally occurring canine osteoarthritis model: a randomized controlled trial. J Orthop Surg Res 16, 290 (2021). https://doi.org/10.1186/s13018-021-02423-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13018-021-02423-4