Abstract
Background
D-dimer, a coagulation-related indicator, has recently been used as a tool for the diagnosis of periprosthetic joint infection (PJI), but its reliability is uncertain. The purpose of this systematic review and meta-analysis was to explore the accuracy of D-dimer in the diagnosis of PJI after joint arthroplasty.
Methods
We systematically searched the MEDLINE, EMBASE, and Cochrane databases for relevant literature about D-dimer in the diagnosis of PJI. QUADAS-2 was used to assess the risk of bias and clinical applicability of each included study. We used the bivariate meta-analysis framework to pool the sensitivity, specificity, positive likelihood ratio (PLR), negative likelihood ratio (NLR), diagnostic odds ratio (DOR), and area under the SROC curve (AUC). Univariate meta-regression and subgroup analyses were performed to explore the sources of heterogeneity.
Results
We included 8 eligible studies. The pooled diagnostic sensitivity and specificity were 0.82 (95% CI, 0.70–0.89) and 0.70 (95% CI, 0.55–0.82), respectively. The pooled PLR, NLR, and DOR were 2.7 (95% CI, 1.7–4.4), 0.26 (95% CI, 0.15–0.46), and 10 (95% CI, 4–25), respectively. The AUC was 0.83 (95% CI, 0.8–0.86). Serum D-dimer might have higher diagnostic accuracy than plasma D-dimer for PJI (pooled sensitivity: 0.88 vs 0.67; pooled specificity: 0.76 vs 0.61).
Conclusions
D-dimer has limited performance for the diagnosis of PJI.
Similar content being viewed by others
Introduction
Periprosthetic joint infection (PJI) is a rare and devastating complication that affects 0.7–2.4% of patients after hip or knee arthroplasty [1,2,3]. PJI not only affects the quality of life of infected patients but also increases the risk of death [4].
Because the typical clinical manifestations of patients with PJI may not appear and pain can be caused by other diseases, PJI is difficult to diagnose. The Musculoskeletal Infection Society (MSIS) formulated diagnostic criteria for PJI and tried to reduce the incidence rate of this dreaded complication [5, 6]. In 2018, the International Consensus Meeting (ICM) modified the criteria and added D-dimer and alpha-defensin into the new definition of PJI for the knee and hip joint [7] (Table 1).
D-dimer is a specific degradation product of fibrin monomer that is crosslinked by activating factor XIII and then hydrolyzed by fibrinolytic enzyme [8]. It is a specific marker of the fibrinolysis process and mainly reflects the function of fibrinolysis [8]. A study suggested that D-dimer could be used to determine prognosis in systemic sepsis [9]. D-dimer levels continue to rise due to the host’s inflammatory response to infection in sepsis [9].
Currently, some studies have examined the diagnostic value of D-dimer for PJI, but diagnostic accuracy varies in different studies. Therefore, the purpose of this systematic review and meta-analysis was to evaluate the diagnostic accuracy of D-dimer for PJI.
Materials and methods
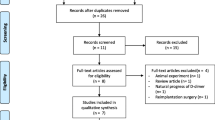
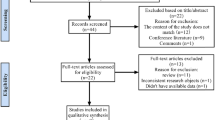
This systematic review and meta-analysis strictly followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [10] (Fig. 1).
Search strategy
We systematically searched all literature about D-dimer in the diagnosis of PJI in MEDLINE, Embase, and the Cochrane Library (from the inception of each database until November 2019), without language restrictions. The search strategies are shown in Table 2.
Eligibility criteria
We included all studies that reported the accuracy of D-dimer in the diagnosis of PJI after hip or knee arthroplasty and used the MISS or modified MISS criteria. Studies lacking sensitivity and specificity values and those that had duplicated data were excluded.
Two authors independently scanned the titles, abstracts, and full texts sequentially and screened the literature based on the eligibility criteria. The third author settled any disagreements that arose.
Data extraction
Two authors independently classified all studies and extracted data using standardized scales. We extracted all baseline data (author name, publication year, country, average age, sex distribution, BMI, joint type, patient exclusion criteria, diagnostic criteria, etc.) and outcome indicators (sensitivity, specificity, PLR, NLR, DOR, AUC, etc.). The third author resolved any disagreements that arose.
Quality evaluation
The quality of each included study was evaluated using the QUADAS-2 tool [11], which mainly includes four parts: patient selection, indicator testing, reference standard, and flow and timing. The first three parts are also needed to evaluate clinical practicability. According to the answers (“yes,” “no,” or “uncertain”) to the relevant landmark questions included in each part, the risk of bias level was determined as “low,” “high,” or “uncertain.” Two authors independently evaluated the quality, and the third author decided the final result in the event of any divergences.
Statistical analysis
We used the bivariate meta-analysis framework to pool the sensitivity, specificity, PLR, NLR, DOR, and AUC by using the “Midas” command [12]. Compared with the traditional summary ROC curve, the bivariate model is a development and expansion [13]. The joint modeling of sensitivity and specificity is used as the starting point for the analysis, and a random effects model is used [13]. Thus, the diagnostic accuracy may be more reliable with this method [14]. The I2 statistic was used to estimate the heterogeneity among studies. The value of I2 is between 0 and 100%. An I2 value of < 50% indicates low heterogeneity, while an I2 value of > 50% indicates high heterogeneity.
When there was high heterogeneity, we evaluated the threshold effect through the Spearman correlation coefficient of the logarithm of sensitivity and 1-specificity. When the P value was < 0.05, the threshold effect was considered significant. At the same time, we used univariate meta-regression to find the potential sources of heterogeneity. Then, we conducted a subgroup analysis to further investigate the source of heterogeneity. A test for publication bias (Deeks’ funnel plot) was also used to analyze the sources of heterogeneity. When the P value was < 0.05, the tests for publication bias were considered statistically significant [15].
Stata 14.0 software and Meta-DiSc 1.4 were used for data analysis.
Result
After a systematic search in the above databases, 34 studies were initially selected, and finally, 8 studies [16,17,18,19,20,21,22,23] were included according to the inclusion and exclusion criteria (Table 3). The 8 included studies were conducted in 2 countries (China and the USA) and included 1587 patients, involving 514 knee joints, 822 hip joints, and 50 extra-articular infections. A total of 457 patients were diagnosed with PJI, and the rate ranged from 17 to 45%. The average age of all the patients in the studies ranged from 61.5 to 68.9 years, with 33–53% males. All 8 studies were published in the last 3 years, and there was no patient overlap in these studies.
Four studies [16, 17, 20, 22] were prospective studies, and the other 4 studies [18, 19, 21, 23] were retrospective studies. In terms of the diagnostic threshold, 4 studies [20,21,22,23] used 850 퓊g/L, which was recommended by the ICM (2018) as the diagnostic threshold of D-dimer. Pannu et al. [23] also used 2300 ng/ml as the cut-off in their study. The remaining 4 studies [16,17,18,19] used 1250 ng/L, 1020 ng/L, 1170 ng/ml, and 760 ng/ml as the diagnostic thresholds. Four studies [21,22,23] determined the diagnostic threshold in advance, and the remaining studies [16,17,18,19,20] obtained the diagnostic threshold from the ROC curve. Three studies [18, 19, 22, 23], all from China, used plasma samples for the quantification of D-dimer, and 5 studies [16, 17, 20, 21, 23] used serum samples. Four studies [16, 17, 21, 22] excluded patients with rheumatoid arthritis, autoimmune diseases, tumors, smoker status, or obesity and the remaining 4 studies [18,19,20, 23] did not.
Quality assessment
According to the QUADAS-2 tool, we evaluated the quality of all included studies (Table 4 and Fig. 2). The risk of bias in reference standards and flow and timing was low in all studies. Six studies [16,17,18, 20,21,22] were at high risk of bias for patient selection because of inappropriate discharge standards and case-control trials. Because retrospective studies and thresholds were not set in advance in 7 studies [16,17,18,19,20,21, 23], the bias of the index test was high. All studies scored between 6 and 9 (the total score is 10 points).
Diagnostic value
The pooled diagnostic sensitivity and specificity were 0.82 (95% CI, 0.70–0.89) and 0.70 (95% CI, 0.55–0.82), respectively (Fig. 3); however, the heterogeneity between studies was obvious, with I2 values of 83.19% (95% CI, 71.75–94.64%) and 94.17% (95% CI, 91.23–97.11%). The pooled PLR, NLR, and DOR were 2.7 (95% CI, 1.7–5.4), 0.26 (95% CI, 0.15–0.46), and 10 (95% CI, 4–25), respectively (Fig. 3). The AUC was 0.83 (95% CI, 0.8–0.86) (Fig. 4). The Spearman correlation coefficient was − 0.071 (P = 0.867). The heterogeneity might be unrelated to the threshold effects.
Heterogeneity analysis
Meta-regression
We performed univariate meta-regression to search for the potential sources of heterogeneity (Fig. 5). For sensitivity and specificity, the sample differences and racial differences had the most significant impacts on the heterogeneity of the results (P < 0.05). Based on these results, we performed subgroup analysis to further explore the source of heterogeneity. When I2 < 50% or P > 0.05, we considered the heterogeneity to be low in the subgroup.
Subgroup analysis
In the subgroup of plasma D-dimer [18, 19, 22], the pooled sensitivity and specificity were 0.67 (95% CI 0.60–0.72) and 0.61 (95% CI 0.57–0.65); in the subgroup of serum D-dimer [16, 17, 20, 21, 23], the pooled sensitivity and specificity were 0.88 (95% CI 0.83–0.92) and 0.76 (95% CI 0.71–0.80). In the subgroup of East Asian races [16–19, 21, 22], the pooled sensitivity and specificity were 0.72 (95% CI 0.67–0.77) and 0.65 (95% CI 0.61–0.68); in the subgroup of Caucasian and African American races [20, 23], the pooled sensitivity and specificity were 0.92 (95% CI 0.86–0.97) and 0.74 (95% CI 0.67–0.80), respectively (Table 5).
Publication bias
The Deeks’ funnel plot asymmetry test of DOR did not show significant asymmetry (P = 0.34), indicating that publication bias might not exist (Fig. 6).
Discussion
The diagnosis of PJI after arthroplasty is a complicated problem for every orthopedist. With early diagnosis, patients can undergo debridement or conservative treatment to treat PJI and avoid 1 or 2 stage revision. Therefore, the quick and accurate diagnosis of PJI is critical. Many potential blood and synovial fluid biomarkers for the diagnosis of PJI have been evaluated, but the clinical gold standard for the diagnosis of the disease has still not been found. Therefore, it is necessary and meaningful to develop a new and accurate diagnostic method for PJI.
D-dimer is familiar to medical workers and has not been valued in the past few decades. It has only been used to screen venous thromboembolism [24, 25]. Recently, some studies showed that D-dimer was associated with inflammation and might be elevated in infected patients [26, 27]. Rodelo et al. found that higher levels of D-dimer were associated with increased 28-day mortality in septic patients [9]. In addition, D-dimer is recommended as a critical diagnostic indicator for infectious diseases such as endocarditis and mycoplasma pneumonia [28, 29]. Subsequently, D-dimer levels attracted the attention of plastic surgeons.
Shahi et al. [20] reported in his study that serum D-dimer has high diagnostic value for PJI in lower limbs, with a sensitivity and specificity of 89% and 93%, respectively, which preluded the diagnosis of PJI by D-dimer. Parvizi et al. [30] believe that the diagnosis of PJI, such as ankylosing spondylitis, rheumatoid arthritis, and endocarditis, should depend on a combination of various diagnoses, so they added D-dimer and redefined the diagnosis of the PJI standard. The new diagnostic criteria were validated in 222 PJI patients and 200 sterile patients. They found that the sensitivity and specificity of the new diagnostic criteria were 97.7% and 99.5%, respectively, while the sensitivities of the MSIS and ICM diagnostic criteria were only 86.9% and 79.3%, and their specificities were both 99.5%. The ICM passed this diagnostic criterion in 2018, but the pass rate was only 68%. Since 2019, an increasing number of articles about D-dimer in the diagnosis of PJI have been reported, and its diagnostic value is suspected.
This is the first systematic review and meta-analysis about the utility of D-dimer for the diagnosis of PJI. We found that D-dimer has limited performance for the diagnosis of PJI, with a pooled sensitivity and specificity of 0.82 and 0.70, respectively, and had a poorer diagnostic value than that of CPR and ESR reported by Carli AV et al. [31]. In this systematic review, the pooled sensitivity and specificity of CRP were 0.85 and 0.81, respectively, and the pooled sensitivity and specificity of ESR were 0.82 and 0.79. The results of the subgroup analysis showed that serum D-dimer might have a higher diagnostic accuracy than plasma D-dimer for PJI (the pooled sensitivity was 0.88 vs 0.67, and the pooled specificity was 0.76 vs 0.61), and D-dimer had better accuracy in subgroups with Caucasian and African American races than in subgroups with East Asian races (the pooled sensitivity was 0.92 vs 0.72, and the pooled specificity was 0.74 vs 0.65).
One possible reason for the variance in the subgroup results was that the samples for the quantification of D-dimer were different: serum vs plasma. Serum is the liquid part of blood after coagulation, while plasma is the liquid part of the blood where coagulation has been prevented. Their density is similar, but their composition is different. The main difference is that there are more fibrinogen and coagulation proteins in plasma [32]. Boisclair et al. [33] reported that there was a very high correlation between plasma and serum D-dimer levels (r = 0.931, P < 0.01), but the diagnostic sensitivity was not consistent. The study reported that the sensitivities of plasma D-dimer for DIC, DVT, and MI were 100%, 90.4%, and 60%, respectively, but the sensitivities of serum D-dimer were 100%, 94.1%, and 22.2%. The different sensitivities of plasma and serum might be due to the more significant uncertainty in assigning a cut-off for elevated levels of serum D-dimer. The D-dimer assay was operating at its lower detection limit when used to measure non-elevated levels in serum [33]. However, whether different sensitivities between plasma and serum exist in PJI is not supported by relevant literature.
Another possible reason was that the level of D-dimer is easily affected by other diseases. Busso et al. [34] reported that the inflammatory synovium secretes a large amount of fibrin in patients with rheumatoid arthritis, and the degradation of this protein subsequently leads to an increase in the level of D-dimer in serum and synovial fluid. In addition, thrombosis [35], malignancies, autoimmune diseases, pregnancy, and heart and brain vascular diseases might affect the determination of D-dimer levels in the blood [36, 37]. Li et al. [19] found that the diagnostic accuracy of D-dimer was poor in the subgroups containing these diseases in their study.
In addition, racial differences may affect the diagnostic accuracy of D-dimer for PJI. Shahi and Pannu’s studies [20, 23] were conducted in the USA, and the population may be predominantly Caucasian and African-American. In the six other studies reported by Chinese scholars, the patients were predominantly of the East Asian race. The studies found that D-dimer levels varied between races, such as between African American and Caucasian patients [38, 39]. We suspect that there are also differences in D-dimer levels between the East Asian population and the other races, which will affect the result. However, there are no studies to support this view.
Synovial fluid viscosity tests and several other plasma biomarkers have been reported to diagnose PJI. The synovial fluid viscosity level was significantly lower in patients with PJI than in patients with aseptic failure, with a sensitivity of 0.99 and a specificity of 0.67 [22]. Both plasma fibrinogen and fibrin degradation product (FDP) are coagulation-related indicators. When the threshold for plasma fibrinogen was 4.01 g/L, the sensitivity and specificity values were 0.763 and 0.862 [19], respectively. FDP has low sensitivity and specificity, with values of 65.12% and 60.33%, respectively [18].
Our meta-analysis has some strengths and potential limitations. The cases included all involved hip and knee joints. In addition, all studies used MSIS standards [5] or modified MSIS standards [6]. Therefore, the classification bias was minimized. The most important factor was that all D-dimer tests were taken before surgery, excluding the interference of a sharp increase in serum D-dimer levels after surgery [40].
The limitations of our meta-analysis included variability in race, age range, sex ratio, and sample size. In addition, none of the studies considered whether patients used antibiotics before admission. Shahi et al. [20] reported that premature antibiotic treatment could affect the results of D-dimer in the blood. Another limitation of our study is that MSIS standards or modified MSIS standards lack the sensitivity to detect chronic and low-grade PJI; patients with “positive” D-dimer results might be classified as uninfected [41]. Additionally, most studies did not provide information about the measurement of D-dimer. D-dimer assays can be categorized into three types [42]: ELISA, immunoturbidimetric automated assay, and latex-based immunoassays. ELISA is more sensitive than immunoturbidimetric automated assays and latex-based immunoassays [42]. In addition, some studies excluded patients with tumors, rheumatoid arthritis, autoimmune diseases, a history of smoking, and obesity. However, the proportion of such patients in joint replacement is still high. The exclusion of these patients will interfere with the accuracy of D-dimer in the diagnosis of PJI. Finally, the diagnostic thresholds in some studies were not determined in advance, and the threshold values were not unified in this meta-analysis.
Conclusion
D-dimer, a coagulation-related indicator, is inexpensive and easy to measure but has limited performance for the diagnosis of PJI, and the pooled sensitivity and specificity were poorer than those of traditional inflammatory markers such as CRP and ESR. Based on our findings, we suggest using serum samples for the quantification of D-dimer. Additionally, the diagnostic accuracy may be better in Caucasian and African American patients.
Availability of data and materials
Not applicable
Abbreviations
- PJI:
-
Periprosthetic joint infection
- PLR:
-
Positive likelihood ratio
- NLR:
-
Negative likelihood ratio
- DOR:
-
Diagnostic odds ratio
- AUC:
-
The area under the SROC curve
- MSIS:
-
Musculoskeletal Infection Society
- ICM:
-
International Consensus Meeting
- CRP:
-
C-reactive protein
- ESR:
-
Erythrocyte sedimentation rate
- WBC:
-
White blood cell
- PMN%:
-
Polymorphonuclear neutrophil percentage
- PRISMA:
-
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
References
Kurtz SM, Lau E, Watson H, Schmier JK, Parvizi J. Economic burden of periprosthetic joint infection in the United States. J Arthroplasty. 2012;27(8 Suppl):61-5.e1.
Huotari K, Peltola M, Jamsen E. The incidence of late prosthetic joint infections: a registry-based study of 112,708 primary hip and knee replacements. Acta Orthop. 2015;86(3):321–5.
Dale H, Fenstad AM, Hallan G, Havelin LI, Furnes O, Overgaard S, et al. Increasing risk of prosthetic joint infection after total hip arthroplasty. Acta Orthop. 2012;83(5):449–58.
Berend KR, Lombardi AV Jr, Morris MJ, Bergeson AG, Adams JB, Sneller MA. Two-stage treatment of hip periprosthetic joint infection is associated with a high rate of infection control but high mortality. Clin Orthop Relat Res. 2013;471(2):510–8.
Zmistowski B, Della Valle C, Bauer TW, Malizos KN, Alavi A, Bedair H, et al. Diagnosis of periprosthetic joint infection. J Orth Res. 2014;32(Suppl 1):S98–107.
Parvizi J, Zmistowski B, Berbari EF, Bauer TW, Springer BD, Della Valle CJ, et al. New definition for periprosthetic joint infection: from the Workgroup of the Musculoskeletal Infection Society. Clin Orthop Relat Res. 2011;469(11):2992–4.
Shohat N, Bauer T, Buttaro M, Budhiparama N, Cashman J, Della Valle CJ, et al. Hip and Knee Section, What is the definition of a Periprosthetic Joint Infection (PJI) of the knee and the hip? Can the same criteria be used for both joints?: Proceedings of International Consensus on Orthopedic Infections. The Journal of arthroplasty. 2019;34(2S):S325–S7.
Adam SS, Key NS, Greenberg CS. D-dimer antigen: current concepts and future prospects. Blood. 2009;113(13):2878–87.
Rodelo JR, De la Rosa G, Valencia ML, Ospina S, Arango CM, Gomez CI, et al. D-dimer is a significant prognostic factor in patients with suspected infection and sepsis. Am J Emerg Med. 2012;30(9):1991-9.
Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol. 2009;62(10):1006–12.
Whiting PF, Rutjes AW, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med. 2011;155(8):529–36.
Dwamena BA. Evidence-based radiology: step 3--diagnostic systematic review and meta-analysis (critical appraisal). Semin Roentgenol. 2009;44(3):170–9.
Dora C, Altwegg M, Gerber C, Bottger EC, Zbinden R. Evaluation of conventional microbiological procedures and molecular genetic techniques for diagnosis of infections in patients with implanted orthopedic devices. J Clin Microbiol. 2008;46(2):824–5.
Kriston L, Harter M, Holzel L. Challenges in reporting meta-analyses of diagnostic accuracy studies. Ann Intern Med. 2009;150(6):430.
Deeks JJ, Macaskill P, Irwig L. The performance of tests of publication bias and other sample size effects in systematic reviews of diagnostic test accuracy was assessed. J Clin Epidemiol. 2005;58(9):882–93.
Qin L, Li F, Gong X, Wang J, Huang W, Hu N. Combined measurement of D-dimer and C-reactive protein levels: highly accurate for diagnosing chronic periprosthetic joint infection. J Arthroplasty. 2020;35(1):229–34.
Xiong L, Li S, Dai M. Comparison of D-dimer with CRP and ESR for diagnosis of periprosthetic joint infection. J Orthop Surg Res. 2019;14(1):240.
Xu H, Xie J, Huang Q, Lei Y, Zhang S, Pei F. Plasma fibrin degradation product and D-dimer are of limited value for diagnosing periprosthetic joint infection. J Arthroplasty. 2019;34(10):2454–60.
Li R, Shao HY, Hao LB, Yu BZ, Qu PF, Zhou YX, et al. Plasma fibrinogen exhibits better performance than plasma D-dimer in the diagnosis of periprosthetic joint infection: a multicenter retrospective study. J Bone Joint Surg Am. 2019;101(7):613–9.
Shahi A, Kheir MM, Tarabichi M, Hosseinzadeh HRS, Tan TL, Parvizi J. Serum D-dimer test is promising for the diagnosis of periprosthetic joint infection and timing of reimplantation. J Bone Joint Surg Am. 2017;99(17):1419–27.
Huang J, Zhang Y, Wang Z, Dong Y, Zhao Y, Zheng J, et al. The serum level of D-Dimer is not suitable for distinguishing between prosthetic joint infection and aseptic loosening. J Orthop Surg Res. 2019;14(1):407.
Fu J, Ni M, Chai W, Li X, Hao L, Chen J. Synovial fluid viscosity test is promising for the diagnosis of periprosthetic joint infection. J Arthroplasty. 2019;34(6):1197–200.
Pannu TS, Villa JM, Patel PD, Riesgo AM, Barsoum WK, Higuera CA. The utility of serum D-dimer for the diagnosis of periprosthetic joint infection in revision total hip and knee arthroplasty. The Journal of arthroplasty. 2020:S0883-5403(20)30071-1.
Khanbhai M, Hansrani V, Burke J, Ghosh J, McCollum C. The early management of DVT in the North West of England: a nation-wide problem? Thromb Res. 2015;136(1):76–86.
Bounameaux H, de Moerloose P, Perrier A, Reber G. Plasma measurement of D-dimer as diagnostic aid in suspected venous thromboembolism: an overview. Thromb Haemost. 1994;71(1):1–6.
Schwameis M, Steiner MM, Schoergenhofer C, Lagler H, Buchtele N, Jilma-Stohlawetz P, et al. D-dimer and histamine in early stage bacteremia: a prospective controlled cohort study. Eur J Intern Med. 2015;26(10):782–6.
Ribera T, Monreal L, Armengou L, Rios J, Prades M. Synovial fluid D-dimer concentration in foals with septic joint disease. J Vet Intern Med. 2011;25(5):1113–7.
Turak O, Canpolat U, Ozcan F, Yayla C, Mendi MA, Oksuz F, et al. D-dimer level predicts in-hospital mortality in patients with infective endocarditis: a prospective single-centre study. Thromb Res. 2014;134(3):587–92.
Mele N, Turc G. Stroke associated with recent mycoplasma pneumoniae infection: a systematic review of clinical features and presumed pathophysiological mechanisms. Front Neurol. 2018;9:1109.
Parvizi J, Tan TL, Goswami K, Higuera C, Della Valle C, Chen AF, et al. The 2018 definition of periprosthetic hip and knee infection: an evidence-based and validated criteria. J Arthroplasty. 2018;33(5):1309-14.e2.
Carli AV, Abdelbary H, Ahmadzai N, Cheng W, Shea B, Hutton B, et al. Diagnostic accuracy of serum, synovial, and tissue testing for chronic periprosthetic joint infection after hip and knee replacements: a systematic review. J Bone Joint Surg Am. 2019;101(7):635–49.
Lima-Oliveira G, Monneret D, Guerber F, Guidi GC. Sample management for clinical biochemistry assays: are serum and plasma interchangeable specimens? Crit Rev Clin Lab Sci. 2018;55(7):480–500.
Boisclair MD, Lane DA, Wilde JT, Ireland H, Preston FE, Ofosu FA. A comparative evaluation of assays for markers of activated coagulation and/or fibrinolysis: thrombin-antithrombin complex, D-dimer and fibrinogen/fibrin fragment E antigen. Br J Haematol. 1990;74(4):471–9.
Busso N, Hamilton JA. Extravascular coagulation and the plasminogen activator/plasmin system in rheumatoid arthritis. Arthritis Rheum. 2002;46(9):2268–79.
Coleman DM, Wakefield TW. Biomarkers for the diagnosis of deep vein thrombosis. Expert Opin Med Diagn. 2012;6(4):253–7.
Olson JD. D-dimer: an overview of hemostasis and fibrinolysis, assays, and clinical applications. Adv Clin Chem. 2015;69:1–46.
O'Neal WT, Soliman EZ, Howard G, Howard VJ, Safford MM, Cushman M, et al. Inflammation and hemostasis in atrial fibrillation and coronary heart disease: the REasons for Geographic And Racial Differences in Stroke study. Atherosclerosis. 2015;243(1):192–7.
Pieper CF, Rao KM, Currie MS, Harris TB, Cohen HJ. Age, functional status, and racial differences in plasma D-dimer levels in community-dwelling elderly persons. J Gerontol A Biol Sci Med Sci. 2000;55(11):M649–57.
Zakai NA, McClure LA, Judd SE, Kissela B, Howard G, Safford M, et al. D-dimer and the risk of stroke and coronary heart disease. The REasons for Geographic and Racial Differences in Stroke (REGARDS) Study. Thromb Haemost. 2017;117(3):618–24.
Lee YS, Lee YK, Han SB, Nam CH, Parvizi J, Koo KH. Natural progress of D-dimer following total joint arthroplasty: a baseline for the diagnosis of the early postoperative infection. J Orthop Surg Res. 2018;13(1):36.
Osmon DR, Berbari EF, Berendt AR, Lew D, Zimmerli W, Steckelberg JM, et al. Diagnosis and management of prosthetic joint infection: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis. 2013;56(1):e1–e25.
Tripodi A. D-dimer testing in laboratory practice. Clin Chem. 2011;57(9):1256–62.
Acknowledgements
Not applicable
Funding
This study was funded by the National Natural Science Foundation of China, China Program (No. 30740089).
Author information
Authors and Affiliations
Contributions
Guangxu Lu, first author, searched the database, performed the meta-analysis, and drafted the manuscript. Tong Li, co-first author, participated in the design of the study, searched the database, and performed the meta-analysis. Haoqi Ye participated in the design of the study, searched the database, and performed the meta-analysis. Shujing Liu participated in the design of the study, searched the database, and performed the meta-analysis. Peng Zhang participated in the design of the study, searched the database, and performed the meta-analysis. Wenliang Wang participated in the design of the study and helped to draft the manuscript. The authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable
Consent for publication
Not applicable
Competing interests
No author associated with this paper has disclosed any potential or pertinent conflicts which may be perceived to have impending conflict with this work.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Lu, G., Li, T., Ye, H. et al. D-dimer in the diagnosis of periprosthetic joint infection: a systematic review and meta-analysis. J Orthop Surg Res 15, 265 (2020). https://doi.org/10.1186/s13018-020-01761-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13018-020-01761-z