Abstract
Background
Hidden blood loss is a major concern for patients undergoing hip surgery for intertrochanteric fracture. The objective of this study was to investigate whether tranexamic acid (TXA) could reduce postoperative hidden blood loss in patients undergoing hip surgery for intertrochanteric fracture.
Methods
A total of 77 patients with intertrochanteric fracture were enrolled in this randomized controlled study. Patients received either 200 mL (1 g) of TXA (n = 37) or normal-saline (NS) (n = 40) i.v. before hip surgery using proximal femoral nail anti-rotation (PFNA). Hemoglobin and hematocrit levels were measured preoperatively and postoperatively at day 1 and 3. Visible and hidden blood loss volumes were calculated at postoperative day 3.
Results
On postoperative day 3, the transfusion rate was significantly lower in the TXA group compared to the NS group, although mean hemoglobin and hematocrit levels were not significantly different between the two groups. However, the estimated hidden blood loss volume (210.09 ± 202.14 mL vs. 359.35 ± 290.12 mL; P < 0.05) and total blood loss volume (279.35 ± 209.11 mL vs. 417.89 ± 289.56 mL; P < 0.05) were significantly less in the TXA group compared to the NS group, respectively.
Conclusion
TXA significantly reduced postoperative hidden blood loss in patients with intertrochanteric fracture who underwent PFNA.
(Registration number: ChiCTR-INR-16008134).
Similar content being viewed by others
Background
Globally, hip fracture is a frequent cause of morbidity and mortality, especially in elderly people [1, 2]. Intertrochanteric fracture is one of the three major types of hip fracture, comprising approximately half of all hip fractures. Intertrochanteric fracture usually occurs in patients with a history of falls or bone disease, and results from a low-energy mechanism such as a fall from standing [3]. Patients typically present with pain and difficulty walking.
Types of intertrochanteric fracture [4] and treatment [5] affect functional outcomes and mortality in patients with hip fracture. Patients with intertrochanteric fractures incur more blood loss than those with femoral neck fractures and have a higher rate of transfusion [6]. In addition, perioperative hemoglobin and hematocrit levels have implications for outcomes, as patients with hip fracture are usually frail and elderly and particularly prone to anemia and hypovolaemia [7,8,9]. Evidence suggests that total blood loss during hip fracture surgery may be much greater than that observed intraoperatively. One study showed that overall blood loss was 1473 mL greater than that observed intraoperatively in patients undergoing hip surgery [6], and another study [10] reported 277.2 ± 7.6 mL hidden blood loss in patients undergoing proximal femoral nail anti-rotation (PFNA) for intertrochanteric fractures. Hidden blood loss could aggravate functional outcomes and increase mortality in patients with hip fracture by lowering hemoglobin levels. Hidden blood loss should be minimized during surgery for intertrochanteric fracture.
Tranexamic acid (TXA) is a synthetic derivative of the amino acid lysine, with antifibrinolytic properties that competitively inhibit lysine-binding sites on plasminogen molecules. TXA has been used for hemostasis in orthopedic surgery [11,12,13,14,15,16]. Previous studies have shown that TXA reduced total blood loss and the need for transfusion in hip arthroplasty and hip fracture surgery [14, 15, 17]. However, the majority of these studies focused on the hemostatic effect of TXA on visible blood loss in hip fracture surgery, rather than on postoperative hidden blood loss [18]. One study showed that TXA decreased external blood loss by 30%, but not hidden blood loss, in total knee replacement [19]. Other reports in total knee arthroplasty show that TXA significantly reduced hidden blood loss and total blood loss [20,21,22], but there have been few studies investigating whether TXA can reduce hidden blood loss in surgery for intertrochanteric fractures [18, 23].
In this study, we hypothesized that TXA administration would lead to decreased postoperative hidden blood loss in patients with intertrochanteric fractures. The objective of this study was to investigate whether intravenous (i.v.) administration of 1 g TXA could reduce postoperative hidden blood loss in patients with intertrochanteric fractures.
Methods
Study population
This prospective study was a single-blinded randomized controlled clinical trial conducted at a single center. Patients with stable and unstable intertrochanteric fractures admitted to our institution through the emergency department between December 1, 2015 and July 5, 2016 were eligible for this study. Inclusion criteria were (1) patients with a definite history of trauma, fall or traffic accident; (2) patients suffering from hip pain, tenderness, dysfunction, local swelling, and vertical percussion pain in the area of the greater trochanter, with limited function in the injured limb; (3) patients with a confirmed diagnosis of intertrochanteric fracture and fracture classified according to AO type on X-ray or computed tomography (CT) [24]; and (4) patients eligible for intertrochanteric fracture surgery using the proximal femoral nail anti-rotation (PFNA) system (TianJin ZhengTian, XiaMen Double), as determined by the senior orthopedic surgeons at our institution.
Exclusion criteria were (1) patients with allergy to TXA; (2) patients with recent or ongoing thromboembolic events (deep venous thrombosis, pulmonary embolism, arterial thrombosis, or cerebral thrombosis stroke); (3) patients who were recently taking or who were taking anticoagulation therapy including vitamin K-antagonists, direct thrombin inhibitors, direct factor X-a inhibitors, and platelet aggregation inhibitors; (4) patients with disseminated intravascular coagulation or patients had hepatic or renal diseases with impairment of coagulation function; or (5) patients with a history of subarachnoid bleeding, malignancy, pathological fracture, or prior surgery on the injured hip.
This study was approved by the Ethics Committee of Xi’an Jiaotong University, and each patient provided written informed consent before surgery. This study was performed in line with the Declaration of Helsinki international ethical guidelines for studies involving human subjects [25].
Intervention
Patients were randomized to a TXA group or a normal-saline (NS) group using a random number table. If patients were anemic (defined as hemoglobin <90 g/L) on admission they received an i.v. infusion of RBC. After anesthesia, but before surgery, patients in the TXA group received i.v. TXA 1 g (200 mL), and patients in the NS group received 200 mL of NS (i.v). A single orthopedic surgeon (LJL) performed surgery on all included patients. Patients were placed in supine position, the fractured bone fragments were identified by X-ray, and PFNA was performed.
Outcome measurements
Patient demographic and clinical characteristics were recorded. Hemoglobin and hematocrit levels 1 day before surgery and on postoperative Day 1 and 3; duration of surgery; and visible blood loss collected with a sterile plastic foil, a funnel, and gauzes were measured. Complications associated with surgery—including hematoma, infection, deep vein thrombosis (examined by ultrasonography on day 3 post-operation), pulmonary embolism, myocardial infarction, ischemic cerebral infarction, respiratory infection, and renal failure—were also recorded.
Nadler’s formulae for blood volume and visible and hidden blood loss were applied after surgery: [26,27,28]
Statistical analysis
Data were analyzed using SPSS v18.0 statistical software (SPSS Inc., Chicago, IL, USA). According to previous literature [13] and a power analysis, at least 72 patients were required for this study. Descriptive data are presented as mean ± SD. The chi-squared test or Student’s t test was used to compare demographic and clinical characteristics. A non-parametric test was used to evaluate ASA classification. P < 0.05 was considered statistically significant.
Results
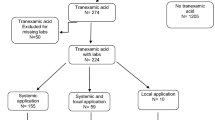
Between December 2015 and July 2016, 215 patients with intertrochanteric fractures were admitted to our institution through the emergency department. Seventy-seven patients who met all of the inclusion criteria and none of the exclusion criteria were randomized to the TXA (n = 37) and NS groups (n = 40) (Fig. 1). Patients’ demographic and clinical characteristics were similar between the two groups as summarized in Table 1. Most patients (81.81%) were female aged 64 to 93 years. Upon admission, the hemoglobin level in 16 patients was <90 g/L; these patients received a total of 48.0 U of packed RBC by intravenous infusion. Four patients in the TXA group and four patients in the NS group each received 4 U packed RBC. Before surgery, hemoglobin level in most patients was between 91 and 137 g/L and hematocrit was between 27.6 and 34.6%.
All surgeries were successful. Mean operative time and mean length of hospital stay were not significantly different between the TXA and NS groups. There was less surgical blood loss in the TXA group compared to the NS group (128.85 ± 123.18 vs. 98.30 ± 59.10; P = 0.31), but the difference was not significant. However, the postoperative transfusion rate was significantly lower in the TXA group compared to the NS group (TXA, 28.20% [2.0 U packed RBC in 10 patients, 4.0 U packed RBC in 1 person] vs. NS, 56.09% [2.0 U packed RBC in 18 patients, 4.0 U packed RBC in 5 patients]; P = 0.01). In the NS group, three patients >90 years of age received an intraoperative transfusion of 2 U packed RBC each.
As shown in Table 2, on postoperative day 1, both mean hematocrit and mean hemoglobin levels were not significantly different in the TXA group compared to the NS group. Postoperative drainage at postoperative day 2 was also not significantly different between the TXA and NS group (89.50 ± 44.77 mL vs. 89.73 ± 33.30 mL, P = 0.98). On postoperative day 3, mean hemoglobin and mean hematocrit levels were comparable. But the calculated hidden RBC loss (210.09 ± 202.14 mL vs. 359.35 ± 290.12 mL, P = 0.049) and total RBC loss (279.35 ± 209.11 mL vs. 417.89 ± 289.56 mL, P = 0.049) were significantly less in the TXA group compared to the NS group (Table 2).
There were no systematic complications related to TXA administration. The incidence of adverse events in the TXA and NS group were not significantly different (Table 3). Patients with hematoma and infection at the surgical site were treated conservatively, but one patient required surgical debridement.
All patients were followed up for 30 days after surgery. Three patients were lost to follow-up due to death (2 of pulmonary embolism and 1 of renal failure). Deep vein thrombosis resolved spontaneously.
Discussion
Hip fracture surgery may result in substantial blood loss in elderly and frail patients, exposing them to postoperative anemia, which could negatively impact clinical outcomes and mortality. Previous studies have shown that TXA reduced hidden blood loss associated with total knee arthroplasty [22]. However, it is not clear whether TXA decreases hidden blood loss in patients undergoing PFNA for intertrochanteric fractures. Our results showed that both postoperative hidden blood loss and total blood loss were significantly reduced in patients with intertrochanteric fractures treated with TXA, suggesting TXA administration is an efficacious approach to reducing blood loss in patients undergoing PFNA for intertrochanteric fractures.
Our results showed that compared to NS, TXA administration reduced postoperative RBC loss to 279.35 mL and hidden blood loss to 700.3 mL (assuming 30% hematocrit) in patients with intertrochanteric fractures. These results are consistent with previous studies reporting on total knee arthroplasty [12], periacetabular osteotomy [11], extracapsular fracture of the hip [13], and total shoulder arthroplasty [16]. Our data also suggests that TXA administration has the potential to decrease the number of orthopedic patients requiring transfusion; administration of 1 g of TXA decreased the transfusion rate from 56.09 to 28.20%. This would contribute to a substantial reduction in healthcare costs and resource utilization for these patients.
TXA competitively blocks a lysine-binding site of plasminogen and thereby inhibits its conversion to the active enzyme plasmin. Plasmin binds to fibrinogen or fibrin structures and promotes fibrinolysis [29]. Additional evidence suggests that plasmin is pro-inflammatory [30]. Currently, it is debatable whether TXA benefits trauma patients through reversal of fibrinolysis or modulating the inflammatory response [31, 32].
In this study, we chose to administer 1 g of TXA or NS i.v. after anesthetization and before surgery. We used a low dose and systemic administration, as reported by Wingerter et al. [33]. In contrast, Drakos et al. [23] administered 3 g of TXA around the fracture site at the end of the surgical procedure in patients undergoing surgery for intertrochanteric fracture, and Tengberg et al. [13] administered 1 g of TXA as an intravenous bolus prior to surgery followed by a postoperative 24-h infusion of 3 g TXA in 1 L of isotonic saline in patients undergoing surgery for extracapsular hip fracture.
The most common complication associated with TXA administration is ischemic cerebral infarction at postoperative 1 month after operation. However, there was no ischemic cerebral infarction in our study and there were no significant differences in the incidence of adverse events between the TXA and NS groups. TXA is a synthetic derivative of the amino acid lysine and may, therefore, be associated with thrombotic complications; however, recent large studies and meta-analyses have not consistently reported an increased risk for thrombosis [34,35,36]. The overall complication rate in our study was comparable with previous reports [13, 23].
To our knowledge, this is the first randomized controlled trial of TXA vs. NS for postoperative hidden blood loss in patients undergoing PFNA for intertrochanteric fractures. Only patients eligible for PFNA were included; therefore, the demographic and clinical characteristics of the TXA and NS groups were comparable.
The study has some limitations. First, the sample size is relatively small. Second, this study was not double-blind. Third, the fracture type was limited to intertrochanteric fractures. Future studies using larger sample sizes and a variety of fracture types are warranted to confirm our findings.
Conclusion
This study demonstrated that TXA could effectively reduce postoperative hidden blood loss in patients undergoing PFNA for intertrochanteric fractures and may decrease the number of patients needing transfusion.
Abbreviations
- CT:
-
Computed tomography
- Hb:
-
Hemoglobin
- Hct:
-
Hematocrit
- NS:
-
Normal saline
- PFNA:
-
Proximal femoral nail anti-rotation
- RBC:
-
Red blood cell
- TXA:
-
Tranexamic acid
References
Peeters CM, Visser E, Van de Ree CL, Gosens T, Den Oudsten BL, De Vries J. Quality of life after hip fracture in the elderly: a systematic literature review. Injury. 2016;47(7):1369–82.
Cooper C, Cole Z, Holroyd C, Earl S, Harvey N, Dennison E, Melton L, Cummings S, Kanis J. Epidemiology ICWGoF: secular trends in the incidence of hip and other osteoporotic fractures. Osteoporos Int. 2011;22(5):1277.
Ahn J, Bernstein J. Fractures in brief: intertrochanteric hip fractures. Clin Orthop Relat Res. 2010;468(5):1450–2.
Carvajal-Pedrosa C, Gómez-Sánchez RC, Hernández-Cortés P. Comparison of outcomes of Intertrochanteric fracture fixation using percutaneous compression plate between stable and unstable fractures in the elderly. J Orthop Trauma. 2016;30(6):e201–e06.
Dhamangaonkar AC, Joshi D, Goregaonkar AB, Tawari AA. Proximal femoral locking plate versus dynamic hip screw for unstable intertrochanteric femoral fractures. J Orthop Surg. 2013;21(3):317–22.
Foss NB, Kehlet H. Hidden blood loss after surgery for hip fracture. Bone Joint J. 2006;88(8):1053–9.
Kannus P, Parkkari J, Sievänen H, Heinonen A, Vuori I, Järvinen M. Epidemiology of hip fractures. Bone. 1996;18(1):S57–63.
Sharrock N. Fractured femur in the elderly: intensive perioperative care is warranted. Br J Anaesth. 2000;84(2):139.
Swain D, Nightingale P, Patel J. Blood transfusion requirements in femoral neck fracture. Injury. 2000;31(1):7–10.
Yu W, Zhang X, Wu R, Zhu X, Hu J, Xu Y, Yi J, Liu Y. The visible and hidden blood loss of Asia proximal femoral nail anti-rotation and dynamic hip screw in the treatment of intertrochanteric fractures of elderly high-risk patients: a retrospective comparative study with a minimum 3 years of follow-up. BMC Musculoskelet Disord. 2016;17(1):269.
Wassilew G, Perka C, Janz V, Krämer M, Renner L. Tranexamic acid reduces the blood loss and blood transfusion requirements following peri-acetabular osteotomy. Bone Joint J. 2015;97(12):1604–7.
Wang H, Shen B, Zeng Y. Blood loss and transfusion after topical tranexamic acid administration in primary total knee arthroplasty. Orthopedics. 2015;38(11):e1007–e16.
Tengberg P, Foss N, Palm H, Kallemose T, Troelsen A. Tranexamic acid reduces blood loss in patients with extracapsular fractures of the hip. Bone Joint J. 2016;98(6):747–53.
Poeran J, Rasul R, Suzuki S, Danninger T, Mazumdar M, Opperer M, Boettner F, Memtsoudis SG. Tranexamic acid use and postoperative outcomes in patients undergoing total hip or knee arthroplasty in the United States: retrospective analysis of effectiveness and safety. BMJ. 2014;349(aug12 8):g4829.
Perel P, Ker K, Morales Uribe CH, Roberts I. Tranexamic acid for reducing mortality in emergency and urgent surgery. Cochrane Database Syst Rev. 2013;1(1):CD010245.
Gillespie R, Shishani Y, Joseph S, Streit JJ, Gobezie R. Neer award 2015: a randomized, prospective evaluation on the effectiveness of tranexamic acid in reducing blood loss after total shoulder arthroplasty. J Shoulder Elb Surg. 2015;24(11):1679–84.
Farrow LS, Smith TO, Ashcroft GP, Myint PK. A systematic review of tranexamic acid in hip fracture surgery. Br J Clin Pharmacol. 2016;82(6):1458–70.
Smith G, Tsang J, Molyneux S, White T. The hidden blood loss after hip fracture. Injury. 2011;42(2):133–5.
Good L, Peterson E, Lisander B. Tranexamic acid decreases external blood loss but not hidden blood loss in total knee replacement. BJA Br J Anaesth. 2003;90(5):596.
Chen X, Zhu X, Yang S, Lin W, Wang L. Tranexamic acid treatment decreases hidden blood loss in total knee arthroplasty. Am J Ther. 2016;23(6):e1397–e405.
Seol Y-J, Seon J-K, Lee S-H, Jin C, Prakash J, Park Y-J, Song E-K. Effect of tranexamic acid on blood loss and blood transfusion reduction after Total knee Arthroplasty. Knee Surg Relat Res. 2016;28(3):188.
Xie J, Ma J, Yao H, Yue C, Pei F. Multiple boluses of intravenous tranexamic acid to reduce hidden blood loss after primary total knee arthroplasty without tourniquet: a randomized clinical trial. J Arthroplast. 2016;31(11):2458–64.
Drakos A, Raoulis V, Karatzios K, Doxariotis N, Kontogeorgakos V, Malizos K, Varitimidis SE. Efficacy of local administration of tranexamic acid for blood salvage in patients undergoing Intertrochanteric fracture surgery. J Orthop Trauma. 2016;30(8):409–14.
Marsh JL, Slongo TF, Agel J, Broderick JS, Creevey W, Decoster TA, Prokuski L, Sirkin MS, Ziran B, Henley B. Fracture and dislocation classification compendium - 2007: orthopaedic trauma association classification, database and outcomes committee. J Orthop Trauma. 2007;21(10 Suppl):1–133.
Foster CG. International ethical guidelines for biomedical research involving human subjects. J Med Ethics. 2002;10(182):17.
Gross JB. Estimating allowable blood loss corrected for dilution. J Am Soc Anesthesiol. 1983;58(3):277–80.
Meunier A, Petersson A, Good L, Berlin G. Validation of a haemoglobin dilution method for estimation of blood loss. Vox Sang. 2008;95(2):120–4.
Nadler SB, Hidalgo JH, Bloch T. Prediction of blood volume in normal human adults. Surgery. 1962;51(2):224–32.
Benoni G, Fredin H. Fibrinolytic inhibition with tranexamic acid reduces blood loss and blood transfusion after knee arthroplasty. Bone Joint J. 1996;78(3):434–40.
Syrovets T, Lunov O, Simmet T. Plasmin as a proinflammatory cell activator. J Leukoc Biol. 2012;92(3):509–19.
Jimenez JJ, Iribarren JL, Lorente L, Rodriguez JM, Hernandez D, Nassar I, Perez R, Brouard M, Milena A, Martinez R. Tranexamic acid attenuates inflammatory response in cardiopulmonary bypass surgery through blockade of fibrinolysis: a case control study followed by a randomized double-blind controlled trial. Crit Care. 2007;11(6):R117.
Hsia T-Y, McQuinn TC, Mukherjee R, Deardorff RL, Squires JE, Stroud RE, Crawford FA, Bradley SM, Reeves ST, Spinale FG. Effects of aprotinin or tranexamic acid on proteolytic/cytokine profiles in infants after cardiac surgery. Ann Thorac Surg. 2010;89(6):1843–52.
Wingerter SA, Keith AD, Schoenecker PL, Baca GR, Clohisy JC. Does tranexamic acid reduce blood loss and transfusion requirements associated with the periacetabular osteotomy? Clin Orthop Relat Res. 2015;473(8):2639–43.
Duncan CM, Gillette BP, Jacob AK, Sierra RJ, Sanchez-Sotelo J, Smith HM. Venous thromboembolism and mortality associated with tranexamic acid use during total hip and knee arthroplasty. J Arthroplast. 2015;30(2):272–6.
Yang Z-G, Chen W-P, Wu L-D. Effectiveness and safety of tranexamic acid in reducing blood loss in total knee arthroplasty: a meta-analysis. J Bone Joint Surg Am. 2012;94(13):1153–9.
Liu Y, Meng F, Yang G, Kong L, Shen Y. Comparison of intra-articular versus intravenous application of tranexamic acid in total knee arthroplasty: a meta-analysis of randomized controlled trials. Arch Med Sci: AMS. 2017;13(3):533.
Acknowledgments
None
Funding
This work was supported by the Science and Technology Project of Shaanxi Social Development (2016SF-312)
Availability of data and materials
The datasets generated and/or analyzed during the current study are not publicly available due to personal reasons, but are available from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Contributions
JL, HW, SH, and BZ designed the study, conducted all searches, appraised all potential studies, and wrote and revised the draft manuscript and subsequent manuscripts. YC, SW, HH, and YZ revised the draft manuscript and subsequent manuscripts. XW and YF assisted with the presentation of findings and with drafting and revising the manuscript. WW, SL, KZ, and PW conceived and designed the study, assisted with searches, appraised relevant studies, and assisted with drafting and revising the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The experimental protocol was established according to the ethical guidelines of the Helsinki Declaration and was approved by the Human Ethics Committee of the Department of Department of Orthopedic Trauma, Xi’an Honghui Hospital, Xi’an Jiaotong University Health Science Center, China.
Consent for publication
Written informed consent was obtained from individual participants.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Lei, J., Zhang, B., Cong, Y. et al. Tranexamic acid reduces hidden blood loss in the treatment of intertrochanteric fractures with PFNA: a single-center randomized controlled trial. J Orthop Surg Res 12, 124 (2017). https://doi.org/10.1186/s13018-017-0625-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13018-017-0625-9