Abstract
Introduction
The first line of treatment for nonfunctioning pituitary adenoma (NFPA) is surgery. Adjuvant radiotherapy or surveillance and new treatment (second surgical operation or salvage radiotherapy) in case of recurrence are options discussed at the multidisciplinary tumor board. The purpose of this study was to evaluate the therapeutic outcome for each option.
Methods
The records of 256 patients followed with NFPA between 2007 and 2018 were retrospectively reviewed. Mean age at initial surgery was 55 years [18–86]. Post-operative MRI found a residual tumor in 87% of patients. Mean follow-up was 12.1 years [0.8–42.7].
Results
After initial surgery, 40 patients had adjuvant radiotherapy. At 5, 10 and 15 years progression-free survival (PFS) was significantly different after surgery alone (77%, 58% and 40%) compared to surgery and adjuvant radiotherapy (84%, 78% and 78%) (HR = 0.24 [0–0.53] p < 0.0005). Overall, after first, second or third surgical operation, 69 patients had adjuvant radiotherapy and 41 salvage radiotherapy. Five-year PFS was similar for adjuvant (90%) and salvage radiotherapy (97%) (p = 0.62). After a second surgical operation, 62% and 71% of patients were irradiated after 2 and 5 years respectively. The risk of corticotropic and thyrotropic deficiency rates were 38% and 59% after second or third surgical operation and 40% and 73% after radiotherapy. Brain tumors occurred in 4 patients: 1 meningioma present at initial surgery, and after radiotherapy, 1 neurinoma which appeared at 5 years, 1 glioblastoma at 13 years and 1 meningioma at 20 years.
Conclusion
Among patients treated by surgery for NFPA, a “wait-and-see” attitude should be an option since adjuvant radiotherapy is not superior to salvage radiotherapy. However, in case of recurrence or progression, the authors recommended delivery of salvage radiotherapy to avoid a second surgical operation.
Similar content being viewed by others
Introduction
The incidence of pituitary adenomas (PA) keeps increasing (3.47/100,000 in 2016 in the USA) while non-functioning pituitary adenomas (NFPA) represent about one-third of all PA [1,2,3]. NFPA are mostly diagnosed because of chronic compressive symptoms (visual field defects or headaches) or hypopopituarism and do not present any secreting clinical manifestation. They are mostly gonadotropic or null cell adenoma, less often adenoma with ACTH, GH, PRL or TSH immunostaining [4].
The standard treatment for symptomatic NFPA’s consists in a debulking transsphenoidal surgery, associated with a 26.5% morbidity rate [5,6,7]. Endoscopic endonasal transsphenoidal approach is associated with toxicity such as worsening in 2.4% of visual functions, 13.7% of pituitary functions and 6.2% permanent diabetes insipidus, 0.8% cerebrospinal fluid leaks with hard reconstructions of the sella [6]. Shorter hospital stays and fewer postoperative complications are associated with higher-volume hospitals and surgeons [7].
The risk of relapse after exclusive surgery is 15.2–66% after 5 years, 41–50.5% after 10 years and 51–72% after 15 years [8,9,10,11,12]. Strategy after surgery is debated [13,14,15]. Post-operative radiotherapy has proven to be efficacious in reducing the risk of relapse: 0–6% after 5 years, 0–9% after 10 years and 7–9% after 15 years [8, 10,11,12, 16,17,18]. However, radiotherapy sometimes causes further pituitary deficiency and may induce cerebrovascular disease, secondary intracranial tumors and psycho-cognitive dysfunction [19,20,21,22,23,24]. Nowadays, high-precision radiotherapy with MRI registration and new intensity-modulated techniques reduce toxicity, notably by hippocampal-sparing [13, 25, 26].
Clinicopathological prognostic classification that combines tumor size, type, and grade accounting for invasion and proliferation criteria could be tools to decide between surveillance and adjuvant radiotherapy [27]. In case of recurrence, a second surgical operation or salvage radiotherapy is discussed at the multidisciplinary tumor board level [14].
Our purpose was to describe treatments for NFPA following initial surgery, including further surgeries and radiotherapy, and to evaluate outcomes after these second-line treatments. Secondary toxicities, especially endocrinological, as well as deciding factors that lead to a decision for radiotherapy were also studied.
Materials and methods
All patients followed (day hospital endocrinology department, radiotherapy department, neurosurgery department) for clinical NFPA at the Bordeaux University Hospital between January 2007 and January 2018 were screened. The cut-off date for the data was fixed at January 1st, 2021. Inclusion criteria were: age ≥ 18 years old, first-line surgery, histologically proven benign pituitary adenoma, > 6 months follow-up. Exclusion criteria were: pituitary carcinoma, NFPA without initial surgery or cerebral radiotherapy before surgery. Progression was defined as the need for further treatment (radiotherapy or surgery). Each treatment modification was decided by the multidisciplinary tumor board including neurosurgeon, radiation oncologist, neuroradiologist and endocrinologist. Salvage treatment was decided in case of objective radiological progression on MRI. Surgery was preferred in case of visual disorder. Salvage radiotherapy was preferred for patients with cavernous invasion and/or without new optic deficiency. Adjuvant radiotherapy consisted of irradiation within 6 months after surgery indicated by residual tumor or histoprognostical considerations. Salvage radiotherapy was defined as irradiation indicated by tumor evolution during follow-up, after surgery. The decision for adjuvant radiotherapy was taken by the multidisciplinary tumor board. Factors associated with adjuvant decision making will be studied. Adjuvant or salvage radiotherapy was done with standard fractionation, at 1.8 Gy to 2 Gy/fraction. In our center, the usual prescribed dose is 48.6 Gy at 1.8 Gy/fraction, 5 days per week, during 5 weeks and 2 days. To maintain a homogeneous treatment schedule, radiosurgery and hypofractionation radiotherapy, for instance with 5 fractions, were excluded. All data were collected retrospectively and in compliance with institutional ethical policies. Based on the documents presented, the publication group of the Ethics Committee of the Bordeaux University Hospital issued a favorable opinion to publish this research (CE-GP-2019/19).
Tumor characteristics
The vast majority of resected tumors were analyzed in the same laboratory. All immuno-histochemical analysis results (ACTH, GH, PRL, TSH, FSH, and sub-unit α2, β-FSH, β-LH) were available except for patients operated before 2000.
Null cell adenomas were defined as tumors without any pituitary hormones or other immunomarkers [28]. NFPAs were classified according to the HYPOPRONOS clinicopathological classification of pituitary endocrine tumors [27].
Follow-up and statistical analysis
As recommended, the postoperative residual tumor status was analyzed following a 3–12 months post-operative MRI [29]. Prior to any treatment decision, all patients were assessed by a multidisciplinary tumor board including endocrinologists, neurosurgeons, neuroradiologists, and radiation oncologists.
All statistical analyses were carried out using XLSTAT® software. Chi-square analysis was used to compare categorical variables, t-test to compare continuous variables, and Mann–Whitney test to compare continuous variables concerning time ranking. Two-sided p-values below 0.05 were considered statistically significant. Survival analysis was done according to the Kaplan Meier method and compared according to the Cox model.
Results
The mean follow-up after initial surgery was 12.1 years [0.8–42.7]. After 3, 5, 10, 15 and 20 years, 243 (95%), 210 (82%), 127 (49%), 68 (27%) and 24 (9%) patients were followed. The first patient initially underwent surgery in February 1978. Most patients (n = 204; 79.6%) were treated after year 2000.
Patients and tumor characteristics
Two hundred and fifty-six patients met the inclusion criteria (Table 1).
Mean age at diagnosis was 55 years, and there were 106 women for 150 men. Visual disorder (67%) and headache (32%) were the most frequent symptoms at diagnosis.
NFPAs were macroadenomas (between 10 and 40 mm) (90%) and giant adenomas (over 40 mm) (10%), with a 28.6 mm [12–60] mean size. Before the first surgery, 70% of patients (86/123 evaluable) presented at least one cavernous sinus invasion and 25% both. After surgery, 87% of patients presented an MRI-residual tumor. Invasion of at least one cavernous sinus concerned 42% of patients (82/195 evaluable).
NFPA were gonadotroph (n = 106, 41.4%), null-cell (n = 93, 36.3%), corticotroph (n = 13, 5%), with pluri-hormonal dominance (n = 13, 5.1%), lactotroph (n = 4, 1.6%), thyrotroph (n = 1, 0.4%) unknown type (n = 26, 10.2%) and no somatotroph adenoma. According to the HYPOPRONOS clinico-pathological classification of pituitary endocrine tumors, on available data, tumors were classified for 20% as grade 1a, 19% grade 1b, 31% grade 2a and 30% grade 2b.
Treatment sequence
Among 256 patients treated by surgery, 40 patients underwent adjuvant radiotherapy and 216 patients underwent wait-and-see attitude. 125 patients (49%) were free of further treatment during the entire follow-up whereas 91 patients underwent a salvage intervention: 35 patients underwent salvage radiotherapy, 56 patients underwent a second surgical operation of whom 28 patients with adjuvant radiotherapy (Fig. 1).
One hundred and ten (43%) actually received radiotherapy. Adjuvant treatment was indicated for 69 patients: 40 following initial surgery and 28 following a second surgical operation and 1 after a third surgical operation. Salvage treatment was indicated in 41 patients: 35 following initial surgery, 5 following a second surgical operation, and 1 after a third surgical operation. After radiotherapy, 9 patients out of 110 underwent another surgical operation.
After a second surgical operation without adjuvant radiotherapy, 5 patients out of 28 underwent salvage radiotherapy, and 3 underwent a third surgical operation.
No patient received any medical treatment such as dopamine agonist, somatostatin analog nor temozolomide.
Adjuvant radiotherapy after first surgery
Among the 40 patients who underwent surgery and adjuvant radiotherapy, 100% had a surgical remnant compared to 85% among 216 who did not underwent adjuvant radiotherapy (p = 0.02) (Additional file 2: Table S1). In the surgery with adjuvant radiotherapy group, patients were also younger (49 years vs. 56 years, p = 0.006) than in the other group, there was more cavernous sinus invasion (65% vs. 39%, p = 0.02), adenoma size before surgery was bigger (33 mm vs. 28 mm, p = 0.025). At last, median treatment period for adjuvant radiotherapy was November 2000 compared to October 2009 for the other group (p < 0.001). In our serie, adjuvant radiotherapy was more indicated in the past compared to nowadays.
Treatment in wait and see sequence after first surgery
Factors associated with failure and salvage treatments were studied for patients without adjuvant radiotherapy (Table 2).
Two factors were significantly associated with second surgery and salvage radiotherapy, compared to first surgery alone: cavernous sinus invasion and residual tumor after surgery. Otherwise, patients with 2 surgeries were significantly younger (mean age: 50.1 years) than patients treated by only one surgery (mean age: 57.2 years) and patients needing salvage radiotherapy (mean age: 59.3 years). The mean time between the first and second surgery was 6.3 years.
Progression-free survival
Five-year, 10-year and 15-year PFS was respectively 77%, 58% and 40% for patients treated by surgery alone compared to 84% and 78% after 5, 10 and 15 years (HR = 0.24 [IC95% 0–0.53]; p < 0.0005) for patients treated by surgery followed by adjuvant radiotherapy (Fig. 2A). Explorative analysis showed that patients with residual tumor and adjuvant radiotherapy had better PFS than unirradiated patients (HR = 3.14 [1.43–6.9] p = 0.004) (Fig. 2B). Moreover unirradiated patients had a worse PFS if they had a residual tumor (HR = 5.74 [1.81–18.2] p = 0.003). PFS was not different between patients with residual tumor and adjuvant radiotherapy, and patients without residual tumor unirradiated. At 10 years, PFS for patients treated by adjuvant radiation and for unirradiated patients without and with residue was respectively 91.6%, 79.2% and 50.5%.
A PFS after initial surgery for the whole cohort. Patients exposed to relapse are shown in lines 1 and 2, and patients followed are shown in third line. B PFS for patients with available data for post-operative residual tumor after first surgery (N = 228). Patients could have wait and see attitude and had post-operative residual tumor (N = 168) or without residual tumor (N = 29) or could be treated with adjuvant RT, all with residual tumor (N = 31). No patient treated by adjuvant radiotherapy had residual tumor. Patients without information concerning their residual tumor were excluded from this analysis (N = 28)
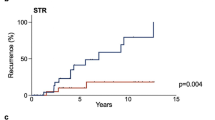
Among 196 patients with available immunohistochemistry and treated by surgery without adjuvant irradiation, PFS for gonadotrophic NFPA (N = 99) was no different compared to other subgroups (null-cell = 74, corticotroph = 9, plurihormonal = 12, lactotroph = 1, thryreotroph = 1)(p = 0.28) (Fig. 3A). Five-year PFS was 90% in patients treated by adjuvant radiotherapy compared to 97% in patients treated by salvage radiotherapy for recurrence (p = 0.62) (Fig. 3B). Radiotherapy free survival after a second surgical operation was 38% after 2 years, 29% after 5 years and 26% after 10 years (shown in Fig. 3C).
A PFS according to histological subtypes: Gonodotrophic: 99 vs. other subtypes (null-cell = 74, corticotroph = 9, plurihormonal = 12, lactotroph = 1, thryreotroph = 1) (p = 0.285) B PFS after adjuvant RT or salvage RT is no different (p = 0.17) C Radiation-free survival after second surgical operation
Radiotherapy was significantly more prescribed in case of a rapid recurrence estimated by the time between two surgical operations (p = 0015, when considered as a continuous variable in Cox model), particularly with a 2-year cutoff (HR = 0.31 [IC95% 0–0.62] p = 0.001) (shown in Additional file 1: Fig. S1).
Radiotherapy
Among 110 irradiated patients, radiotherapy dose and fractionation data were available for 94 patients (85%). Radiotherapy was delivered according to standard daily fractionation, five days a week. The most common dose prescribed was 48.6 Gy (27 × 1.8 Gy) in 80 patients (85%). Other schemes were 45–51 Gy with 1.8–2.2 Gy per fraction.
Among 107 patients irradiated, 91 (83%) were treated in one center. The radiotherapy modalities evolved over time: except for one patient (1%) treated with a 60Co source in 1989, all 25 patients (27%) treated before 2007 received conformational non stereotactic radiotherapy. All other patients (71%) received “modern” radiotherapy technique as defined by Minniti et al. [13]. Since 2008, 24 patients (26%) had conformational stereotactic treatment. Since 2012, 20 patients (22%) were treated by intensity-modulated stereotactic radiotherapy and then 21 patients (23%) with non-coplanar arcs allowing for hippocampal sparing [26].
Hormonal deficiencies
Postoperative deficiencies concerned corticotropic, thyrotropic and gonadotrophic axis for respectively 35%, 49% and 46% of patients. GH deficiencies as well as pre-operative deficiencies were not systematically recorded. Among the patients who were treated with only one surgery, 42%, 52% and 48% of patients had corticotropic, thyrotropic and/or gonadotropic deficiencies. Irradiated patients had corticotropic, thyrotropic and/or gonadotropic deficiencies in respectively 40%, 73% and 63% of cases. Forty-one percent of patients (42/103) suffered from panhypopituitarism after surgery and radiotherapy with a 14.6-year mean follow-up (1.1–42.7). After two to three surgical operations, post-operative deficiencies on corticotropic, thyrotropic and gonadotrophic axis accounted for respectively 38%, 59% and 60% of patients (Additional file 3: Table S2).
Non-hormonal toxicities
No radiation-induced optic neuropathy, nor any nerve toxicity were reported. Similarly no grade > 2 acute toxicities were reported following radiotherapy whereas 9 grade 2 toxicities (7 asthenia and 2 headaches) were reported. One patient had a meningioma diagnosed at initial surgery, and 3 patients had cerebral tumors after radiotherapy: a neurinoma (after 2 years), a glioblastoma (after 13 years) and a meningioma (after 20 years).
Among 256 patients, 19 patients (7.5%) had a stroke without any significant difference between irradiated patients (9%) and non-irradiated patients (6%) (p = 0.47, NS).
Discussion
After initial surgery: “wait-and-see” attitude
We present here one of the largest postoperative NFPA studies and the first to specify the timeline of postoperative management. Among 256 surgically treated NFPA patients, 60% needed a second treatment in a 15-year follow-up.
A complete NFPA surgery is difficult because of its anatomic position: resections are often sub-complete. The pooled results of 12 studies including 1455 patients suggest a 57% MRI residual tumor rate after surgery reaching 70% in the most recent study whereas we even found an 87% (199/228) post-operative residual tumor rate [12, 23]. These high rates could justify the indication for adjuvant radiotherapy. After exclusive surgery, Chen et al. reported a PFS of 71% at 5 years and 59% at 10 years, compared with our own 77%, 58% at 5 and 10 years [30].
Conversely, the combination of surgery and radiotherapy shows good and consistent efficacy with relapse rates between 0 and 6% after 5 years, 0 and 9% after 10 years and 6 and 9% after 15 years [8, 10,11,12, 18]. In our series, the relapse rate was 10% at 5 years for patients after adjuvant radiotherapy. Adjuvant radiotherapy might have been a good option after surgery.
However, radiotherapy could be responsible for late toxicities such as pituitary deficiencies that remain important sequelae after surgery and radiotherapy. Our results showing 32% and 48% of corticotropic and thyrotropic deficiencies after surgery and 46% and 58% after surgery and radiotherapy are consistent with the existing literature [19, 23]. The self-reported quality of life is stable or improved in almost all patients [31].
In addition, radiotherapy is thought to increase the risk of long-term cerebrovascular accidents by a 2–4.1 factor according to most studies [32, 33]. Another study denies this risk, based on a review of 11 studies and 4394 patients [22]. Our study did not observe a significantly increased stroke risk estimated at 6–9%. Radiotherapy is also believed to cause second tumors as redefined by Sheehan et al. [34]. We found 3 brain tumors in our cohort, including 2 out of 110 after radiotherapy despite a 12-year mean postoperative follow-up. Previously, Sheehan et al. described no second tumor among 1621 patients while Brada et al. with five patients, mention a 1.3% and 1.9% 10- and 20-year cumulative risk [24, 34]. Burman et al. based on 8917 adenoma analyses, including 3751 NFPA and 3236 radiotherapy treatments, found a 3.3-fold higher relative risk of developing de novo malignant brain tumors and a fourfold higher risk of meningiomas in patients who received radiotherapy [35]. Our results corroborate the idea that, if developing a brain tumor after radiotherapy is possible, the risk remains nevertheless low and delayed.
Most NFPA has a slow growth rate with a 3.4-year mean doubling time, without radiotherapy [30]. Nowadays, it appears that advances in pituitary imaging and generalization of multidisciplinary tumor boards are also tools to explore tumor progression precisely to avoid emergency surgery.
It is admitted that deferring radiotherapy is a safe attitude in case of complete resection if the patient is included in an MRI surveillance protocol [10, 14]. Therefore, a radiological relapse will rarely need immediate treatment [36].
Differing radiotherapy with a “wait-and-see” attitude appears all the more reason for all patients and particularly in non-deficient patients and women of childbearing age (gonadic deficiency, expensive and imperfect lifetime substitution treatment). Conversely, for patients already suffering from hormonal deficiencies, differed radiotherapy would induce no hormonal preservation benefits.
Ferrante et al. studied as we did the time leading up to new medical treatment [37]. Their cohort included 98% of patients treated by surgery and 41% who had adjuvant radiotherapy. With a mean follow-up of 9.3 years, 73 relapses out of 226 patients (32%) occurred. In the group of patients treated by surgery alone, the mean time before the second surgical operation was 5.2 years (± 4.7 years) consistent with our results (6.3 years). This time interval was compatible with a “wait-and-see” attitude. Even in the population of patients with residual tumor, we found in our serie that more than half of patients (50.5%) will not need further treatment (surgery or salvage radiotherapy) at 10 years.
In a multicenter matched cohort study, Pomeraniec et al. compared early (< 6 months) and late (> 6 months after resection) radiation by Gamma Knife after NFPA resection [38]. At 4 years, radiological progression was 6.1% in the early radiation group vs. 14.3% in the late radiation group. In this study, new endocrinopathies were constated in 27–30% of people during the follow-up period. In our study, after the first surgery, 77% and 58% of patients were free from new treatment (surgery/radiotherapy) at 5 and 10 years respectively. Increasing the radiological efficacy by 8.2% at 4 years does not seem to us sufficient to favor an adjuvant treatment which will cause 1 patient out of 3 or 4 to have permanent endocrine treatment.
Salvage radiotherapy in case of progression
Interestingly, fractionated radiotherapy was as effective when used as adjuvant or as salvage treatment, which is consistent with other published results [10, 13, 39]. Pomeraniec et al. showed less radiological progression after early radiation, but it is difficult to know if there is a clinical impact in the available data [38]. Regarding radiotherapy, in our series, radiation technique is consistent with most treatments at a dose of 48.6 Gy [45–50.4] at 1.8–2 Gy/fraction. This dose conformed with Grigsby et al. who showed a dose–response relationship with a better local control above 45 Gy over a three-decade dose-escalation experience after prior surgery [40]. This could also be a limitation because radiosurgery is preferred by many for low treatment volumes not too close to the optic tract. However, radiosurgery has a cost in cranial nerve deficit risk (0.8–6.6%) with mixed reports in the existing literature [41,42,43]. For instance, Cifarelli et al. described 4% of dysfunction in 217 pituitary adenomas treated by Gammaknife, one-third of which was permanent [44]. Regarding optic nerve toxicity, a review of historical series of fractioned radiotherapy found an incidence of 11 radiation optic neuropathies out of 2063 treated patients (0.53%) [45]. More recently, no radiation-induced neuropathy has been reported in 76 patients treated with fractionated radiotherapy (50.4 Gy/1.8 Gy/fraction) for pituitary adenoma with a minimum follow-up of 5 years [46]. Standard fractionation is not limited by size or optic nerve proximity, and our results seem to validate this approach. Efficacy of fractionated radiotherapy and stereotactic is judged to be not different [13, 15]. As a matter of fact, a comparison of fractionated radiotherapy and radiosurgery suggests that radiosurgery should be preferred for small tumors away from the optic chiasma, whereas fractioned stereotactic is more convenient for > 2.5–3 cm tumor or adenomas located near optic pathways [47].
Stereotactic radiotherapy in 3–5 fractions could be proposed for tumors located near visual pathways if radiosurgery is contraindicated. This possibility needs to be demonstrated by long term safety and efficacy studies [13].
Only randomized trials could recommend one or another technique in this pathology with a low relapse rate. In conclusion, salvage radiotherapy gives excellent results with 97% PFS at 5 years, and should be preferred to adjuvant radiotherapy.
After a second surgical operation, in our series, 62% of patients had radiotherapy within 2 years, and 71% within 5 years. This surgical intervention is often required to decompress the optic tract but it is not a way to avoid radiotherapy.
Study limitation
Our study presents some intrinsic limitations. Even if this study has one of the longest follow-up in the literature, it is monocentric and retrospective. Stereotactic radiosurgery was not available until recently and all patients were treated with conventional fractionation, even if most then were treated by “modern” radiotherapy. Only functioning adenomas were addressed to radiosurgery by GammaKnife®. The main limitation of this study is that we did not have access to the indication of radiotherapy or further surgeries. Finally, only partial data from endocrinologic status were available limiting interpretation of surgery and radiotherapy impact on it.
Conclusion
Over a long follow-up of 256 patients with operated NFPA, we reported that 60% of patients needed further treatment in a 15-year follow-up time. Better PFS above 90% at 5 years was similarly reported using adjuvant or salvage radiotherapy. Follow-up with MRI justifying a “wait-and-see” attitude. We consider that a watch and wait attitude after initial surgery is a good option. Even in a population of patients with residual tumor, more than half of patients will not need further treatment at 10 years. This is compatible with a “wait-and-see” attitude. If this second treatment consisted of surgery, about 3/4th of patients would receive radiotherapy within the next 5 years, whereas after radiotherapy less than 10% needed surgery within the next 5 years. We recommend that when significant growth is observed, leading to a risk of surgery within the following years, salvage radiotherapy should be proposed to reduce progression but carries with it some risks that must be weighed for individual patients alongside other variables like age, endocrine function, and residual tumor size/location to make personalized decisions for individual patients.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
McNeill KA. Epidemiology of brain tumors. Neurol Clin. 2016;34(4):981–98.
Molitch ME. Diagnosis and treatment of pituitary adenomas: a review. JAMA. 2017;317(5):516.
Loiseau H, Huchet A, Baldi I. Épidémiologie des tumeurs cérébrales primitives. Neurologie.com. 2010;4:83–6.
Jaffe CA. Clinically non-functioning pituitary adenoma. Pituitary. 2006;9(4):317–21.
Lucas JW, Bodach ME, Tumialan LM, Oyesiku NM, Patil CG, Litvack Z, et al. Congress of neurological surgeons systematic review and evidence-based guideline on primary management of patients with nonfunctioning pituitary adenomas. Neurosurgery. 2016;79(4):E533-535.
Magro E, Graillon T, Lassave J, Castinetti F, Boissonneau S, Tabouret E, et al. Complications related to the endoscopic endonasal transsphenoidal approach for nonfunctioning pituitary macroadenomas in 300 consecutive patients. World Neurosurg. 2016;89:442–53.
Barker FG, Klibanski A, Swearingen B. Transsphenoidal surgery for pituitary tumors in the United States, 1996–2000: mortality, morbidity, and the effects of hospital and surgeon volume. J Clin Endocrinol Metab. 2003;88(10):4709–19.
Gittoes NJ, Bates AS, Tse W, Bullivant B, Sheppard MC, Clayton RN, et al. Radiotherapy for non-function pituitary tumours. Clin Endocrinol. 1998;48(3):331–7.
Woollons AC, Hunn MK, Rajapakse YR, Toomath R, Hamilton DA, Conaglen JV, et al. Non-functioning pituitary adenomas: indications for postoperative radiotherapy. Clin Endocrinol. 2000;53(6):713–7.
Park P, Chandler WF, Barkan AL, Orrego JJ, Cowan JA, Griffith KA, et al. The role of radiation therapy after surgical resection of nonfunctional pituitary macroadenomas. Neurosurgery. 2004;55(1):100–7.
Losa M, Mortini P, Barzaghi R, Ribotto P, Terreni MR, Marzoli SB, et al. Early results of surgery in patients with nonfunctioning pituitary adenoma and analysis of the risk of tumor recurrence. J Neurosurg. 2008;66:525–32.
Brochier S, Galland F, Kujas M, Parker F, Gaillard S, Raftopoulos C, et al. Factors predicting relapse of nonfunctioning pituitary macroadenomas after neurosurgery: a study of 142 patients. Eur J Endocrinol. 2010;163(2):193–200.
Minniti G, Flickinger J, Tolu B, Paolini S. Management of nonfunctioning pituitary tumors: radiotherapy. Pituitary. 2018;21(2):154–61.
Sheehan J, Lee CC, Bodach ME, Tumialan LM, Oyesiku NM, Patil CG, et al. Congress of neurological surgeons systematic review and evidence-based guideline for the management of patients with residual or recurrent nonfunctioning pituitary adenomas. Neurosurgery. 2016;79(4):E539–40.
Chanson P, Dormoy A, Dekkers OM. Use of radiotherapy after pituitary surgery for non-functioning pituitary adenomas. Eur J Endocrinol. 2019;181(1):D1-13.
van den Bergh ACM, van den Berg G, Schoorl MA, Sluiter WJ, van der Vliet AM, Hoving EW, et al. Immediate postoperative radiotherapy in residual nonfunctioning pituitary adenoma: beneficial effect on local control without additional negative impact on pituitary function and life expectancy. Int J Radiat Oncol Biol Phys. 2007;67(3):863–9.
Dekkers OM, Pereira AM, Roelfsema F, Voormolen JHC, Neelis KJ, Schroijen MA, et al. Observation alone after transsphenoidal surgery for nonfunctioning pituitary macroadenoma. J Clin Endocrinol Metab. 2006;91(5):1796–801.
Weber DC, Momjian S, Pralong FP, Meyer P, Villemure JG, Pica A. Adjuvant or radical fractionated stereotactic radiotherapy for patients with pituitary functional and nonfunctional macroadenoma. Radiat Oncol. 2011;6(1):169.
Erridge SC, Conkey DS, Stockton D, Strachan MWJ, Statham PFX, Whittle IR, et al. Radiotherapy for pituitary adenomas: long-term efficacy and toxicity. Radiother Oncol. 2009;93(3):597–601.
Brown PD, Blanchard M, Jethwa K, Flemming KD, Brown CA, Kline RW, et al. The incidence of cerebrovascular accidents and second brain tumors in patients with pituitary adenoma: a population-based study. Neuro-Oncol Pract. 2014;1(1):22–8.
Erfurth EM, Bülow B, Svahn-Tapper G, Norrving B, Odh K, Mikoczy Z, et al. Risk factors for cerebrovascular deaths in patients operated and irradiated for pituitary tumors. J Clin Endocrinol Metab. 2002;87(11):4892–9.
van Westrhenen A, Muskens IS, Verhoeff JJC, Smith TRS, Broekman MLD. Ischemic stroke after radiation therapy for pituitary adenomas: a systematic review. J Neurooncol. 2017;135(1):1–11.
Molitch ME. Nonfunctioning pituitary tumors. Handb Clin Neurol. 2014;124:167–84.
Brada M, Ford D, Ashley S, Bliss JM, Crowley S, Mason M, et al. Risk of second brain tumour after conservative surgery and radiotherapy for pituitary adenoma. BMJ. 1992;304(6838):1343–6.
Gondi V, Tolakanahalli R, Mehta MP, Tewatia D, Rowley H, Kuo JS, et al. Hippocampal-sparing whole-brain radiotherapy: a “how-to” technique using helical tomotherapy and linear accelerator–based intensity-modulated radiotherapy. Int J Radiat Oncol Biol Phys. 2010;78(4):1244–52.
Uto M, Mizowaki T, Ogura K, Miyabe Y, Nakamura M, Mukumoto N, et al. Volumetric modulated dynamic WaveArc therapy reduces the dose to the hippocampus in patients with pituitary adenomas and craniopharyngiomas. Pract Radiat Oncol. 2017;7(6):382–7.
The members of HYPOPRONOS, Trouillas J, Roy P, Sturm N, Dantony E, Cortet-Rudelli C, et al. A new prognostic clinicopathological classification of pituitary adenomas: a multicentric case–control study of 410 patients with 8 years post-operative follow-up. Acta Neuropathol. 2013;126(1):123–35.
Raverot G, Dantony E, Beauvy J, Vasiljevic A, Mikolasek S, Borson-Chazot F, et al. Risk of recurrence in pituitary neuroendocrine tumors: a prospective study using a five-tiered classification. J Clin Endocrinol Metab. 2017;6:66.
Dina TS, Feaster SH, Laws ER, Davis DO. MR of the pituitary gland postsurgery: serial MR studies following transsphenoidal resection. Am J Neuroradiol. 1993;14(3):763–9.
Chen Y, Wang CD, Su ZP, Chen YX, Cai L, Zhuge QC, et al. Natural history of postoperative nonfunctioning pituitary adenomas: a systematic review and meta-analysis. Neuroendocrinology. 2012;96(4):333–42.
Rieken S, Habermehl D, Welzel T, Mohr A, Lindel K, Debus J, et al. Long term toxicity and prognostic factors of radiation therapy for secreting and non-secreting pituitary adenomas. Radiat Oncol. 2013;8(1):18.
Flickinger JC, Nelson PB, Taylor FH, Robinson A. Incidence of cerebral infarction after radiotherapy for pituitary adenoma. Cancer. 1989;63(12):2404–8.
Brada M, Burchell L, Ashley S, Traish D. The incidence of cerebrovascular accidents in patients with pituitary adenoma. Int J Radiat Oncol Biol Phys. 1999;45(3):693–8.
Sheehan JP, Niranjan A, Sheehan JM, Jane JA, Laws ER, Kondziolka D, et al. Stereotactic radiosurgery for pituitary adenomas: an intermediate review of its safety, efficacy, and role in the neurosurgical treatment armamentarium. J Neurosurg. 2005;102(4):678–91.
Burman P, van Beek AP, Biller BMK, Camacho-Hübner C, Mattsson AF. Radiotherapy, especially at young age, increases the risk for de novo brain tumors in patients treated for pituitary/sellar lesions. J Clin Endocrinol Metab. 2017;102(3):1051–8.
Raverot G, Burman P, McCormack A, Heaney A, Petersenn S, Popovic V, et al. European Society of Endocrinology Clinical Practice Guidelines for the management of aggressive pituitary tumours and carcinomas. Eur J Endocrinol. 2018;178(1):G1-24.
Ferrante E, Ferraroni M, Castrignanò T, Menicatti L, Anagni M, Reimondo G, et al. Non-functioning pituitary adenoma database: a useful resource to improve the clinical management of pituitary tumors. Eur J Endocrinol. 2006;155(6):823–9.
Pomeraniec IJ, Kano H, Xu Z, Nguyen B, Siddiqui ZA, Silva D, et al. Early versus late Gamma Knife radiosurgery following transsphenoidal surgery for nonfunctioning pituitary macroadenomas: a multicenter matched-cohort study. J Neurosurg. 2018;129(3):648–57.
Tsang RW, Brierley JD, Panzarella T, Gospodarowicz MK, Sutcliffe SB, Simpson WJ. Radiation therapy for pituitary adenoma: treatment outcome and prognostic factors. Int J Radiat Oncol Biol Phys. 1994;30(3):557–65.
Grigsby PW, Stokes S, Marks JE, Simpson JR. Prognostic factors and results of radiotherapy alone in the management of pituitary adenomas. Int J Radiat Oncol Biol Phys. 1988;15(5):1103–10.
Ding D, Starke RM, Sheehan JP. Treatment paradigms for pituitary adenomas: defining the roles of radiosurgery and radiation therapy. J Neurooncol. 2014;117(3):445–57.
Park KJ, Kano H, Parry PV, Niranjan A, Flickinger JC, Lunsford LD, et al. Long-term outcomes after gamma knife stereotactic radiosurgery for nonfunctional pituitary adenomas. Neurosurgery. 2011;69(6):1188–99.
Sheehan JP, Starke RM, Mathieu D, Young B, Sneed PK, Chiang VL, et al. Gamma Knife radiosurgery for the management of nonfunctioning pituitary adenomas: a multicenter study. J Neurosurg. 2013;66:446–56.
Cifarelli CP, Schlesinger DJ, Sheehan JP. Cranial nerve dysfunction following Gamma Knife surgery for pituitary adenomas: long-term incidence and risk factors. J Neurosurg. 2012;116(6):1304–10.
van den Bergh ACM, Schoorl MA, Dullaart RPF, van der Vliet AM, Szabó BG, ter Weeme CA, et al. Lack of radiation optic neuropathy in 72 patients treated for pituitary adenoma. J Neuroophthalmol. 2004;24(3):200–5.
Kim JO, Ma R, Akagami R, McKenzie M, Johnson M, Gete E, et al. Long-term outcomes of fractionated stereotactic radiation therapy for pituitary adenomas at the BC Cancer Agency. Int J Radiat Oncol Biol Phys. 2013;87(3):528–33.
Minniti G, Clarke E, Scaringi C, Enrici RM. Stereotactic radiotherapy and radiosurgery for non-functioning and secreting pituitary adenomas. Rep Pract Oncol Radiother. 2016;21(4):370–8.
Acknowledgements
Not applicable.
Funding
The funders had no role in the design of the study; in the collection, analysis, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Author information
Authors and Affiliations
Contributions
TC: data collecting, original manuscript, manuscript reviewing, and editing; CD: statistical analysis, original manuscript, manuscript reviewing and editing; VV, AH, RT, AF, AT, VJ, HL: manuscript reviewing and editing. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Publication group of the Ethics Committee of the Bordeaux Hospital issued a favorable opinion to publish this research (CE-GP-2019/19).
Consent for publication
Not applicable.
Competing interests
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1. Fig. S1
: Radiation free survival according to duration between 2 surgical operations. It is statistically significant when this duration is considered as a continuous variable (p=0.015) or with a cut-off at 2 years (p=0.001).
Additional file 2. Table S1
: Patient and tumor characteristics according to treatment strategy.
Additional file 3. Table S2
: Final deficiencies according to intervention. (Radiotherapy encompasses adjuvant and salvage radiotherapy).
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Charleux, T., Vendrely, V., Huchet, A. et al. Management after initial surgery of nonfunctioning pituitary adenoma: surveillance, radiotherapy or surgery?. Radiat Oncol 17, 165 (2022). https://doi.org/10.1186/s13014-022-02133-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13014-022-02133-z