Abstract
Background
Curative treatment of inoperable post-irradiation sarcoma is often challenging, especially using radiotherapy, wherein curative dose administration is difficult because the organs around the tumor have already been irradiated during the first cancer treatment. Carbon-ion radiotherapy (C-ion RT) might be useful in the treatment of post-irradiation sarcomas because it allows re-irradiation with high-dose localization properties and also demonstrates higher cytotoxic effects on radioresistant tumors compared with X-rays. This study presents the long-term survival of two patients with inoperable post-irradiation pelvic osteosarcoma treated with C-ion RT after uterine cervical cancer treatment.
Case presentation
The durations from prior radiotherapy to the diagnosis of post-irradiation osteosarcoma were 112.8 and 172.2 months, respectively. Both patients received 70.4 Gy (relative biological effectiveness) in 16 fractions of C-ion RT, and chemotherapy was performed before and after C-ion RT. Both patients achieved a complete response 1 year after the initiation of C-ion RT. However, one patient developed single lung metastasis 12.6 months after the initiation of C-ion RT and underwent thoracoscopic lobectomy. After 63.7 and 89.0 months from the initiation of C-ion RT, respectively, the patients were alive with no evidence of local recurrence, other distant metastasis, or fatal toxicities.
Conclusions
The study findings suggest that C-ion RT is a suitable treatment option for inoperable post-irradiation osteosarcoma.
Similar content being viewed by others
Background
Radiotherapy (RT) is widely known to be an oncologic risk factor, and post-irradiation sarcomas can develop in patients who receive RT for another malignancy [1, 2]. In 1948, Cahan et al. defined the criteria for the diagnosis of post-irradiation sarcomas as follows: (1) history of RT, (2) asymptomatic latent period of several years, (3) development of sarcoma within a previous RT field, and (4) histological confirmation of the sarcomatous nature of the post-RT lesion [3]. These criteria have since been used for the diagnosis of post-irradiation sarcomas [4].
Uterine cervical cancer is expected to be highly curable with RT, and many patients survive for a long time after treatment [5]. However, post-irradiation sarcoma is sometimes a problem in these patients. The incidence of post-irradiation sarcomas after RT for uterine cervical cancer is 0.6%, representing a 22.0-fold increased risk of developing post-irradiation sarcomas compared with the general population [2]. Patients with clinically inoperable post-irradiation sarcomas have limited treatment options [6]. Although RT is an option for such patients, it may be difficult to administer a curative dose because the tolerable dose to the healthy surrounding normal organs may have been exceeded due to the first irradiation. Additionally, post-irradiation sarcomas are considered radioresistant, and local control by RT may be difficult.
Carbon-ion (C-ion) RT might be useful for post-irradiation sarcomas because it allows re-irradiation with high dose localization properties due to the distal tail-off by the Bragg peak and sharp lateral penumbra [7, 8]. Additionally, it shows a higher cytotoxic effect than X-rays on radioresistant tumors, such as sarcomas, due to a higher linear energy transfer [9,10,11]. With these advantages, post-irradiation sarcomas that are usually difficult to cure with conventional RT could be cured with C-ion RT. Here, we present two patients who underwent C-ion RT for inoperable post-irradiation osteosarcoma after uterine cervical cancer treatment.
Case presentation
Patients and treatment
Two patients with inoperable post-irradiation pelvic osteosarcoma after uterine cervical cancer treatment were referred to our department; both received postoperative RT without chemotherapy for uterine cervical cancer. The durations from prior RT to the diagnosis of post-irradiation osteosarcoma were 112.8 and 172.2 months, respectively. Both patients underwent magnetic resonance imaging, contrast-enhanced computed tomography, and 2-deoxy-2-[18F]fluoro-D-glucose-positron emission tomography (FDG-PET). Figures 1 and 2 show diagnostic imaging results before C-ion RT. Both patients had locally advanced disease, were unsuitable for surgery, and had no distant metastases or direct infiltration to the gastrointestinal tract. The patient characteristics and treatments are summarized in Table 1. The eighth edition of the Union for International Cancer Control/American Joint Committee on Cancer TNM staging system was used for tumor staging [12].
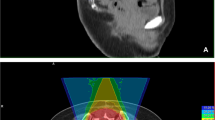
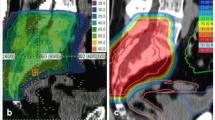
Radiological images before and after carbon-ion radiotherapy (C-ion RT) and dose distribution of Case 1. a Contrast-enhanced magnetic resonance imaging (MRI) before C-ion RT. The tumor (50 × 80 × 95 mm) was located in the left iliac bone and had good contrast enhancement (red arrow). b 2-deoxy-2-[18F]fluoro-D-glucose-positron emission tomography (FDG-PET) before C-ion RT. The red arrow shows the tumor with abnormal FDG uptake. c Dose distribution on axial computed tomography images. The area within the red outline is the gross tumor volume of the osteosarcoma. The 95% (red), 90% (orange), 80% (yellow), 65% (green), 50% (blue), and 20% (purple) isodose curves are highlighted (100% = 70.4 Gy [relative biological effectiveness]). d FDG-PET 1 year after C-ion RT. FDG uptake was decreased compared to that before treatment (green arrow). (E) FDG-PET 5 years after C-ion RT. FDG uptake was decreased compared to that before treatment (green arrow)
Radiological images before and after carbon-ion radiotherapy (C-ion RT) and dose distribution of Case 2. a Contrast-enhanced magnetic resonance imaging (MRI) before C-ion RT. The tumor (51 × 52 × 68 mm) was located in the sacral bone and had good contrast enhancement (red arrow). b 2-deoxy-2-[18F]fluoro-D-glucose-positron emission tomography (FDG-PET) before C-ion RT. The red arrow shows the tumor with abnormal FDG uptake. c Dose distribution on axial computed tomography images. The area within the red outline is the gross tumor volume of the osteosarcoma. The 95% (red), 90% (orange), 80% (yellow), 65% (green), 50% (blue), and 20% (purple) isodose curves are highlighted (100% was 70.4 Gy [relative biological effectiveness]). d FDG-PET 1 year after C-ion RT. FDG uptake was decreased compared with that before treatment (green arrow). e FDG-PET 7 years after C-ion RT. FDG uptake was decreased compared with that before treatment (green arrow)
Both patients received 70.4 Gy (relative biological effectiveness [RBE]) in 16 fractions for 4 weeks. The microdosimetric kinetic model was used to calculate the RBE, and doses of C-ion RT were expressed as RBE-weighted dose [Gy (RBE)], which was defined as the physical dose multiplied by the RBE of the C-ions [13]. C-ion RT was performed using passive scattering irradiation, with the beams in one direction per fraction. The patients received C-ion RT once daily for 4 days a week (Tuesday to Friday). Figures 1c and 2c show the dose distribution of C-ion RT. The tumor response was assessed using the Response Evaluation Criteria in Solid Tumors (version 1.1) and FDG-PET [14, 15]. Toxicities were assessed using the Common Terminology Criteria for Adverse Effects (version 4.0) [16].
Case 1
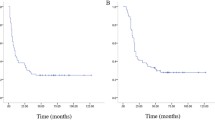
One year after C-ion RT, a complete metabolic response was observed on FDG-PET (Fig. 1d). However, the patient developed a single lung metastasis 12.6 months after C-ion RT initiation and underwent thoracoscopic lobectomy. The patient is alive 63.7 months after C-ion RT initiation with no evidence of local recurrence, other distant metastasis, or grade 3 or higher toxicities.
Case 2
One year after C-ion RT, a complete metabolic response was observed on FDG-PET (Fig. 2d). The patient is alive 89 months after C-ion RT initiation with no evidence of local recurrence or distant metastasis. The patient developed grade 3 sacral bone fracture where the sarcoma was located, grade 3 edema of the lower extremities associated with sacral bone fracture, and grade 2 peripheral neuropathy requiring high-dose opioids.
Discussion and conclusions
The two patients experienced favorable clinical outcomes after C-ion RT for inoperable post-irradiation pelvic osteosarcoma that arose after uterine cervical cancer treatment. These results suggest that C-ion RT, which has a high dose concentration and higher cell-killing effect than other RT modalities, exerts a safe and favorable local effect, even as the second irradiation and for radioresistant tumors, and contributes to long-term survival.
There have been several reports of initial treatment with C-ion RT and proton beam therapy with C-ion RT boost for sarcomas in patients with no history of irradiation which showed favorable clinical outcomes despite including patients with inoperable tumors; the median survival time was 31.2–49.4 months, indicating that some patients had long-term survival [9,10,11, 17]. The survival times of our patients were longer than this range, despite both patients having previously received irradiation to the pelvis. In a previous report of post-irradiation sarcoma, the median survival was only 37 months, including patients who underwent curative surgery. For patients with inoperable tumors, survival was significantly poorer (median survival: 15 months) [18]. One reason for poor survival in patients with inoperable tumors is that curative irradiation is not possible for post-irradiation sarcomas because of the risks associated with re-irradiation. However, the high dose localization property of C-ion RT enables a curative dose administration with a smaller risk to the surrounding organs, and higher cell-killing effect of C-ion RT provides local control of radioresistant post-irradiation osteosarcoma. Our two patients achieved long-term survival. Therefore, we believe that C-ion RT might be a curative treatment option for inoperable post-irradiation osteosarcoma.
Generally, dose constraints for the gastrointestinal tract in re-irradiated patients are stricter than those at the time of initial irradiation. However, there are no data on these dose constraints or on the recovery of normal tissues in the period between the first and second irradiations. We designed our treatment plan to reduce the gastrointestinal tract dose as much as possible. The total maximum dose to the gastrointestinal tract in the worst-case calculation could have exceeded 100 Gy (RBE) due to overlap with the gastrointestinal tract within the irradiation area of the previous RT and C-ion RT. However, we considered that the dose to the gastrointestinal tract was tolerable because of the long period between the previous RT and C-ion RT and the small high-dose volume in the gastrointestinal tract. Neither patient developed gastrointestinal toxicities.
C-ion RT for head and neck sarcomas, including post-irradiation sarcomas, was previously reported [19]. However, this report included a small number of patients, including those with locally recurrent sarcoma after surgery without RT, and those who received a combination of proton-beam therapy and C-ion RT. Therefore, this cannot be considered a coherent report of C-ion RT for post-irradiation sarcomas. To our knowledge, this is the first report of C-ion RT for post-irradiation osteosarcoma.
A limitation of this report is that it is not an analysis to confirm the safety and efficacy of C-ion RT for post-irradiation sarcomas. Further investigation with a larger sample size is warranted to establish the safety and efficacy of C-ion RT.
In conclusion, the study findings suggest that C-ion RT is a potential treatment option for inoperable post-irradiation osteosarcoma.
Availability of data and materials
The datasets generated for this report are available from the corresponding author on reasonable request..
Abbreviations
- RT:
-
Radiotherapy
- C-ion:
-
Carbon-ion
- FDG-PET:
-
2-Deoxy-2-[18F]fluoro-D-glucose-positron emission tomography
- RBE:
-
Relative biological effectiveness
References
Tubiana M. Can we reduce the incidence of second primary malignancies occurring after radiotherapy? A critical review. Radiother Oncol. 2009;91:4–15.
Ohno T, Kato S, Sato S, Fukuhisa K, Nakano T, Tsujii H, et al. Long-term survival and risk of second cancers after radiotherapy for cervical cancer. Int J Radiat Oncol Biol Phys. 2007;69:740–5.
Cahan WG, Woodard HQ, Higinbotham NL, Stewart FW, Coley BL. Sarcoma arising in irradiated bone; report of 11 cases. Cancer. 1948;1:3–29.
Kirova YM, Vilcoq JR, Asselain B, Sastre-Garau X, Fourquet A. Radiation-induced sarcomas after radiotherapy for breast carcinoma: a large-scale single-institution review. Cancer. 2005;104:856–63.
Kato S, Ohno T, Thephamongkhol K, Chansilpa Y, Cao J, Xu X, et al. Long-term follow-up results of a multi-institutional phase 2 study of concurrent chemoradiation therapy for locally advanced cervical cancer in east and Southeast Asia. Int J Radiat Oncol Biol Phys. 2013;87:100–5.
Ozaki T, Flege S, Kevric M, Lindner N, Maas R, Delling G, et al. Osteosarcoma of the pelvis: experience of the cooperative osteosarcoma study group. J Clin Oncol. 2003;21:334–41.
Kanai T, Endo M, Minohara S, Miyahara N, Koyama-ito H, Tomura H, et al. Biophysical characteristics of HIMAC clinical irradiation system for heavy-ion radiation therapy. Int J Radiat Oncol Biol Phys. 1999;44:201–10.
Shiba S, Okonogi N, Kato S, Wakatsuki M, Kobayashi D, Kiyohara H, et al. Clinical impact of Re-irradiation with carbon-ion radiotherapy for lymph node recurrence of gynecological cancers. Anticancer Res. 2017;37:5577–83.
Mohamad O, Imai R, Kamada T, Nitta Y, Araki N. Working Group for Bone and Soft Tissue Sarcoma. Carbon ion radiotherapy for inoperable pediatric osteosarcoma. Oncotarget. 2018;9:22976–85.
Imai R, Kamada T, Araki N, Working Group for Carbon Ion Radiotherapy for Bone and Soft Tissue Sarcomas. Carbon ion radiotherapy for unresectable localized axial soft tissue sarcoma. Cancer Med. 2018;7:4308–14.
Shiba S, Okamoto M, Kiyohara H, Okazaki S, Kaminuma T, Shibuya K, et al. Impact of carbon ion radiotherapy on inoperable bone sarcoma. Cancers (Basel). 2021;13:1099.
Brierley JD, Gospodarowicz MK, Wittekind C, editors. International union against cancer (UICC): TNM classification of malignant tumours. Oxford: Wiley-Blackwell; 2017.
Inaniwa T, Kanematsu N, Matsufuji N, Kanai T, Shirai T, Noda K, et al. Reformulation of a clinical-dose system for carbon-ion radiotherapy treatment planning at the National Institute of Radiological Sciences Japan. Phys Med Biol. 2015;60:3271–86.
Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 11). Eur J Cancer. 2009;45:228–47.
Wahl RL, Jacene H, Kasamon Y, Lodge MA. From RECIST to PERCIST: Evolving Considerations for PET response criteria in solid tumors. J Nucl Med. 2009;50;Suppl 1 [Suppl:122S–50S]:122S–50S.
NCI common terminology criteria for adverse events (CTCAE) version 4.0. Data File. http://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm. Accessed 1 Sep 2020
Seidensaal K, Mattke M, Haufe S, Rathke H, Haberkorn U, Bougatf N, et al. The role of combined ion-beam radiotherapy (CIBRT) with protons and carbon ions in a multimodal treatment strategy of inoperable osteosarcoma. Radiother Oncol. 2021;159:8–16.
Spałek MJ, Czarnecka AM, Rutkowski P. The management of radiation-induced sarcomas: a cohort analysis from a sarcoma tertiary center. J Clin Med. 2021;10:694.
Yang J, Gao J, Wu X, Hu J, Hu W, Kong L, et al. Salvage carbon ion radiation therapy for locally recurrent or radiation-induced second primary sarcoma of the head and neck. J Cancer. 2018;9:2215–23.
Acknowledgements
The authors would like to thank the patients who were involved in this study, our colleagues at the Gunma University Heavy Ion Medical Center and Department of Radiation Oncology Gunma University Graduate School of Medicine, and Editage (www.editage.com) for English language editing.
Funding
This work was supported by the following grant: JSPS KAKENHI, Grant Number 20K16751.
Author information
Authors and Affiliations
Contributions
Conceptualization, S.S., M.O., and T.O.; methodology, S.S., M.O., and T.O.; investigation, S.S. and M.O.; resources, S.S. M.O., and T.Y.; data curation, S.S.; writing—original draft preparation, S.S., M.O. and T.O.; writing—review and editing, T.Y., I.K., K.S. S.O., Y.M. and H.C.; visualization, S.S.; supervision, T.O.; project administration, M.O. The author(s) read and approved the final manuscript. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was approved by the Ethics Committee of the Gunma University Graduate School of Medicine and was performed in accordance with the Declaration of Helsinki. Informed consent for this treatment was obtained from the patients before therapy initiation.
Consent for publication
All patients provided consent for data usage in the study and its publication.
Competing interests
T. O. received research funding from Hitachi. All other authors have no conflicts of interest to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Shiba, S., Okamoto, M., Yanagawa, T. et al. Long-term survival of two patients with inoperable post-irradiation osteosarcoma treated with carbon-ion radiotherapy: a case report. Radiat Oncol 17, 68 (2022). https://doi.org/10.1186/s13014-022-02040-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13014-022-02040-3