Abstract
Background
To investigate the relationship between radiotherapy (RT) and the risk of cerebrovascular mortality (CVM) in head and neck cancer (HNC) survivors aged ≥ 65 years.
Methods
Patients with HNC survivors aged ≥ 65 years diagnosed between 2000 and 2012 were included from the Surveillance, Epidemiology, and End Results database. Kaplan–Meier analysis, Log-rank tests, and Cox proportional-hazards regression models were performed for statistical analyses.
Results
We included 16,923 patients in this study. Of these patients, 7110 (42.0%) patients received surgery alone, 5041 (29.8%) patients underwent RT alone, and 4772 (28.2%) patients were treated with surgery and RT. With a median follow-up time of 87 months, 1005 patients died with cerebrovascular disease. The 10-years CVM were 13.3%, 10.8%, and 11.2% in those treated with RT alone, surgery alone, and surgery plus RT, respectively (P < 0.001). The mean time for CVM was shorter in RT alone compared to surgery alone and surgery plus RT (52 months vs. 56–60 months). After adjusting for covariates, patients receiving RT alone had a significantly higher risk of developing CVM compared to those receiving surgery alone (hazard ratio [HR] 1.703, 95% confidence interval [CI] 1.398–2.075, P < 0.001), while a comparable risk of CVM was found between those treated with surgery alone and surgery plus RT (HR 1.106, 95% CI 0.923–1.325, P = 0.274). Similar trends were found after stratification age at diagnosis, gender, tumor location, and marital status.
Conclusions
Definitive RT but not postoperative RT can increase the risk of CVM among older HNC survivors. Long-term follow-up and regular screening for CVD are required for HNC patients who received definitive RT to decrease the risk of CVM.
Similar content being viewed by others
Background
Head and neck cancer (HNC) accounts for approximately 3–5% of newly diagnosed cancers each year [1]. Surgery and radiotherapy (RT) remain the cornerstone for local treatment of HNC, and postoperative RT should be administrated in those with high-risk recurrence factors [2, 3]. However, several studies have shown that receiving RT increases the risk of cerebrovascular disease (CVD) or cerebrovascular mortality (CVM) in HNC patients [4,5,6], and the excess risk of CVD among HNC survivors increases with attained age [7]. It might be due to the chronic damage of the vasculatures by the use of RT in the head and neck region [8, 9]. Therefore, once RT has been completed, physicians, patients, and even insurance practitioners should jointly evaluate the risk of CVD or CVM.
Although several prior studies have assessed the CVD or CVM risk after RT, several limitations should be noticed in these studies [4, 6, 10, 11], including no distinction between definitive and adjuvant RT [6, 10], short follow-up time for survivors [4], and excluded laryngeal cancer patients [4]. Thus, a well-designed study with long-term follow-up is needed to reflect the current relationship between RT and CVD or CVM. In light of this, we used a large cohort of HNC survivors from the Surveillance, Epidemiology, and End Results (SEER) program to analyze the relationship between RT and the risk of CVM.
Material and methods
SEER database and patients
The SEER database is the authoritative source of information regarding the incidence and survival of cancer in the United States (US), which covers approximately 35 percent of the US population [12]. The study cohort consisted of patients with HNC survivors aged ≥ 65 years who had undergone surgery, RT, or surgery plus adjuvant RT between 2000 and 2012. Patients were excluded if they (1) had no positive histology, (2) had died with other causes (non-cerebrovascular mortality), (3) had no anti-cancer local treatment or unknown the local treatment status, (4) had non-beam RT technologies including radioisotopes and radioactive implants, (5) had follow-up time ≤ 12 months. Since this SEER program includes the de-identified data of patients, the current study was exempted from approval by the Institutional Review Board.
Measurements
We included the following demographic, clinicopathological variables, and survival outcomes of each patient: age, gender, race/ethnicity, tumor grade, histology, SEER stage, tumor location, marital status. Treatment variables, including surgery, definitive RT, adjuvant RT, and chemotherapy were also included. The primary outcome in the study was CVM, which was defined as the time from the initial diagnosis of HNC to death from CVD.
Statistical analysis
We compared the risk of CVM among the three treatment groups including surgery alone, RT alone, and surgery plus RT. The differences in categorical variables among the three treatment cohorts were tested by Pearson's chi-squared test. The rate of CVM was estimated by the Kaplan–Meier method and compared by the log-rank test. Multivariate Cox proportional hazards models were used to determine the risk factors associated with CVM. Sensitivity analyses focused on age, gender, tumor sites, and marital status were performed. Statistical analyses were conducted with SPSS statistical software (version 22.0, IBM Corporation, Armonk, NY, USA). A P value of < 0.05 was considered statistically significant.
Results
Patient characteristics
We included 16,923 patients in this study (Table 1). Figure 1 depicts the patient selection flowchart of the study. The Median age at the time of diagnosis was 71 years (range, 65–100 years), 67.2% (n = 11,380) were male, 79.9% (n = 13,518) were Non-Hispanic White and 82.9% (n = 14,031) had squamous histology. At diagnosis, 58.3% (n = 9869), 32.4% (n = 5479), 6.6% (n = 1111) of patients had localized, regional, and distant stage, respectively. Most cancers developed in the oral cavity (35.3%) and larynx (26.4%). Of these patients, 7110 (42.0%) patients received surgery alone, 5041 (29.8%) patients underwent RT alone, and 4772 (28.2%) patients were treated with surgery and RT. Most patients with tumors located in the oral cavity had undergone surgery alone (57.1%), whereas patients with tumors developed in the oropharynx (48.6%), nasopharynx (77.6%), hypopharynx (68.6%), and larynx (50.8%) were more likely to treat with RT alone. In addition, patients with tumors developed in the salivary gland (55.8%), and nasal cavity and sinus (45.5%) were more likely to received surgery and RT. Moreover, only 23.5% (n = 3974) of patients received chemotherapy.
The incidence of CVM
With a median follow-up time of 87 months (range, 13–203 months), 1005 patients died with CVD: 423 (42.1%) in the surgery alone cohort, 324 (32.2%) in the RT alone cohort, and 258 (25.7%) in the surgery plus RT cohort. The 3-, 5-, 10-, and 15-years of CVM was 1.8%, 3.3%, 7.2%, and 11.5%, respectively.
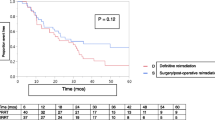
The risk of CVM was highest in patients receiving RT alone than those receiving surgery alone or surgery plus RT (P < 0.001). The 5-years CVM was 8.1% in patients with RT alone compared with 6.9% in the surgery alone cohort and 6.8% in the surgery plus RT cohort. The corresponding 10-years CVM were 13.3%, 10.8%, and 11.2%, respectively (Fig. 2; Table 2). The mean time for CVM was shorter in RT alone compared to surgery alone and surgery plus RT (52 months vs. 56–60 months).
Risk factors associated with CVM
We used the Cox proportional hazard regression model to investigate the risk factors associated with CVM (Table 3). After adjustment by age, gender, race/ethnicity, histology, SEER stage, grade, tumor location, chemotherapy, and marital status, our results showed that patients receiving RT alone had a significantly higher risk of developing CVM compared to those receiving surgery alone (hazard ratio [HR] 1.703, 95% confidence interval [CI] 1.398–2.075, P < 0.001), which comparable risk of CVM was found between those treated with surgery alone and surgery plus RT (HR 1.106, 95% CI 0.923–1.325, P = 0.274). In addition, age at diagnosis, chemotherapy, race/ethnicity, tumor grade, SEER stage, and marital status were also the independent risk factors associated with cerebrovascular mortality. Similar trends were found after stratification by age at diagnosis, gender, tumor location, SEER stage, and marital status (Table 3). In patients without chemotherapy, we also found a significant effect of definitive RT on the increase of CVM. As only 70 patients receiving chemotherapy in the surgery alone group, we used surgery plus RT as a reference in the final Cox proportional hazard regression model. We also found a significant effect of definitive RT on the increase of CVM compared to those receiving surgery plus RT (HR 1.779, 95% CI 1.210–2.615, P = 0.003), and comparable risk of CVM between those treated with surgery alone and surgery plus RT (HR 2.320, 95% CI 0.903–5.957, P = 0.080) (Table 3).
Discussion
In the current study, we analyzed the effect of RT on CVM in HNC survivors aged ≥ 65 years using a population-based cohort. Our results showed that older HNC survivors receiving RT alone had an increased risk of CVM compared to those receiving surgery alone or surgery plus RT. However, comparable CVM was found between surgery alone and surgery plus RT cohorts. Our findings are significant because CVM is one of the few long-term fatal toxicities of RT. Moreover, most previous studies utilized the CVD as study endpoint, no CVM.
An analysis using traditional RT technology included younger patients aged < 60 years from 1977 to 1998. It was found that any RT could significantly increase the risk of stroke (relative risk 10.1, 15-years risk of stroke 12.0%) [5]. A previous SEER-Medicare study included 6862 patients aged > 65 years (diagnosed between 1992 and 2002), with a median follow-up time was 3.2 years for those who survived, the 5-years incidence of cerebrovascular events was 14–19% [4]. A large cohort from Korea included 5570 HNC patients diagnosed between 2003 and 2005, 77.0% of patients receiving 2-dimensional RT, the 10-years ischemic cerebrovascular disease was approximately 5% [10]. A comprehensive study of 17 related literature published from 1981 to 2009 showed that the prevalence of transient ischemic attack and ischemic stroke after RT was 2.3–17.7% [13]. In our study, we included 16,923 HNC survivors with a median follow-up of 87 months, the 5-, 10-, and 15-years of CVM was 3.3%, 7.2%, and 11.5%, respectively. The study period of the present study is in the mode of contemporary radiation technology, although we use the CVM as the end-point of follow-up, it seems to be no significant difference in the incidence of cerebrovascular events or CVM among different RT technologies. In our study, 75% of patients had tumors developed in the oral cavity, pharynx, or larynx, which were prone to cervical lymph node metastasis and most of the radiation target volume included the neck.
Several studies have tried to answer the question regarding the relationship between RT and the risk of CVD or CVM in HNC. The results from the Korean cohort found that the receipt of RT increased the CVD risk by 40.8% than the surgery alone group [10]. However, RT did not increase the risk of CVM. In addition, they did not group the patients for definitive RT or postoperative RT. The study by Arthurs et al. included 14,609 patients from Canada, they found that RT alone (HR 1.70) or any RT cohort (HR 1.46) had a significantly higher risk of ischemic stroke compared to surgery alone [6]. However, they also did not group the patients for definitive RT or postoperative RT. In a study from Taiwan province of China included 10,172 HNC patients, they found that in patients aged < 55 years, RT alone had a higher risk of stroke event than those treated with surgery alone or surgery plus RT, and comparable risk of stroke event was found between surgery alone and surgery plus RT [11]. However, no statistical difference was found regarding stroke risk among different treatment modalities in those aged ≥ 55 years. A previous SEER-Medicare study included 6862 patients aged > 65 years (diagnosed between 1992 and 2002), the median follow-up months was 3.2 years for those who survived, they found that definitive RT but not adjuvant RT was associated with a higher CVD risk in these patients. The 10-years risk of cerebrovascular events was 34%, 25%, and 26% in those receiving RT alone, surgery plus RT, and surgery alone, respectively (4). However, laryngeal cancer patients were excluded from this study. In our study, approximately 25% of HNC patients were tumors located in the larynx, so the lack of analysis of the data regarding the larynx may have a potential impact on the results. Two studies from early-stage glottic cancer have found that the risk of CVD or CVM in patients receiving definitive RT was significantly higher than that in patients receiving surgery alone [14, 15].
In our study, older HNC patients receiving RT alone had increased CVM risk compared to those receiving surgery with or without RT. Our finding is consistent with the independent effect of RT on CVD or CVM in other cancers, including breast cancer [16, 17] and brain tumors [18, 19], which may expose high RT dose to carotid vessels or central arterial circulations.
Although patients receiving definitive RT have a higher risk of CVM (15-year 13.3%), we should note that the 15-year CVM of patients receiving surgery alone and surgery plus RT was 10.8% and 11.2%, respectively. In a previous large cohort study including 14,069 HNC patients at all ages (68.8% of patients were < 65 years old), the 15-year CVM of patients who underwent surgery alone was about 7.0% [6]. In addition, a previous SEER study showed a 10-year CVM of 0.64% in brain tumors patients who received postoperative RT [18], which was significantly lower than our study. Older HNC patients have more vascular risk factors compared to their younger counterparts, such as diabetes and hypertension affect the risk of CVM. Therefore, older age is a risk factor for the increased risk of CVM [7]. Moreover, it is important to note that complications caused by surgery, including fibrosis and adhesions, may also have adverse effects in the smaller vessels and carotid, leading to the increased risk of CVM in the surgery alone group [20]. Brown et al. found that the risk of carotid artery stenosis was 32% in patients who underwent neck lymph node dissection, which was significantly higher than those who had not (4%) [21]. Therefore, complete assessment of the modifiable risk factors and regular surveillance should be performed in older HNC patients who received different treatment strategies for early detection and intervention of CVD.
In terms of mechanism, RT-related atherosclerotic changes in the carotid arteries, hypertrophy of the intima, thickening and fibrosis of the endothelium and muscular wall thickening, and dysfunction of the elastic membrane may contribute to the vascular compromise and ischemic events in HNC patients [8, 22, 23]. However, patients who were treated with surgery plus RT did not associate with a higher CVM risk in our study. The reduction in the total postoperative RT dose to the head and neck regions may explain this phenomenon [4, 24, 25]. Haynes et al. included 413 patients who received RT to the head and neck. A total of 20 patients developed experienced CVD and all of these 20 patients had received more than 60 Gy, which suggested a significant link between RT dose and the development of CVD [26]. The study from Duke Cancer Institute also found that the higher risk of carotid artery stenosis was associated with a higher RT dose to carotid bulb plus 2 cm [27]. In addition, the dose to the carotid arteries was also had an independent effect on the risk of CVD (HR = 1.11, P < 0.001) [28]. In the current clinical practice, HNC patients who received postoperative RT typically received total RT doses of approximately 54–63 Gy (60–66 Gy in patients with adverse features), whereas patients treated with definitive RT typically received 66–70 Gy [29]. The previous study including older breast cancer patients reported no increased risk of CVD in patients receiving postoperative supraclavicular irradiation with an RT dose typically between 45 and 50 Gy [30]. The RT dose is highest in those receiving definitive RT or radiochemotherapy. Therefore, we can make a hypothesis that the dose threshold for developing clinically significant CVD may be between 66 and 70 Gy and the incidence of CVD or CVM is a dose-dependency. For HNC patients, the effect of surgery or postoperative RT on vascular stenosis is considered insignificant, while definitive RT contributed to a higher risk of CVD or CVM.
The mean time for CVM was shorter in the RT alone group than those in the surgery alone group or surgery plus RT group (52 months vs. 56–60 months). The study by Lee et al. showed a mean time to CVD was 64 and 62 for those treated with non-RT and RT groups, respectively [10]. Since the chronic damage to the artery caused by RT may be long-term, the incidence of CVD or CVM after RT is still possible during the long-term follow-up. Therefore, it is necessary to perform a long-term follow-up and regular screening of CVD risks for HNC patients who received definitive RT. However, the CVD risk caused by RT should not be potentially exaggerated or overestimated beyond the hazards by traditional risk factors.
Several limitations warrant mention in this study. First, SEER only records CVM but not CVD as isolated events. Second, the details of RT, including RT technology, RT dose, fractionation utilized, specific RT fields, and target volume definition are not captured in the SEER database. Therefore, treatment groups in the present study may not reflect completely homogeneous therapeutic strategies. Third, it is a large difference in RT fields and dosages if an early-stage laryngeal cancer has been treated by RT alone (for example 66 Gy) or a locally advanced laryngeal cancer has been treated by radiochemotherapy (70 Gy or above). Therefore, the effects of different treatment strategies among HNC patients on the CVM may be different by stage. However, we also found a significant effect of definitive RT on the increase of CVM regardless of the SEER stage in the stratified analyses. Interestingly, we found that with the increase in the SEER stages, the effect of definitive RT on CVM increased significantly. Moreover, a previous SEER-Medicare study including 6862 HNC patients (age > 65 years), and approximately 40% of patients had comorbidities, patients with severe comorbid disease were more likely to receive RT alone [4]. Due to the lack of comorbidity status in the SEER database, it is worth noting that unidentified confounding factors may exist for the relation to CVM in patients receiving RT alone. Future studies with more detailed RT and comorbidity status are required to further rule out potential confounding factors to determine the true magnitude of relation in patients receiving RT alone. Finally, information regarding the comorbidities, alcohol consumption, and smoking status are also not available in the SEER database. However, a previous compliance study on the HNC RT trials found that nearly 90% of patients completely received the prescribed RT doses [31].
Conclusion
In conclusion, our study indicates that definitive RT can increase the risk of CVM among older HNC survivors. Long-term follow-up and regular screening for CVD are required for HNC patients who received definitive RT to decrease the risk of CVM. It is necessary to further optimize the RT technology and the delineation of the target volume to minimize the dose to the carotid artery to reduce the risk of CVM.
Availability of data and materials
Any request for data and material may be sent to the corresponding author.
Abbreviations
- HNC:
-
Head and neck cancer
- RT:
-
Radiotherapy
- CVD:
-
Cerebrovascular disease
- CVM:
-
Cerebrovascular mortality
- SEER:
-
Surveillance, Epidemiology, and End Results
- US:
-
United States
- HR:
-
Hazard ratio
- CI:
-
Confidence interval
References
Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2021. CA Cancer J Clin. 2021;71(1):7–33.
Chow LQM. Head and neck cancer. N Engl J Med. 2020;382(1):60–72.
Marur S, Forastiere AA. Head and neck squamous cell carcinoma: update on epidemiology, diagnosis, and treatment. Mayo Clin Proc. 2016;91(3):386–96.
Smith GL, Smith BD, Buchholz TA, Giordano SH, Garden AS, Woodward WA, et al. Cerebrovascular disease risk in older head and neck cancer patients after radiotherapy. J Clin Oncol. 2008;26(31):5119–25.
Dorresteijn LD, Kappelle AC, Boogerd W, Klokman WJ, Balm AJ, Keus RB, et al. Increased risk of ischemic stroke after radiotherapy on the neck in patients younger than 60 years. J Clin Oncol. 2002;20(1):282–8.
Arthurs E, Hanna TP, Zaza K, Peng Y, Hall SF. Stroke after radiation therapy for head and neck cancer: What is the risk? Int J Radiat Oncol Biol Phys. 2016;96(3):589–96.
Bright CJ, Hawkins MM, Guha J, Henson KE, Winter DL, Kelly JS, et al. Risk of cerebrovascular events in 178 962 five-year survivors of cancer diagnosed at 15 to 39 years of age: the TYACSS (Teenage and Young Adult Cancer Survivor Study). Circulation. 2017;135(13):1194–210.
Fernández-Alvarez V, López F, Suárez C, Strojan P, Eisbruch A, Silver CE, et al. Radiation-induced carotid artery lesions. Strahlenther Onkol. 2018;194(8):699–710.
Murphy ES, Xie H, Merchant TE, Yu JS, Chao ST, Suh JH. Review of cranial radiotherapy-induced vasculopathy. J Neurooncol. 2015;122(3):421–9.
Lee JY, Kim YA, Kim HS, Back JH, Jung YH, Lee DH, et al. Radiotherapy can increase the risk of ischemic cerebrovascular disease in head and neck cancer patients: a Korean population-based cohort study. Radiother Oncol. 2020;142:85–91.
Huang YS, Lee CC, Chang TS, Ho HC, Su YC, Hung SK, et al. Increased risk of stroke in young head and neck cancer patients treated with radiotherapy or chemotherapy. Oral Oncol. 2011;47(11):1092–7.
Surveillance, Epidemiology, and End Results (SEER) Program (www.seer.cancer.gov) SEER*Stat Database: Incidence—SEER Research Plus Data, 18 Registries, Nov 2020 Sub (2000–2018)—Linked To County Attributes—Total U.S., 1969–2019 Counties, National Cancer Institute, DCCPS, Surveillance Research Program, released April 2021, based on the November 2020 submission.
Plummer C, Henderson RD, O’Sullivan JD, Read SJ. Ischemic stroke and transient ischemic attack after head and neck radiotherapy: a review. Stroke. 2011;42(9):2410–8.
Hong JC, Kruser TJ, Gondi V, Mohindra P, Cannon DM, Harari PM, et al. Risk of cerebrovascular events in elderly patients after radiation therapy versus surgery for early-stage glottic cancer. Int J Radiat Oncol Biol Phys. 2013;87(2):290–6.
Swisher-McClure S, Mitra N, Lin A, Ahn P, Wan F, O’Malley B, et al. Risk of fatal cerebrovascular accidents after external beam radiation therapy for early-stage glottic laryngeal cancer. Head Neck. 2014;36(5):611–6.
Killander F, Anderson H, Kjellén E, Malmström P. Increased cardio and cerebrovascular mortality in breast cancer patients treated with postmastectomy radiotherapy–25 year follow-up of a randomised trial from the South Sweden Breast Cancer Group. Eur J Cancer. 2014;50(13):2201–10.
Stokes EL, Tyldesley S, Woods R, Wai E, Olivotto IA. Effect of nodal irradiation and fraction size on cardiac and cerebrovascular mortality in women with breast cancer treated with local and locoregional radiotherapy. Int J Radiat Oncol Biol Phys. 2011;80(2):403–9.
Aizer AA, Du R, Wen PY, Arvold ND. Radiotherapy and death from cerebrovascular disease in patients with primary brain tumors. J Neurooncol. 2015;124(2):291–7.
Lo AC, Howard AF, Nichol A, Hasan H, Martin M, Heran M, et al. A cross-sectional cohort study of cerebrovascular disease and late effects after radiation therapy for craniopharyngioma. Pediatr Blood Cancer. 2016;63(5):786–93.
Landeen KC, Spanos WC, Gromer L. Topical superoxide dismutase in posttreatment fibrosis in patients with head and neck cancer. Head Neck. 2018;40(7):1400–5.
Brown PD, Foote RL, McLaughlin MP, Halyard MY, Ballman KV, Collie AC, et al. A historical prospective cohort study of carotid artery stenosis after radiotherapy for head and neck malignancies. Int J Radiat Oncol Biol Phys. 2005;63(5):1361–7.
Bowers DC, Liu Y, Leisenring W, McNeil E, Stovall M, Gurney JG, et al. Late-occurring stroke among long-term survivors of childhood leukemia and brain tumors: a report from the Childhood Cancer Survivor Study. J Clin Oncol. 2006;24(33):5277–82.
Bowers DC, McNeil DE, Liu Y, Yasui Y, Stovall M, Gurney JG, et al. Stroke as a late treatment effect of Hodgkin’s Disease: a report from the Childhood Cancer Survivor Study. J Clin Oncol. 2005;23(27):6508–15.
Storey MR, Garden AS, Morrison WH, Eicher SA, Schechter NR, Ang KK. Postoperative radiotherapy for malignant tumors of the submandibular gland. Int J Radiat Oncol Biol Phys. 2001;51(4):952–8.
Cooper JS, Pajak TF, Forastiere AA, Jacobs J, Campbell BH, Saxman SB, et al. Postoperative concurrent radiotherapy and chemotherapy for high-risk squamous-cell carcinoma of the head and neck. N Engl J Med. 2004;350(19):1937–44.
Haynes JC, Machtay M, Weber RS, Weinstein GS, Chalian AA, Rosenthal DI. Relative risk of stroke in head and neck carcinoma patients treated with external cervical irradiation. Laryngoscope. 2002;112(10):1883–7.
Dorth JA, Patel PR, Broadwater G, Brizel DM. Incidence and risk factors of significant carotid artery stenosis in asymptomatic survivors of head and neck cancer after radiotherapy. Head Neck. 2014;36(2):215–9.
van Aken ESM, van der Laan HP, Bijl HP, Van den Bosch L, van den Hoek JGM, Dieters M, et al. Risk of ischaemic cerebrovascular events in head and neck cancer patients is associated with carotid artery radiation dose. Radiother Oncol. 2021;157:182–7.
National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: head and neck cancer; 2021. Accessed 14 Aug 2021.
Woodward WA, Giordano SH, Duan Z, Hortobagyi GN, Buchholz TA. Supraclavicular radiation for breast cancer does not increase the 10-year risk of stroke. Cancer. 2006;106(12):2556–62.
Khalil AA, Bentzen SM, Bernier J, Saunders MI, Horiot JC, Van Den Bogaert W, et al. Compliance to the prescribed dose and overall treatment time in five randomized clinical trials of altered fractionation in radiotherapy for head-and-neck carcinomas. Int J Radiat Oncol Biol Phys. 2003;55(3):568–75.
Acknowledgements
The authors acknowledge the efforts of the Surveillance, Epidemiology, and End Results (SEER) Program tumor registries in the creation of the SEER database.
Funding
None available.
Author information
Authors and Affiliations
Contributions
QSH, ZPW, ZJL, SFC, and SGW drafted the manuscript. SGW performed the statistical analyses. PZ and CLL participated in the design of the study. SFC and SGW conceived of the study. SGW acquired the datasets. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
This study was based on the publicly available SEER database and we have got permission to access them for research only (Reference number: 11025-Nov2016). As the SEER database consists of de-identified information, the study was exempt from the approval process of the Institutional Review Boards of the First Affiliated Hospital of Xiamen University.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
He, QS., Wang, ZP., Li, ZJ. et al. Increased risk of cerebrovascular mortality in head and neck cancer survivors aged ≥ 65 years treated with definitive radiotherapy: a population-based cohort study. Radiat Oncol 16, 185 (2021). https://doi.org/10.1186/s13014-021-01913-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13014-021-01913-3