Abstract
Objectives
To report overall survival and local control for patients identified in the RSSearch® Patient Registry with metastatic cancer to the lung treated with SBRT.
Methods
Seven hundred two patients were identified with lung metastases in the RSSearch® Registry. Of these patients, 577 patients had SBRT dose and fractionation information available. Patients were excluded if they received prior surgery, radiation, or radiofrequency ablation to the SBRT treated area. Between April 2004-July 2015, 447 patients treated with SBRT at 30 academic and community-based centers were evaluable for overall survival (OS). Three hundred four patients with 327 lesions were evaluable for local control (LC). All doses were converted to Monte Carlo equivalents and subsequent BED Gy10 for dose response analysis.
Results
Median age was 69 years (range, 18–93 years). Median Karnofsky performance status (KPS) was 90 (range 25/75% 80–100). 49.2% of patients had prior systemic therapy. Median metastasis volume was 10.58 cc (range 25/75% 3.7–25.54 cc). Site of primary tumor included colorectal (25.7%), lung (16.6%), head and neck (11.4%), breast (9.2%), kidney (8.1%), skin (6.5%) and other (22.1%). Median dose was 50 Gy (range 25/75% 48–54) delivered in 3 fractions (range 25/75% 3–5) with a median BED of 100Gy10 (range 25/75% 81–136).
Median OS for the entire group was 26 months, with actuarial 1-, 3-, and 5-year OS of 74.1%, 33.3, and 21.8%, respectively. Patients with head and neck and breast cancers had longer median OS of 37 and 32 months respectively, compared to colorectal (30 months) and lung (26 months) which corresponded to 3-year actuarial OS of 51.8 and 47.9% for head and neck and breast respectively, compared to 35.8% for colorectal and 31.2% for lung.
The median LC for all patients was 53 months, with actuarial 1-, 3-, and 5-year LC rates of 80.4, 58.9, and 46.3%, respectively. There was no difference in LC by primary histologic type (p = 0.49). Improved LC was observed for lung metastases that received SBRT doses of BED ≥100Gy10 with 3-year LC rate of 77.1% compared to 45% for lung metastases treated with BED < 100Gy10 (p = 0.01). Smaller tumor volumes (<11 cc) had improved LC compared to tumor volumes > 11 cc. (p = 0.005) Two-year LC rates for tumor volumes < 11 cc, 11–27 cc and > 27 cc were 72.9, 64.2 and 45.6%, respectively. This correlated with improved OS with 2-year OS rates of 62.4, 60.9 and 46.2% for tumor volumes < 11 cc, 11–27 cc and > 27 cc, respectively (p = 0.0023). In a subset of patients who received BED ≥100Gy10, 2-year LC rates for tumor volumes < 11 cc, 11–27 cc and > 27 cc were 82.8, 58.9 and 68.6%, respectively (p = 0.0244), and 2-year OS rates were 66.0, 58.8 and 28.5%, respectively (p = 0.0081).
Conclusion
Excellent OS and LC is achievable with SBRT utilizing BED ≥100Gy10 for lung metastases according to the RSSearch® Registry data. Patients with small lung metastases (volumes < 11 cc) had better LC and OS when using SBRT doses of BED ≥100Gy10. Further studies to evaluate a difference, if any, between various tumor types will require a larger number of patients.
Similar content being viewed by others
Introduction
Pulmonary metastases are a very frequent occurrence in patients with cancer. One series of a thousand patients found that 50% who suffered a malignancy-related death had the presence of pulmonary metastases at the time of autopsy [1]. A large surgical series of cancer patients with lung metastases treated with metastasectomy revealed a 15-year survival of 22%, an unexpected outcome for patients with stage 4 disease [2]. Researchers have found genomic differences in microRNA expression of these limited metastatic tumors compared to their widely metastatic counterparts, lending credence to the idea that our binary system of local or metastatic disease might be incorrect [3, 4]. Hellman et al. coined a limited metastatic state titled oligometastases where aggressive surgical and ablative therapies could potentially lead to long disease free intervals [5].
Metastasectomy for lung metastases has been the standard of care but is often not possible due to medical comorbidities, extrathoracic disease, unresectable metastases, or short disease free intervals. An ablative therapy such as stereotactic body radiation therapy (SBRT) has been reported in many retrospective reports for lung metastases however, with limited sample size [6]. We present a large series of metastatic lung tumors treated with SBRT using the RSSearch® Registry.
Materials and methods
The RSSearch® Registry is an international, web-based registry designed for SBRT and stereotactic radiosurgery (SRS) research with currently over 18,000 patients enrolled (www.clinicaltrials.gov/NCT01885299) [7]. RSSearch® was designed to standardize the collection of patient screening, treatment and outcome data for patients treated with SBRT and SRS with the goal of conducting research outcomes analysis to identify the most effective and appropriate clinical uses of SRS/SBRT. RSSearch® is managed by the Radiosurgery Society, a non-profit, professional medical society (www.therss.org) and adheres to the Health Insurance Portability and Accountability Act (HIPAA) in all domains including database security, data transmission, and confidentiality. The database is contracted and maintained by Advertek (Nashville, TN). An audit was performed by the study investigators of sites participating in this study which outlined missing data points. Centers were asked to provide missing data which was generally successful in recapturing this data; however we are unable to quantify its success rate.
Data collected in RSSearch® includes the following categories: patient demographics, treated lesion (size, volume, location), treatment plan including use of surgery or chemotherapy, information on SBRT delivery including dose and fractionation, toxicity, symptom control, lesion response, survival data, and progression data. Aggregate de-identified data is accessible by RSSearch® administrators. Requests for retrospective data analysis are sent to the RSSearch® Review Committee, which approves or denies all requests for data.
Lesion locations and SBRT treatment sites are described using the World Health Organization (WHO) International Classification of Diseases (ICD), version 9 codes. Toxicity data is coded using the Common Toxicity Criteria for Adverse Event Reporting, version 3. The majority of patients were treated with the CyberKnife™ Robotic Radiosurgery System (Accuray Inc., Sunnyvale, CA) and 2 patients were treated with Truebeam (Varian Medical Systems, Palo Alto, CA). Due to the nature of the current study using registry data, no pre-defined treatment planning criteria were enforced and instead relied upon individual institutional guidelines. After consensus review of the physicians from the treatment sites, the majority of patients were simulated in the supine position using computed tomography (CT) scanning above and below the region of interest during inspiration, expiration, and free breathing. One millimeter slice reconstruction of the treatment planning area was transferred to the treatment planning station. Positron emission tomography (PET) scans were used to aid in target volume delineation via image fusion to the CT scan. Target volumes were delineated by physician (radiation oncologist, pulmonologist, or surgeon) using CT and PET scans. Gross tumor volume (GTV) was often used as the clinical target volume (CTV), with a 3–10 mm margin added circumferentially to define the planning target volume (PTV).
Real time tumor tracking was incorporated for patients treated with CyberKnife using the Synchrony® Respiratory Motion Tracking System. Radiation dosimetry on patients treated on the CyberKnife system was planned using the MultiPlan® System (Accuray Incorporated, Sunnyvale, CA) which incorporated non-isocentric and non-coplanar radiation delivery using Monte Carlo or Ray Tracing algorithms. Ray Tracing generally overestimates tumor dose in the lung due to lack of capacity to account for the lung-tumor density heterogeneity. Therefore Ray Tracing dose was converted into a more accurate Monte Carlo equivalent dose using an equation based on the tumor size. Patients who did not have tumor size information available within the RSSearch® Registry were excluded from BED analysis (n = 20). In comparing various fractionation schema and doses, biologically effective dose (BED Gy10) was calculated using the linear quadratic model.
All centers performing SBRT/SRS are able to participate in RSSearch®. No compensation is given to patient participants or participating centers. Institutional Review Board (IRB) approval is required at each participating center, and patients must give informed consent. Data is entered into RSSearch® usually in a prospective fashion however retrospective data entry is allowed and coded as such.
Patient follow-up was performed per institutional guidelines and the date of last follow up used for actuarial analysis with all time intervals considered. Patients were censored for survival at time of death and for local control at time of local failure. All participating centers reported follow-up clinical and imaging data. Local progression was evaluated independently for each lesion at the participating institution following a modified RECIST (Response Evaluation and Criteria in Solid Tumors) criteria which defined local progression as at least a 20% increase in the size of lesions and/or appearance of one or more lesions in target treatment location and local control was defined as disappearance of, decrease in, or no increase in size of the treated lesions.
Statistical analysis and Kaplan-Meier survival curves were performed using GraphPad and Instat Software, La Jolla, CA. Overall survival was calculated for each patient using the first date of SBRT to date of death or date of last follow up. Specific cause of death was not reported for all patients in RSSearch® and therefore not evaluated in this study. Local failure was determined for each treated tumor using last date of SBRT to date of physician reported failure. Subgroups were compared using X 2, log-rank tests and Gehan-Breslow Wilcoxon tests. Values of p < 0.05 were considered statistically significant.
For more information on the background on the RSSearch® Registry, please see the previous descriptive paper on its creation and use [7, 8].
Results
Patient characteristics
Between April 2004 and July 2015, 702 patients with lung metastases from 28 centers in the US, one center from Germany and one center from Australia, were identified in the RSSearch®Patient Registry. Of those patients, 577 had dose and fractionation information available. One hundred thirty patients were excluded from the study because of previous surgery, SBRT, or radiofrequency ablation (RFA) to the SBRT-treated area. This resulted in 447 patients with lung metastases treated with SBRT evaluable for survival. We are unable to say with certainty that these patients had no other metastatic sites due to the nature of our data collection. There were 304 of these patients with 327 lesions evaluable for LC. Median age of the group was 69 years (range 18–93). Additional patient demographics and characteristics are found in Table 1.
Tumor characteristics
Primary tumor types included colorectal (CRC) (25.7%), lung (16.6%), head and neck (H&N) (11.4%), breast (9.2%), skin (6.5%), gynecologic (6%), sarcoma (4%), bladder/ureter (4%), and other (16%). Median Karnofsky Performance Status (KPS) was 90 (range 25%/75% 80–100). Median follow up was 13 months (range 25%/75% 6–26 months, max 123 months). Median number of metastases was 1 (range 1–3). Median metastasis size was 10.58 cc (range 0.1–654.5 cc).
Treatment characteristics
Median dose of SBRT to target metastasis was 50 Gy (range 25%/75% 48–54 Gy). The median corrected Monte Carlo dose was 45.6 Gy (range 25%/75% 40.6–50.7 Gy). The median bioequivalent dose was 100Gy10 (range 25%/75% 81–136). Median number of fractions was 3 (range 25%/75% 3–5). The median Monte Carlo dose per fraction was 13 Gy/fraction (range 25%/75% 10–17 Gy/fraction).
Outcome data
Median overall survival (OS) for the entire group was 26 months. The 1-, 3- and 5- year OS were 74.1, 33.3, and 21.8% of patients, respectively (Fig. 1). Median LC for the entire group was 53 months. The 1-, 3-, and 5-year LC rates were 80.4, 58.9, and 46.2%, respectively (Fig. 2).
We investigated whether primary tumor type (breast, lung, colorectal cancer (CRC), head and neck or other) had an effect on LC and OS following SBRT treatment. There was no statistical difference in LC rates (p = 0.485 by log-rank test; p = 0.181 by Gehan-Breslow Wilcoxon test, Fig. 3a) for the primary tumor types. The 2-year LC rates for breast, lung, CRC, head and neck, and other primaries were 72.4, 55.6, 65.4, 74.4 and 63.1%, respectively. A statistical difference in OS rates was observed for primary tumor types, with head and neck patients having a median OS of 37 months, breast cancer patients had a median OS of 32 months, CRC patients had a median OS of 30 months, lung cancer patients had a median OS of 26 months, and all other primary tumors had a median OS of 20 months (p = 0.004 by log-rank test; p = 0.0024 by Gehan-Breslow Wilcoxon test, Fig. 3b). The 3-year OS rates for breast, CRC, lung, head and neck, and other primary tumors were 47.9, 31.3, 35.8, 51.8, and 23.1%, respectively.
We next investigated whether lung metastases tumor volume was associated with LC and/or OS. Because median and average metastasis volumes were 10.58 cc and 26.72 cc, respectively, lesions were stratified into three groups: tumor volume <11 cc, 11–27 cc, and >27 cc. A statistical difference was noted between the three groups, with improved LC for smaller tumors. Two-year LC was 72.9, 64.2 and 45.6% for tumor volumes < 11 cc, 11–27 cc and > 27 cc, respectively (p = 0.0005 by log-rank test; p = 0.0011 by Gehan-Breslow Wilcoxon test Fig. 4a). This translated into improved OS, with 2-year OS of 62.4, 60.9, 46.1% for tumor volumes < 11 cc, 11–27 cc, and > 27 cc, respectively, and median OS for lesions <11 cc, 11–27 cc, and >27 cc was 29, 31, and 21 months respectively (p = 0.0023 by log-rank test; p = 0.0011 by Gehan-Breslow Wilcoxon test, Fig. 4b).
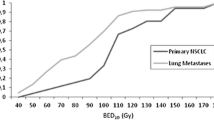
All patients were stratified by BED < 100Gy10 and BED ≥100Gy10 and Kaplan-Meier analysis was used to assess LC and OS. Lung metastases treated with BED ≥100Gy10 had improved LC compared to lesions that received BED ≤ 100Gy10 (Fig. 5a). LC rates at 1-, 3-, and 5-years were 82.6, 71.1, and 66.4%, respectively, for lesions treated with BED ≥ 100Gy10, and 77.6, 44.9 and 30.7%, respectively, for lesions treated with BED < 100Gy10 (p = 0.0148 by log-rank test). This did not translate into a significant difference in OS (p = 0.2064) (Fig. 5b).
Stratification was then performed using only lung metastases treated with BED ≥100Gy10 stratified by lesion volume <11 cc, 11–27 cc, and ≥27 cc. Two-year LC rates for tumors < 11 cc, 11–27 cc and > 27 cc were 82.8% and 58.9% and 68.6%, respectively (p = 0.0244 by log-rank test; p = 0.0122 by Gehan-Breslow Wilcoxon test, Fig. 6a). Two-year OS for patients with tumors < 11 cc, 11–27 cc and 27 cc were 66.0 and 58.8% and 28.5%, respectively (p = 0.008 by log-rank test; p = 0.0133 by Gehan-Breslow Wilcoxon test, Fig. 6b).
Other factors that were evaluated during statistical analysis included age, KPS, gender, and use of chemotherapy, which were all found to be not statistically significant for LC or OS.
Discussion
This RSSearch® Patient Registry analysis represents a report of a large cohort of patients treated with SBRT for lung metastases (n = 447 patients). In comparison, another large study includes 217 patients from a single-institutional prospective series and 95 patients analyzed in a single-institution retrospective study [6, 9, 10]. A meta-analysis reported by Ashworth et al. included 757 patients all with lung cancer; however this study only included 88 patients treated with SBRT and included metastases to all body sites and not only lung metastases [11]. The current study includes a heterogeneous population of patients including large sample sizes of various primary sites, tumor sizes, doses, and patient populations from across the United States, Germany and Australia.
The use of SBRT for lung metastases allows for high ablative doses of radiation with the potential for extended local control and survival as shown in the current analysis. Multiple studies since have found LC rates of isolated or few lung metastases to be 70–100% at 1 year [12–15]. Our LC rate compares favorably at 80.37%. The two-year weighted OS rate compiled in an article review by Alongi et al. was 54% (range 39–84%) [16]. In comparison, the 2-year OS rate for all patients reported in our study compares similarly at 53.02%.
In assessing LC, most studies support using a BED of at least 100Gy10 to have LC comparable to metastasectomy of pulmonary metastases [17, 18]. Doses of less than 100 Gy10 however are still used in clinical practice with high LC rates and minimal toxicity [13]. In our current study, we saw a statistically significant difference with higher LC rates in the BED ≥ 100Gy10 group, adding further evidence for its use. This LC rate did not translate into improved OS for all size lesions, but when stratifying by metastasis volume there was a trend for improved OS in lesions smaller than 11 cc.
In the current study, we saw differences in OS between primary histology types, favoring improved OS for H&N, breast, and CRC but without difference in local control by primary histology. Takeda et al. found CRC lung oligometastases to have poorer LC than other histologies (lung, H&N) [10]. These results do not corroborate with our results, as oligometastatic tumors from lung primaries fared worse in the present study compared to Takeda et al. where other histologies (including lung primary oligometastatic tumors) fared better. Takeda et al. was limited to a small sample size of patients with lung oligometastases (n = 44) of which half were from colorectal cancer compared with our study with a much larger cohort. In addition, other studies have found no relationship between oligometastatic lung tumors from CRC primary vs other primary oligometastatic lung tumors on multivariate analysis (MVA) [14, 19]. Our study also compares oligometastatic lung tumors by primary individually instead of grouping primaries together for comparison.
Our study also found differences in LC and OS rates based on tumor volume, with significantly higher LC and longer OS for smaller volumes. McCammon et al. had similar findings on univariate (UVA) and multivariate (MVA) analysis, finding significant differences in rates of LC comparing values above and below their median tumor volume (8.9 cc), but this study did not see differences in OS [19]. In comparison, two previous studies treated large volume metastases with SBRT, both averaging a median volume of 41 cc per lesion treated, and found a LC rate comparable to small volume lesions, again without reporting OS [20, 21].
Approximately 25% of patients undergoing metastasectomy have long term survival [2, 22, 23]. The remaining patients usually see progression of their disease and development of new metastases within 6 months of ablative therapy [24, 25]. A greater time interval between locoregional disease and metastatic disease portends better prognosis and longer disease-free survival [2, 22]. Other factors portending to longer disease-free survival include having fewer metastases, non-synchronous metastases, stable disease before ablative therapies, estrogen-positive receptor breast cancer primaries, and complete ablations [24–26]. Because this represents a registry for patients treated with SBRT for lung metastases, many of these parameters could not be evaluated.
It is difficult to properly evaluate OS using SBRT for lung metastases in context of comparing it to metastasectomy. There is an absence of phase III randomized controlled trials, and the phase I/II trials that have been completed have patients with widely variable clinical characteristics [16]. Only 1 retrospective study by Yu et al. compared SBRT to metastasectomy in 58 patients with osteosarcoma, with OS at 40% in both groups [27]. In addition, there is a bias in selection of patients for SBRT - they are generally judged to be inoperable due to their medical comorbidities which could significantly affect OS rates [16]. We did not find significant survival differences by KPS however our dataset lacks more rigorous comorbidity scores such as the Charlson/Deyo score needed to provide survival for potentially operable patients [28].
There are weaknesses to the current study including the short median follow-up of 13 months however this follow-up is comparable to other single institution series [29, 30]. Our study includes no predefined treatment planning criteria, defined individually by the participating centers, with variability in dose and fractionation. This variability however allowed for dose response analysis due to wide ranges in dose but may have lowered the local control and survival rates possible if more uniform high dose was utilized in all patients. Our study also has the standard limitations intrinsic to registry studies: allocation of patients is not random and data collection is less robust than randomized clinical trials.
There are ongoing prospective trials that will hopefully answer if a survival benefit is found for patients treated with SBRT for oligometastases. Only one prospective trial from MD Anderson reported in abstract form found a median PFS advantage with three or less sites of oligometastases treated with SBRT, conventional external beam radiation or surgery for non-small cell lung cancer from 3.9 to 11.9 months (p = 0.005) [31]. In addition, other prospective trials continue to accrue including the SABR-COMET, NRG BR002, and the UK CORE trials. Until results of those trials are reported, we will have to rely on prospective registry series like the current study to guide treatment decisions.
Conclusions
SBRT provides extended survival in patients with lung metastases, with the current study providing a 5-year actuarial survival of 21.8%. The 1-, 3-, and 5-year LC rates were 80.4%, 58.9%, and 46.2%, respectively. Smaller tumor size and the primary tumor type (H&N/colon/breast) were associated with prolonged survival. High dose BED (≥100Gy10) and smaller tumor size were associated with prolonged local control.
References
Abrams HL, Spiro R, Goldstein N. Metastases in carcinoma; analysis of 1000 autopsied cases. Cancer. 1950;3:74–85. Available from: http://www.ncbi.nlm.nih.gov/pubmed/15405683.
Pastorino U, Buyse M, Friedel G, Ginsberg RJ, Girard P, Goldstraw P, et al. Long-term results of lung metastasectomy: prognostic analyses based on 5206 cases. J Thorac Cardiovasc Surg. 1997;113:37–49. Available from: http://dx.doi.org/10.1016/S0022-5223(97)70397-0.
Lussier YA, Khodarev NN, Regan K, Corbin K, Li H, Ganai S, et al. Oligo- and polymetastatic progression in lung metastasis(es) patients is associated with specific microRNAs. PLoS One. 2012;7:e50141. Available from: http://dx.doi.org/10.1371/journal.pone.0050141.
Lussier YA, Xing HR, Salama JK, Khodarev NN, Huang Y, Zhang Q, et al. MicroRNA expression characterizes oligometastasis(es. PLoS One. 2011;6:e28650. Available from: http://dx.doi.org/10.1371/journal.pone.0028650.
Hellman S, Weichselbaum RR. Oligometastasis Editorial. J Clin Oncol January 1995;13:8–10.
Boyer MJ, Ricardi U, Ball D, Salama JK. Ablative approaches for pulmonary metastases. Thorac Surg Clin. 2016;26:19–34. Available from: http://dx.doi.org/10.1016/j.thorsurg.2015.09.004.
Davis JN, Medbery C, Sharma S, Danish A, Mahadevan A. The RSSearch™ Registry: patterns of care and outcomes research on patients treated with stereotactic radiosurgery and stereotactic body radiotherapy. Radiat Oncol. 2013;8:275. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3904782&tool=pmcentrez&rendertype=abstract.
Davis JN, Medbery C, Sharma S, Pablo J, Kimsey F, Perry D, et al. Stereotactic body radiotherapy for centrally located early-stage non-small cell lung cancer or lung metastases from the RSSearch® patient registry. Radiat Oncol. 2015;10:1–10. Available from: http://dx.doi.org/10.1186/s13014-015-0417-5.
Wang Z, Kong Q-T, Li J, Wu X-H, Li B, Shen Z-T, et al. Clinical outcomes of cyberknife stereotactic radiosurgery for lung metastases. J Thorac Dis. 2015;7:407–12. Available from: http://dx.doi.org/10.3978/j.issn.2072-1439.2015.01.09.
Takeda A, Kunieda E, Ohashi T, Aoki Y, Koike N, Takeda T. Stereotactic body radiotherapy (SBRT) for oligometastatic lung tumors from colorectal cancer and other primary cancers in comparison with primary lung cancer. Radiother Oncol. 2011;101:255–9. Available from: http://dx.doi.org/10.1016/j.radonc.2011.05.033.
Ashworth AB, Senan S, Palma DA, Riquet M, Chan Ahn Y, Ricardi U, et al. An Individual Patient Data Metaanalysis of Outcomes and Prognostic Factors After Treatment of Oligometastatic Non-Small-Cell Lung Cancer. Clin. Lung Cancer [Internet]. Elsevier Inc; 2014;1–10. Available from: http://dx.doi.org/10.1016/j.cllc.2014.04.003.
Ricardi U, Filippi AR, Guarneri A, Ragona R, Mantovani C, Giglioli F, et al. Stereotactic body radiation therapy for lung metastases. Lung Cancer. 2012;75:77–81. Available from: http://dx.doi.org/10.1016/j.lungcan.2011.04.021.
Okunieff P, Petersen AL, Philip A, Milano MT, Katz AW, Boros L, et al. Stereotactic Body Radiation Therapy (SBRT) for lung metastases. Acta Oncol. 2006;45:808–17. Available from: http://dx.doi.org/10.1080/02841860600908954.
Wulf J, Baier K, Mueller G, Flentje MP. Dose-response in stereotactic irradiation of lung tumors. Radiother Oncol. 2005;77:83–7. Available from: http://dx.doi.org/10.1016/j.radonc.2005.09.003.
Nuyttens JJ, van der Voort van Zyp NCMG, Verhoef C, Maat A, van Klaveren RJ, van der Holt B, et al. Stereotactic body radiation therapy for oligometastases to the lung: a phase 2 study. Int J Radiat Oncol Biol Phys. 2015;91:337–43. Available from: http://dx.doi.org/10.1016/j.ijrobp.2014.10.021.
Alongi F, Arcangeli S, Filippi AR, Ricardi U, Scorsetti M. Review and uses of stereotactic body radiation therapy for oligometastases. Oncologist. 2012;17:1100–7. Available from: http://dx.doi.org/10.1634/theoncologist.2012-0092.
Guckenberger M, Wulf J, Mueller G, Krieger T, Baier K, Gabor M, et al. Dose–response relationship for image-guided stereotactic body radiotherapy of pulmonary tumors: relevance of 4D dose calculation. Int J Radiat Oncol Biol Phys. 2009;74:47–54. Available from: http://dx.doi.org/10.1016/j.ijrobp.2008.06.1939.
Norihisa Y, Nagata Y, Takayama K, Matsuo Y, Sakamoto T, Sakamoto M, et al. Stereotactic body radiotherapy for oligometastatic lung tumors. Int J Radiat Oncol Biol Phys. 2008;72:398–403. Available from: http://dx.doi.org/10.1016/j.ijrobp.2008.01.002.
Mccammon R, Schefter TE, Gaspar LE, Zaemisch R, Gravdahl D, Kavanagh B. Observation of a dose–control relationship for lung and liver tumors after stereotactic body radiation therapy. Int J Radiat Oncol Biol Phys. 2009;73:112–8. Available from: http://dx.doi.org/10.1016/j.ijrobp.2008.03.062.
Corbin KS, Ranck MC, Hasselle MD, Golden DW, Partouche J, Wu T, et al. Feasibility and toxicity of hypofractionated image guided radiation therapy for large volume limited metastatic disease. Pract Radiat Oncol. 2013;3:316–22. Available from: http://dx.doi.org/10.1016/j.prro.2012.08.006.
Milano MT, Katz AW, Muhs AG, Abraham P, Buchholz DJ, Schell MC, et al. A prospective pilot study of curative-intent stereotactic body radiation therapy in patients with 5 or fewer oligometastatic lesions. Cancer. 2008;112:650–8. Available from: http://dx.doi.org/10.1002/cncr.23209.
Fong Y, Cohen AM, Fortner JG, Enker WE, Turnbull AD, Coit DG, et al. Liver resection for colorectal metastases. J Clin Oncol. 1997;15:938–46. Available from: http://www.ncbi.nlm.nih.gov/pubmed/9060531.
Tanvetyanon T, Robinson LA, Schell MJ, Strong VE, Kapoor R, Coit DG, et al. Outcomes of adrenalectomy for isolated synchronous versus metachronous adrenal metastases in Non–small-cell lung cancer: a systematic review and pooled analysis. J Clin Oncol. 2008;26:1142–7. Available from: http://jco.ascopubs.org/content/26/7/1142.abstract.
Salama JK, Hasselle MD, Chmura SJ, Malik R, Mehta N, Yenice KM, et al. Stereotactic body radiotherapy for multisite extracranial oligometastases: final report of a dose escalation trial in patients with 1 to 5 sites of metastatic disease. Cancer. 2012;118:2962–70. Available from: http://dx.doi.org/10.1002/cncr.26611.
de Vin T, Engels B, Gevaert T, Storme G, De Ridder M. Stereotactic radiotherapy for oligometastatic cancer: a prognostic model for survival. Ann Oncol. 2014;25:467–71. Available from: http://dx.doi.org/10.1093/annonc/mdt537.
Abbott DE, Brouquet A, Mittendorf EA, Andreou A, Meric-Bernstam F, Valero V, et al. Resection of liver metastases from breast cancer: estrogen receptor status and response to chemotherapy before metastasectomy define outcome. Surgery. 2012;151:710–6. Available from: http://dx.doi.org/10.1016/j.surg.2011.12.017.
Yu W, Tang L, Lin F, Li D, Wang J, Yang Y, et al. Stereotactic radiosurgery, a potential alternative treatment for pulmonary metastases from osteosarcoma. Int J Oncol. 2014;44:1091–8. Available from: http://dx.doi.org/10.3892/ijo.2014.2295.
Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45:613–9. Available from: http://dx.doi.org/10.1016/0895-4356(92)90133-8.
Yoon SM, Choi EK, Lee S-W, Yi BY, Ahn SD, Shin SS, et al. Clinical results of stereotactic body frame based fractionated radiation therapy for primary or metastatic thoracic tumors. Acta Oncol. 2006;45:1108–14. Available from: http://dx.doi.org/10.1080/02841860600812685.
Hamamoto Y, Kataoka M, Yamashita M, Shinkai T, Kubo Y, Sugawara Y, et al. Local control of metastatic lung tumors treated with SBRT of 48 Gy in four fractions: in comparison with primary lung cancer. Jpn J Clin Oncol. 2010;40:125–9. Available from: http://dx.doi.org/10.1093/jjco/hyp129.
Gomez DR, Blumenschein GR, Lee JJ. Local consolidative therapy (LCT) to improve progression-free survival (PFS) in patients with oligometastatic non-small cell lung cancer (NSCLC) who receive …. ASCO Annual [Internet]. meeting.ascopubs.org; 2016; Available from: http://meeting.ascopubs.org/cgi/content/abstract/34/15_suppl/9004
Acknowledgements
We would like to thank the numerous institutions, physicians, health care personnel who enrolled patients in this registry, as well as the patients who agreed to participate in the RSSearch® Patient Registry.
Funding
Educational grant awarded to the Radiosurgery Society from the Radiosurgical Research Institute to support clinical research associates who obtained missing follow-up for this study.
Availability of data and materials
The dataset from which these results have been gleamed is available upon application to participating centers and is contained within the RSSearch® Patient Registry, [insert https://clinicaltrials.gov/ct2/show/NCT01885299?term=RSSearch&rank=1]. Applications are reviewed by the Radiosurgical Society Education Committee.
Authors’ contributions
AR, RL and JY provided data interpretation and manuscript writing. JD provided data collection, data analysis, and statistical support. All authors contributed to patient data collection, and read and approved the final manuscript.
Competing interests
JD was previously employed by Accuray Inc. in the Department of Medical Affairs from March 2006–January 2013. RL and JY are partial stockholders of Philadelphia Cyberknife. All other co-authors have no competing interests.
Consent for publication
Not applicable.
Ethics approval and consent to participate
All participating centers require IRB approval prior to participating in the registry.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Ricco, A., Davis, J., Rate, W. et al. Lung metastases treated with stereotactic body radiotherapy: the RSSearch® patient Registry’s experience. Radiat Oncol 12, 35 (2017). https://doi.org/10.1186/s13014-017-0773-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13014-017-0773-4