Abstract
Background
This report evaluated the breastfeeding status in a Tasmanian cohort and its effects on infant and maternal anthropometry and body composition.
Methods
An observational-cohort analysis of self-reported feeding data from 175 Tasmanian mother-baby dyads (recruited via in-person contact between September 2017 and October 2019), was executed. Only mothers who were ≥ 18 years of age, who had a singleton pregnancy and were able to speak and understand English, were included in the study. Infants outside a gestational age range between 37+ 0 and 41+ 6 weeks were excluded. Infant (using Air Displacement Plethysmography) and maternal body composition was assessed at 0, 3 and 6 months. Analysis of variance with relevant statistical corrections were utilised for cross-sectional and longitudinal comparisons between non-exclusively breastfed (neBF) and exclusively breastfed (eBF) groups.
Results
Fat-free mass was significantly higher [t = 2.27, df = 98, P = 0.03, confidence interval (CI) 0.03, 0.48] in neBF infants at 6 months (5.59 ± 0.59 vs 5.33 ± 0.50 kg) despite a higher mean fat-free mass in eBF infants at birth (2.89 ± 0.34 vs 3.01 ± 0.35 kg). Weak evidence for different fat mass index trajectories was observed for eBF and neBF infants in the first 6 months of life (ANOVA, F = 2.42, df = 1.9, P = 0.09) with an inversion in fat mass index levels between 3 and 6 months. Body Mass Index (BMI) trajectories were significantly different in eBF and neBF mothers through pregnancy and the first 6 months postpartum (ANOVA, F = 5.56, df = 30.14, P = 0.01). Compared with eBF mothers, neBF mothers retained significantly less weight (t = − 2.754, df = 158, P = 0.02, CI -6.64, − 1.09) at 3 months (0.68 ± 11.69 vs 4.55 ± 6.08 kg) postpartum. Prevalence for neBF was incrementally higher in mothers with a normal BMI compared to mothers with obesity, and mothers who underwent surgical or medical intervention during birth were less likely to exclusively breastfeed.
Conclusions
Infants with different feeding patterns may display varying growth patterns in early life and sustained breastfeeding can contribute to greater postpartum maternal weight loss.
Similar content being viewed by others
Background
Prenatal and early postnatal nutrition plays a significant role in reducing the risk of childhood obesity [1, 2]. In this context, breast milk has been identified as one of the most efficient, natural, cost-effective means of optimising nutrition in early life [3, 4]. Although the exact mechanisms are yet to be elucidated, a substantial body of literature supports the preventative capacity of breastfeeding during infancy on later life incidence of obesity and related comorbidities [5,6,7,8]. The benefit to infants from prolonged breast milk consumption is not limited to prevention of obesity; it is also associated with cognitive, immune, and healthy digestive system development [9,10,11].
Breastfeeding/ lactation can also have a profound impact on maternal health. Evidence indicates that breastfeeding can reduce the incidence of numerous metabolic and physiological complications in mothers, including type 2 diabetes [12, 13], metabolic syndrome [14] and cardiovascular disease [15]. Pregnancy is commonly associated with an increase in visceral fat, insulin production and circulating lipid levels [16], and a delayed return to pre-pregnancy levels may place mothers at an elevated risk of developing deleterious metabolic conditions. According to the ‘Reset Hypothesis’, lactation can reverse some of these trends via mobilization of fat stores accumulated during pregnancy and be a potential vehicle for reductions in the incidence of metabolic disease [17]. Lactation also plays an important role in postpartum maternal weight management with exclusive breastfeeding (eBF) acting as an effective means of weight loss following childbirth [18, 19].
Despite the acknowledged importance of eBF as the best possible nutritional start to life for infants, 78 million babies worldwide (estimated in 2018) are not breastfed within the first hour of life [20]. The ideal timeframe for eBF lacks global consensus; however, the World Health Organization (WHO) recommends eBF for at least 6 months for all infants [21,22,23]. Initiation, duration, continuity, and cessation of breastfeeding depend on a multitude of factors including maternal obesity, use of medications (particularly ones that are prescribed at labour), perceived insufficient milk supply, and mode of birth/birth complications [24,25,26,27,28,29]. In addition, socio-economic status is considered to be a major determinant of breastfeeding pattern [30]. Previous reports have suggested that socioeconomically disadvantaged regions in Australia, including in Tasmania, have low rates of breastfeeding compared with other parts of the country [31, 32]. According to the Department of Health and Human Services, 75–85% (as opposed to the recommended 90%) of Tasmanian mothers breastfeed at the time of discharge from hospital following childbirth [33]. Further, the number of women who breastfeed drops over the first 6 months with only 44% of mothers partially breastfeeding (as opposed to the recommended 80%) at 6 months postpartum [33]. This report evaluated breastfeeding trends in a Tasmanian cohort and its effects on both infant and maternal anthropometry and body composition.
Methods
Participants
Participant recruitment and data collection was undertaken as part of a larger study (‘Developing better information globally on young children’s body composition’) at the maternity ward of the Launceston General Hospital in the north of Tasmania, Australia. Briefly, mothers admitted to the postnatal ward at Launceston General Hospital were approached by trained research staff and midwives and given a plain language description of the study. Subsequently, subject to the provision of written informed consent, all interested, eligible mothers were enrolled in the study. Only mothers who were ≥ 18 years of age, who had a singleton pregnancy and were able to speak and understand English, were included in the study. Infants outside a gestational age range between 37+ 0 and 41+ 6 weeks were excluded. Further, women who experienced significant complications at labour (according to the assessment of the attending clinician), and infants born with a congenital anomaly, were also not included. According to self-reported data from consenting mothers, approximately 90% were Caucasian (Tasmanian average 83.5%). Average maternal age was 30.3 years (range 18–48) and infants were 1.7, 87.7 and 180.4 days old (on average) at birth, 3-month and 6-month data collection points, respectively.
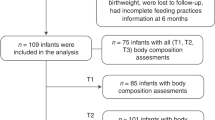
Exclusive breastfeeding was defined as the infant has received only breast milk from his/her mother, or expressed breast milk, and no other substantial amount of liquids or solids with the exception of drops or syrups consisting of vitamins, mineral supplements or medicines since birth [34, 35]. Sixty-three percent of infants were reported to have been eBF to 6 months of age. The remaining 37% were included in the neBF group, who consumed formula or a mixture of breast milk, formula, and solids. A detailed breakdown of participant numbers is shown in Fig. 1.
Body composition and anthropometric measurements
Infant body weight, fat mass and fat-free mass were assessed via air displacement plethysmography (PEA POD, COSMED, Rome, Italy). Briefly, body weight was measured (using the integrated scale) in unclothed infants and hair flattened (with a hair cap or baby oil) prior to placing them in the automatic volume measurement capsule. Subscapular (SS) and triceps (TS) skinfolds were obtained from infants at 3 and 6 months using a calibrated skinfold caliper (Holtain Limited, Croswell, UK). Left mid-upper arm circumference (MUAC) was measured in all infants using a tape at the measured halfway mark between the acromion and olecranon processes.
Prenatal body mass index (BMI) was calculated using self-reported height and weight of participating mothers. For relevant analyses, mothers were allocated into BMI categories (underweight < 18.5; healthy weight 18.5–24.9; overweight 25.0–29.9 and obese, > 30.0 kg m− 2) according to World Health Organisation BMI criteria. Maternal postpartum body weight retention, length corrected fat mass and weight-for-length (WFL) in infants were enumerated as follows:
Maternal body weight and height measures (apart from prenatal height and weight which was self-reported) were completed in duplicate (to maintain reliability) at each of the visits. Body weight was recorded to the nearest gram using a digitized scale (SECA Corp. Hamburg, Germany) and height was measured to the nearest millimetre using a stadiometer (SECA Corp. Hamburg, Germany).
Statistical analyses
All statistical analyses were conducted using the Statistical Package for the Social Sciences (SPSS) software (SPSS Version 26, Inc., Chicago, IL, USA). Results are presented as means and standard deviations, unless specified otherwise. Cross-sectional comparisons between eBF and neBF mothers and infants were executed using independent sample t-tests. Longitudinal differences in WFL, fat mass index and BMI between eBF and neBF groups were evaluated using a repeated measures analysis of variance (ANOVA). In the event of violation of sphericity assumptions, Greenhouse–Geisser or Huynh Feldt corrections were applied as appropriate. Relationship between skinfold measures, MUAC, maternal pre-pregnancy BMI and infant fat mass content was assessed using Pearson correlation coefficient. Statistical significance was set at p < 0.05.
Results
Infant body mass and composition
Body weight, fat mass, skinfold thickness and MUAC did not differ between eBF and neBF infants throughout the first 6 months of life (Table 1). Nevertheless, fat-free mass was significantly higher [t = 2.27, df = 98, P = 0.03, confidence interval (CI) 0.03, 0.48] in neBF infants at 6 months despite a higher mean fat-free mass in eBF infants at birth (2.89 ± 0.34 vs 3.01 ± 0.35 kg) (Table 1). Both groups of infants displayed almost identical measurements for all anthropometric and body composition parameters at 3 months (Table 1). Repeated measures analysis of variance revealed weak evidence (fat mass index lower in eBF at birth but higher at 3 months compared with neBF; Fig. 2a) for different fat mass index trajectories for eBF and nEBF infants in the first 6 months of life (ANOVA, F = 2.42, df = 1.9, P = 0.09). No such effect (WFL lower in eBF throughout the 6 months; Fig. 2b) was observed for WFL (ANOVA, F = 0.32, df = 1.7, P = 0.69). Furthermore, we observed an inversion in fat mass index levels between eBF and neBF infants from 3 to 6 months, alongside a divergence of WFL trajectory (Fig. 2a and b). There were significant correlations between SS, TS, MUAC and fat mass at 3 and 6 months, in both groups of infants (Table 5).
Maternal body weight, composition, breastfeeding status and birth complications
At 3 months postpartum, the mean body weight of mothers who eBF showed weak evidence (t = 1.92, df = 160, P = 0.06, CI -0.17, 11.07) to be lower than counterparts who were neBF, and at 6 months postpartum, eBF mothers were significantly lighter (t = 3.49, df = 166, P = 0.001, CI 4.44, 15.99) (Table 2). Repeated measures analysis of variance revealed significant differences in BMI trajectories of eBF and neBF mothers through pregnancy and the first 6 months postpartum (ANOVA, F = 5.56, df = 1.8, P = 0.01, Fig. 3). Mothers in the neBF group, retained significantly less weight (t = − 2.75, df = 158, P = 0.02, CI -6.64, − 1.09) at 3 months (0.68 ± 11.69 vs 4.55 ± 6.08 kg) at 3 months compared with the eBF women but the amount of weight retained at 6 months was similar between groups (Table 2). Prevalence of neBF showed an increment from mothers with a normal BMI to mothers with obesity (Table 3). Further, mothers who underwent surgical or medical intervention during birth were less likely to eBF (Table 4). Maternal pre-pregnancy BMI was not significantly related to infant fat mass at 3 and 6 months, regardless of breastfeeding status (Table 5).
Discussion
This study illustrates the impact of eBF on mothers and infants in a selected cohort of mother-baby dyads from Tasmania, Australia. Both fat mass index and WFL had similar trajectories in the first 90 days of life, regardless of the type of feeding. In contrast, there was a deviation (from one another) in fat mass index and WFL in the second 90 days of life. Specifically, fat mass index in eBF infants, which was lower in the first 3 months, began to track higher than that of the neBF infants in the second 90 days. WFL trajectories of the two groups appear to separate from the 3-month time point onwards, after displaying identical increments in the first 90 days. Mothers with a higher BMI were less likely to breastfeed exclusively. Further, sustained breastfeeding was associated with greater postpartum maternal weight loss, confirmation of the efficacy of lactation as a weight loss strategy. Breastfeeding (and associated energy expenditure through milk production) intensifies lipolysis whereby accumulated fat tissue during pregnancy is mobilized, resulting in subsequent reductions in postpartum weight loss in mothers [36]. Medical intervention at birth also appears to be a major contributing factor that determines the pattern of breastfeeding. Although we do not have the capacity to draw on definitive conclusions in the current instance, existing empirical evidence indicate that factors such as stressful labour/birth, caesarean births, psychosocial stress/ pain due to childbirth and the consequent endocrine (e.g., oxytocin secretion) or mechanical (e.g., milk ejection reflex) changes associated with them can significantly delay lactogenesis and cause reductions in breastfeeding [37, 38].
Our observation (through fat mass index analysis) that exclusivity of breastfeeding results in increased fat mass accumulation in infants, is consistent with Gridneva et al.’s findings regarding compositional changes in the first 12 months of life [39]. As indicated in the results, overall, fat mass index was higher in the eBF infants compared with the neBF infants. On the other hand, neBF infants displayed higher fat-free mass accretion in the same period (Fig. 2). This pattern corroborates previous findings in neBF infants where significantly higher gains in fat-free mass has been observed compared with breastfed infants [40]. This suggests that the early life compositional changes that contribute to body weight gain is dependent on the feeding status of the infant. We also observed significant correlations between proxy measures of subcutaneous fat (Subscapular skinfold [SS] and Triceps skinfold [TS]) and fat mass in both groups of infants. Our findings partially concurred with existing empirical evidence that eBF is associated with preferential accretion of subcutaneous fat [41]. This notion is particularly important as localization of adipose tissue dictates its functionality and plays a major role in its contribution to the aetiology of obesity and metabolic disease [42]. Specifically, infant subcutaneous fat (between 0 and 24 months) has been shown to associated with general adiposity during adolescence and with cardio-metabolic risk factors in children as young as 6 years of age [43, 44]. The fact that we did not observe any significant associations between maternal pre pregnancy BMI and infant body composition is an anomaly as previous research has indicated an intergenerational link between maternal and infant body composition [45,46,47]. Fat mass and fat-free mass are sensitive indicators of the intrauterine environment, where foetal tissue developmental and regulatory planning happens [48, 49]. Methodological issues may explain at least part of the differences observed in this study. Previous Australian data that indicate associations between pre gravid BMI and neonatal body fat, had a much larger sample size (n = 599) compared to the current study [50].
Despite a dearth of mechanistic evidence regarding the influence of breastfeeding on infant adiposity, some research suggests that the composition of breast milk can have a significant influence on infant growth [51,52,53]. For instance, Gridneva et al. reported that higher total carbohydrate level in human milk is linked with greater fat-free mass whereas higher oligosaccharide content is related to greater fat mass in the first 12 months of life [54]. Other research purports a differential effect of leptin and adiponectin concentrations in breast milk on the development of infant lean and fat mass in the first year of life [55]. Further, secretory immunoglobulins are also thought to play a major role in breast milk mediated modulation of infant body composition [56].
According to the most recent Department of Health and Human Services data, only 44% of Tasmanian infants are at least partially breastfed at 6 months with entrenched socioeconomic disparities being a potential contributing factor [32, 57]. In stark contrast, 63% of the infants in our healthy cohort were eBF for the first 6 months of infancy. It is possible that the high education (i.e., ~ > 70 was university/ tertiary educated - data not reported) attainment of the current cohort of mothers may have contributed towards the higher eBF rates. Existing Australian evidence suggest that university-educated women are twice as likely to breastfeed your child for their first 6 months of their life than non-tertiary-educated women [58]. Furthermore, based on the well documented differences in growth patterns of infants from different ethnicities [59], including in the Tasmanian context, further examination to enable wider generalizability of the findings is warranted.
We observed that exclusivity of breastfeeding decreases with increasing levels of adiposity, only 46% of mothers with obesity exclusively breastfed in the first 6 months postpartum. This finding concurs with existing empirical evidence of lower rates of breastfeeding initiation and exclusivity in women with obesity [60]. Contributing factors in obesity-mediated reductions in breastfeeding include mechanical factors such as larger breasts/areolas [61, 62], suboptimal endocrine activity, [63,64,65] and inefficient lactogenesis [66]. Complications during labour may also affect breastfeeding patterns by delaying the onset of lactogenesis [67]. Interestingly, in the current study, mothers who experienced assisted vaginal birth or caesarean section had relatively lower levels of exclusive breastfeeding. Previous reports have linked caesarean section with reduced the likelihood/prevalence of all forms of breastfeeding from discharge to 6 months postpartum [68]. Another important observation was that mothers who maintained eBF continued to lose weight postpartum, a trend that is corroborated by previous reports [69, 70]. Specifically, between 3- and 6-months postpartum, neBF mothers showed an increment in body weight compared to their breastfeeding counterparts. It is important to note that although (overall) it appears that both neBF and eBF women gained ~ 3 kg during the study, the trajectories associated with arrival at the 6-month body weights are unique to the respective groups. Deducing from suggested mechanisms in the current literature, it could be assumed that much of this weight loss in eBF mothers is likely to be from increases in energy expenditure and distribution/mobilization of adipose tissue depots that were accumulated during pregnancy [71,72,73]. An ongoing challenge in this context is to differentiate between whether sustained postpartum weight loss is due to lactation per se or general/overall increments in energy expenditure, as it is likely that women who were habitually active pre-pregnancy may shed the weight gained during gestation faster than their relatively inactive counterparts [74]. Regardless, weight loss following childbirth is particularly important as excess postpartum weight retention may be associated with numerous complications including adverse metabolic conditions and entering subsequent pregnancies at successively unhealthy weight and fatness levels [75, 76].
Limitations
Dietary self-reporting is thought to be marred by social desirability bias [77]. As such, we acknowledge there may have been limitations/ biases in some of the self-reported parameters by mothers in the current study. Nevertheless, existing literature indicate that breastfeeding initiation and duration information derived from maternal recall can be considered valid and reliable [78]. Further, volunteer bias, a well-documented impediment for participant recruitment and retention in research studies [79] may have contributed to the relatively low sample size in this instance. Reasons beyond the research team’s control such as being time poor, and relocation (to other parts of the state) were cited for the inability to continue participating in the study. In addition, due to the descriptive nature of this report, the influence of potential contributors toward endemic patterns of breastfeeding such as parity, diet, sedentary occupation, and socio-economic status, were not considered thus limiting comparability of current results with other populations.
Conclusions
There is an urgent need for a concerted effort from all key stakeholders in maternal and infant health to promote optimal breastfeeding practices across all populations. As illustrated by the results of the current study, infants with different feeding patterns may display varying growth patterns in early life. Education and support for eBF at a population level should be accompanied by the simultaneous assessment of body composition (as opposed to widespread exclusive use of anthropometry) to obtain a more comprehensive understanding of infant growth. Accurate quantification of body composition status can provide important insights regarding qualitative and quantitative differences in specific tissue types. Logical extensions of the current research include the utilization of objective measurement approaches (e.g., deuterium dilution dose-to-mother technique) and a comprehensive evaluation of the role of different components of breast milk in shaping the longitudinal health, including body composition, of infants and young children.
Availability of data and materials
The datasets generated and/or analysed during the current study are not publicly available due privacy protection and ethical obligations but are available (in deidentified form) from the corresponding author on reasonable request.
Abbreviations
- BMI:
-
Body mass index
- eBF:
-
Exclusive breastfeeding
- MUAC:
-
Mid-upper arm circumference
- neBF:
-
Non-exclusive breastfeeding
- SS:
-
Subscapular skinfold
- TS:
-
Triceps skinfold
- WFL:
-
Weight-for-length
References
Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: a pooled analysis of 2416 population-based measurement studies in 128.9 million children, adolescents, and adults. Lancet 2017;390(10113):2627–42. https://doi.org/10.1016/S0140-6736(17)32129-3.
Woo Baidal JA, Locks LM, Cheng ER, Blake-Lamb TL, Perkins ME, Taveras EM. Risk factors for childhood obesity in the first 1,000 days: a systematic review. Am J Prev Med. 2016;50(6):761–79. https://doi.org/10.1016/j.amepre.2015.11.012.
Geddes DT, Prescott SL. Developmental origins of health and disease: the role of human milk in preventing disease in the 21(st) century. J Hum Lact. 2013;29(2):123–7. https://doi.org/10.1177/0890334412474371.
Kelishadi R, Farajian S. The protective effects of breastfeeding on chronic non-communicable diseases in adulthood: a review of evidence. Adv Biomed Res. 2014;3(1):3. https://doi.org/10.4103/2277-9175.124629.
Gillman MW. Commentary: breastfeeding and obesity--the 2011 scorecard. Int J Epidemiol. 2011;40(3):681–4. https://doi.org/10.1093/ije/dyr085.
Wells JC, Chomtho S, Fewtrell MS. Programming of body composition by early growth and nutrition. Proc Nutr Soc. 2007;66(3):423–34. https://doi.org/10.1017/S0029665107005691.
Savino F, Liguori SA, Fissore MF, Oggero R. Breast milk hormones and their protective effect on obesity. Int J Pediatr Endocrinol. 2009;2009(1):327505. https://doi.org/10.1186/1687-9856-2009-327505.
Bartok CJ. Babies fed breastmilk by breast versus by bottle: a pilot study evaluating early growth patterns. Breastfeed Med. 2011;6(3):117–24. https://doi.org/10.1089/bfm.2010.0055.
Kramer MS, Aboud F, Mironova E, Vanilovich I, Platt RW, Matush L, et al. Breastfeeding and child cognitive development: new evidence from a large randomized trial. Arch Gen Psychiatry. 2008;65(5):578–84. https://doi.org/10.1001/archpsyc.65.5.578.
Victora CG, Bahl R, Barros AJ, Franca GV, Horton S, Krasevec J, et al. Breastfeeding in the 21st century: epidemiology, mechanisms, and lifelong effect. Lancet. 2016;387(10017):475–90. https://doi.org/10.1016/S0140-6736(15)01024-7.
Le Doare K, Holder B, Bassett A, Pannaraj PS. Mother's milk: a purposeful contribution to the development of the infant microbiota and immunity. Front Immunol. 2018;9:361. https://doi.org/10.3389/fimmu.2018.00361.
Stuebe AM, Rich-Edwards JW, Willett WC, Manson JE, Michels KB. Duration of lactation and incidence of type 2 diabetes. JAMA. 2005;294(20):2601–10. https://doi.org/10.1001/jama.294.20.2601.
Horta BL, Loret de Mola C, Victora CG. Long-term consequences of breastfeeding on cholesterol, obesity, systolic blood pressure and type 2 diabetes: a systematic review and meta-analysis. Acta Paediatr. 2015;104(467):30–7. https://doi.org/10.1111/apa.13133.
Ram KT, Bobby P, Hailpern SM, Lo JC, Schocken M, Skurnick J, et al. Duration of lactation is associated with lower prevalence of the metabolic syndrome in midlife--swan, the study of women's health across the nation. Am J Obstet Gynecol. 2008;198(3):268.e261–6.
Nguyen B, Jin K, Ding D. Breastfeeding and maternal cardiovascular risk factors and outcomes: a systematic review. PLoS One. 2017;12(11):e0187923. https://doi.org/10.1371/journal.pone.0187923.
Einstein FH, Fishman S, Muzumdar RH, Yang XM, Atzmon G, Barzilai N. Accretion of visceral fat and hepatic insulin resistance in pregnant rats. Am J Physiol Endocrinol Metab. 2008;294(2):E451–5. https://doi.org/10.1152/ajpendo.00570.2007.
Stuebe AM, Rich-Edwards JW. The reset hypothesis: lactation and maternal metabolism. Am J Perinatol. 2009;26(1):81–8. https://doi.org/10.1055/s-0028-1103034.
Hatsu IE, McDougald DM, Anderson AK. Effect of infant feeding on maternal body composition. Int Breastfeed J. 2008;3:1.
López-Olmedo N, Hernández-Cordero S, Neufeld LM, García-Guerra A, Mejía-Rodríguez F, Gómez-Humarán IM. The associations of maternal weight change with breastfeeding, diet and physical activity during the postpartum period. Matern Child Health J. 2016;20(2):270–80. https://doi.org/10.1007/s10995-015-1826-7.
World Health Organisation: 3 in 5 babies not breastfed in the first hour of life. 2018. Available from: https://www.who.int/news/item/31-07-2018-3-in-5-babies-not-breastfed-in-the-first-hour-of-life
Kramer MS, Kakuma R. Optimal duration of exclusive breastfeeding. Cochrane Database Syst Rev. 2012;8:CD003517.
World Health Organisation: Infant and young child feeding: Model chapter for textbooks for medical students and allied health professionals. 2009. Available from: https://www.who.int/nutrition/publications/infantfeeding/9789241597494/en/
Gupta A, Suri S, Dadhich JP, Trejos M, Nalubanga B. The world breastfeeding trends initiative: implementation of the global strategy for infant and young child feeding in 84 countries. J Public Health Policy. 2019;40(1):35–65. https://doi.org/10.1057/s41271-018-0153-9.
Scott JA, Binns CW. Factors associated with the initiation and duration of breastfeeding: a review of the literature. Breastfeed Rev. 1999;7(1):5–16.
Lepe M, Bacardi Gascon M, Castaneda-Gonzalez LM, Perez Morales ME, Jimenez Cruz A. Effect of maternal obesity on lactation: systematic review. Nutr Hosp. 2011;26(6):1266–9. https://doi.org/10.1590/S0212-16112011000600012.
Kent JC, Hepworth AR, Sherriff JL, Cox DB, Mitoulas LR, Hartmann PE. Longitudinal changes in breastfeeding patterns from 1 to 6 months of lactation. Breastfeed Med. 2013;8(4):401–7. https://doi.org/10.1089/bfm.2012.0141.
Hobbs AJ, Mannion CA, McDonald SW, Brockway M, Tough SC. The impact of caesarean section on breastfeeding initiation, duration and difficulties in the first four months postpartum. BMC Pregnancy Childbirth. 2016;16:1.
Lyons S, Currie S, Smith DM. Learning from women with a body mass index (bmi)≥ 30 kg/m 2 who have breastfed and/or are breastfeeding: a qualitative interview study. Matern Child Health J. 2019;23(5):648–56. https://doi.org/10.1007/s10995-018-2679-7.
Jordan S, Emery S, Bradshaw C, Watkins A, Friswell W. The impact of intrapartum analgesia on infant feeding. BJOG. 2005;112(7):927–34. https://doi.org/10.1111/j.1471-0528.2005.00548.x.
Ayton J, van der Mei I, Wills K, Hansen E, Nelson M. Cumulative risks and cessation of exclusive breast feeding: Australian cross-sectional survey. Arch Dis Child. 2015;100(9):863–8. https://doi.org/10.1136/archdischild-2014-307833.
Donath S, Amir L. Rates of breastfeeding in Australia by state and socio-economic status: evidence from the 1995 National Health Survey. J Paediatr Child Health. 2000;36(2):164–8. https://doi.org/10.1046/j.1440-1754.2000.00486.x.
Hughes R. A study of five-year retrospective data on breastfeeding practices and introduction of solids amongst Tasmanian children. Nutr Diet. 2001;58(3):169–74.
The Department of Health: Breast feeding. 2019. Available from: https://www1.health.gov.au/internet/main/publishing.nsf/Content/health-pubhlth-strateg-brfeed-index.htm
World Health Organisation: Indicators for assessing breast-feeding practices: Report of an informal meeting, 11–12 june 1991, Geneva, Switzerland.1991. Available from https://apps.who.int/iris/handle/10665/62134
Binns CW, Fraser ML, Lee AH, Scott J. Defining exclusive breastfeeding in Australia. J Paediatr Child Health. 2009;45(4):174–80. https://doi.org/10.1111/j.1440-1754.2009.01478.x.
Goldberg GR, Prentice AM, Coward WA, Davies HL, Murgatroyd PR, Sawyer MB, et al. Longitudinal assessment of the components of energy balance in well-nourished lactating women. Am J Clin Nutr. 1991;54(5):788–98. https://doi.org/10.1093/ajcn/54.5.788.
Dewey KG. Maternal and fetal stress are associated with impaired lactogenesis in humans. J Nutr. 2001;131(11):3012S–5S. https://doi.org/10.1093/jn/131.11.3012S.
Hurst NM: Recognizing and treating delayed or failed lactogenesis II. J Midwifery Womens Health. 52(6):588–94. https://doi.org/10.1016/j.jmwh.2007.05.005.
Gridneva Z, Rea A, Hepworth AR, Ward LC, Lai CT, Hartmann PE, et al. Relationships between breastfeeding patterns and maternal and infant body composition over the first 12 months of lactation. Nutrients. 2018;10(1):45.
Bell KA, Wagner CL, Feldman HA, Shypailo RJ, Belfort MB. Associations of infant feeding with trajectories of body composition and growth. Am J Clin Nutr. 2017;106(2):491–8. https://doi.org/10.3945/ajcn.116.151126.
Breij LM, Abrahamse-Berkeveld M, Acton D, Rolfe EDL, Ong KK, Hokken-Koelega AC. Impact of early infant growth, duration of breastfeeding and maternal factors on total body fat mass and visceral fat at 3 and 6 months of age. Ann Nutr Metab. 2017;71(3–4):203–10. https://doi.org/10.1159/000481539.
Goossens GH. The metabolic phenotype in obesity: fat mass, body fat distribution, and adipose tissue function. Obes Facts. 2017;10(3):207–15. https://doi.org/10.1159/000471488.
Santos S, Gaillard R, Oliveira A, Barros H, Hofman A, Franco OH, et al. Subcutaneous fat mass in infancy and cardiovascular risk factors at school-age: the generation R study. Obesity. 2016;24(2):424–9. https://doi.org/10.1002/oby.21343.
Golab BP, Voerman E, van der Lugt A, Santos S, Jaddoe VW. Subcutaneous fat mass in infancy and abdominal, pericardial and liver fat assessed by magnetic resonance imaging at the age of 10 years. Int J Obes. 2019;43(2):392–401. https://doi.org/10.1038/s41366-018-0287-7.
Tikellis G, Ponsonby A, Wells J, Pezic A, Cochrane J, Dwyer T. Maternal and infant factors associated with neonatal adiposity: results from the Tasmanian infant health survey (TIHS). Int J Obes. 2012;36:496.
Sewell MF, Huston-Presley L, Super DM, Catalano P. Increased neonatal fat mass, not lean body mass, is associated with maternal obesity. Am J Obstet Gynecol. 2006;195(4):1100–3. https://doi.org/10.1016/j.ajog.2006.06.014.
Castillo-Laura H, Santos IS, Quadros LC, Matijasevich A. Maternal obesity and offspring body composition by indirect methods: a systematic review and meta-analysis. Cad Saude Publica. 2015;31(10):2073–92. https://doi.org/10.1590/0102-311X00159914.
Sedaghat K, Zahediasl S, Ghasemi A. Intrauterine programming. Iran J Basic Med Sci. 2015;18(3):212.
Catalano PM, Thomas A, Huston-Presley L, Amini SB. Increased fetal adiposity: a very sensitive marker of abnormal in utero development. Am J Obstet Gynecol. 2003;189(6):1698–704. https://doi.org/10.1016/S0002-9378(03)00828-7.
Au CP, Raynes-Greenow CH, Turner RM, Carberry AE, Jeffery H. Fetal and maternal factors associated with neonatal adiposity as measured by air displacement plethysmography: a large cross-sectional study. Early Hum Dev. 2013;89(10):839–43. https://doi.org/10.1016/j.earlhumdev.2013.07.028.
Butte NF, Wong WW, Hopkinson JM, Smith EO, Ellis KJ. Infant feeding mode affects early growth and body composition. Pediatrics. 2000;106(6):1355–66. https://doi.org/10.1542/peds.106.6.1355.
Luque V, Closa-Monasterolo R, Escribano J, Ferre N. Early programming by protein intake: the effect of protein on adiposity development and the growth and functionality of vital organs. Nutr Metab Insights. 2015;8(Suppl 1):49–56. https://doi.org/10.4137/NMI.S29525.
Kon IY, Shilina NM, Gmoshinskaya MV, Ivanushkina TA. The study of breast milk igf-1, leptin, ghrelin and adiponectin levels as possible reasons of high weight gain in breast-fed infants. Ann Nutr Metab. 2014;65(4):317–23. https://doi.org/10.1159/000367998.
Gridneva Z, Rea A, Tie WJ, Lai CT, Kugananthan S, Ward LC, et al. Carbohydrates in human milk and body composition of term infants during the first 12 months of lactation. Nutrients. 2019;11(7):1472.
Gridneva Z, Kugananthan S, Rea A, Lai CT, Ward LC, Murray K, et al. Human milk adiponectin and leptin and infant body composition over the first 12 months of lactation. Nutrients. 2018;10(8):1125.
Atanassov C, Viallemonteil E, Lucas C, Perivier M, Claverol S, Raimond R, et al. Proteomic pattern of breast milk discriminates obese mothers with infants of delayed weight gain from normal-weight mothers with infants of normal weight gain. FEBS Open Bio. 2019;9(4):736–42. https://doi.org/10.1002/2211-5463.12610.
Amir LH, Donath SM. Socioeconomic status and rates of breastfeeding in Australia: evidence from three recent national health surveys. Med J Aust. 2008;189(5):254–6. https://doi.org/10.5694/j.1326-5377.2008.tb02016.x.
Holowko N, Jones M, Koupil I, Tooth L, Mishra G. High education and increased parity are associated with breast-feeding initiation and duration among Australian women. Public Health Nutr. 2016;19(14):2551–61. https://doi.org/10.1017/S1368980016000367.
Kamoun C, Spatz D. Influence of Islamic traditions on breastfeeding beliefs and practices among African American Muslims in West Philadelphia: a mixed-methods study. J Hum Lact. 2018;34(1):164–75. https://doi.org/10.1177/0890334417705856.
Babendure JB, Reifsnider E, Mendias E, Moramarco MW, Davila YR. Reduced breastfeeding rates among obese mothers: a review of contributing factors, clinical considerations and future directions. Int Breastfeed J. 2015;10:1.
Li R, Jewell S, Grummer-Strawn L. Maternal obesity and breast-feeding practices. Am J Clin Nutr. 2003;77(4):931–6. https://doi.org/10.1093/ajcn/77.4.931.
Katz KA, Nilsson I, Rasmussen KM. Danish health care providers' perception of breastfeeding difficulty experienced by women who are obese, have large breasts, or both. J Hum Lact. 2010;26(2):138–47. https://doi.org/10.1177/0890334409349805.
Rasmussen KM, Kjolhede CL. Prepregnant overweight and obesity diminish the prolactin response to suckling in the first week postpartum. Pediatrics. 2004;113(5):e465–71. https://doi.org/10.1542/peds.113.5.e465.
Lovelady CA. Is maternal obesity a cause of poor lactation performance. Nutr Rev. 2005;63(10):352–5. https://doi.org/10.1111/j.1753-4887.2005.tb00113.x.
Nommsen-Rivers LA, Dolan LM, Huang B. Timing of stage II lactogenesis is predicted by antenatal metabolic health in a cohort of primiparas. Breastfeed Med. 2012;7(1):43–9. https://doi.org/10.1089/bfm.2011.0007.
Chapman DJ, Perez-Escamilla R. Identification of risk factors for delayed onset of lactation. J Am Diet Assoc. 1999;99(4):450–6. https://doi.org/10.1016/S0002-8223(99)00109-1.
Bogaerts A, Witters I, Van den Bergh BR, Jans G, Devlieger R. Obesity in pregnancy: altered onset and progression of labour. Midwifery. 2013;29(12):1303–13. https://doi.org/10.1016/j.midw.2012.12.013.
Hoang Nguyen PT, Binns CW, Vo Van Ha A, Nguyen CL, Khac Chu T, Duong DV, et al. Caesarean delivery associated with adverse breastfeeding practices: a prospective cohort study. J Obstet Gynaecol. 2020;40(5):644–8. https://doi.org/10.1080/01443615.2019.1647519.
Van Raaij J, Schonk CM, Vermaat-Miedema SH, Peek M, Hautvast J. Energy cost of lactation, and energy balances of well-nourished Dutch lactating women: reappraisal of the extra energy requirements of lactation. Am J Clin Nutr. 1991;53(3):612–9. https://doi.org/10.1093/ajcn/53.3.612.
Sadurskis A, Kabir N, Wager J, Forsum E. Energy metabolism, body composition, and milk production in healthy Swedish women during lactation. Am J Clin Nutr. 1988;48(1):44–9. https://doi.org/10.1093/ajcn/48.1.44.
Dewey KG, Heinig MJ, Nommsen LA. Maternal weight-loss patterns during prolonged lactation. Am J Clin Nutr. 1993;58(2):162–6. https://doi.org/10.1093/ajcn/58.2.162.
Sohlström A, Forsum E. Changes in adipose tissue volume and distribution during reproduction in Swedish women as assessed by magnetic resonance imaging. Am J Clin Nutr. 1995;61(2):287–95. https://doi.org/10.1093/ajcn/61.2.287.
Dewey KG. Energy and protein requirements during lactation. Annu Rev Nutr. 1997;17(1):19–36. https://doi.org/10.1146/annurev.nutr.17.1.19.
Pivarnik JM, Chambliss HO, Clapp JF, Dugan SA, Hatch MC, Lovelady CA, et al. Impact of physical activity during pregnancy and postpartum on chronic disease risk. Med Sci Sports Exerc. 2006;38(5):989–1006.
Villamor E, Cnattingius S. Interpregnancy weight change and risk of adverse pregnancy outcomes: a population-based study. Lancet. 2006;368(9542):1164–70. https://doi.org/10.1016/S0140-6736(06)69473-7.
Rooney BL, Schauberger CW, Mathiason MA. Impact of perinatal weight change on long-term obesity and obesity-related illnesses. Obstet Gynecol. 2005;106(6):1349–56. https://doi.org/10.1097/01.AOG.0000185480.09068.4a.
Hebert JR, Hurley TG, Peterson KE, Resnicow K, Thompson FE, Yaroch AL, et al. Social desirability trait influences on self-reported dietary measures among diverse participants in a multicenter multiple risk factor trial. J Nutr. 2008;138(1):226S–34S. https://doi.org/10.1093/jn/138.1.226S.
Li R, Scanlon KS, Serdula MK. The validity and reliability of maternal recall of breastfeeding practice. Nutr Rev. 2005;63(4):103–10. https://doi.org/10.1111/j.1753-4887.2005.tb00128.x.
Jordan S, Watkins A, Storey M, Allen SJ, Brooks CJ, Garaiova I, et al. Volunteer bias in recruitment, retention, and blood sample donation in a randomised controlled trial involving mothers and their children at six months and two years: a longitudinal analysis. PLoS One. 2013;8(7):e67912. https://doi.org/10.1371/journal.pone.0067912.
Acknowledgements
We thank Dr. Steve Street and Anne Hanley for co-managing the project, University of Tasmania/ Launceston General Hospital research staff for assistance in the collection of data, Launceston General Hospital midwifery team for assisting recruitment of participants and the Clifford Craig Foundation for providing space for testing/ housing of research equipment.
Funding
This work was supported, in part, by the International Atomic Energy Agency (CRP E43028 Contract Number 20880), the Bill & Melinda Gates Foundation (OPP1143641) and St.LukesHealth.
Author information
Authors and Affiliations
Contributions
APH, NMB and KA – Conceptualisation/design of the study, review and editing of the manuscript. SJ and APH - Formulating research questions, writing and editing drafts, data collection/analysis. JMB – Review and editing of manuscript drafts. MPH – Data collection, review and editing of manuscript drafts. The author(s) read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Ethics approval was obtained for all procedures from the Human Research Ethics Committee (Tasmania) Network; H0016117 and written informed consent was obtained from mothers prior to enrolment in the study.
Competing interests
There are no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Jayasinghe, S., Herath, M.P., Beckett, J.M. et al. Exclusivity of breastfeeding and body composition: learnings from the Baby-bod study. Int Breastfeed J 16, 41 (2021). https://doi.org/10.1186/s13006-021-00389-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13006-021-00389-x