Abstract
Recent epidemiological evidence connects ambient air pollutants to adverse neurobehavioural effects in adults. In animal models, subchronic controlled exposures to diesel exhaust (DE) have also showed evidence of neuroinflammation. Evidence suggests that DE not only affects outcomes commonly associated with cognitive dysfunction, but also balance impairment. We conducted a controlled human exposure experiment with 28 healthy subjects (average age = 28 years (SD = 7.1; range = 21–49); and 40% female) who were exposed to two conditions, filtered air (FA) and DE (300 μg PM2.5/m3) for 120 min, in a double-blinded crossover study with randomized exposures separated by four weeks. Postural stability was assessed by the Balance Error Scoring System (BESS), a brief, easily-administered test of static balance. The BESS consists of a sequence of three stances performed on two surfaces. With hands on hips and eyes closed, each stance is held for 20 s. “Error” points are awarded for deviations from those stances. Pre- and immediately post-exposure BESS “error” point totals were calculated and the difference between the two timepoints were compared for each of the two exposure conditions. A mixed effect model assessed the significance of the association. While our data demonstrates a trend of reduced postural stability in response to exposure to DE, exposure was not significantly associated with BESS value. This is the first study to investigate changes in postural stability as a result of exposure to DE in human subjects.
Similar content being viewed by others
Introduction
Traffic-related air pollution (TRAP) is a major contributor to the outdoor air pollution mix and has been heavily implicated in cardiovascular and respiratory disease [1, 2]. Emerging epidemiological evidence connects ambient air pollutants to adverse cognitive and neurobehavioural effects in adults [3]. Human studies have further demonstrated that living in areas with elevated air pollution is associated with decreased cognitive function [4,5,6,7,8], and elevated risk of dementia [9] and autism [10].
Controlled exposure to diesel exhaust (DE), meanwhile, has been reported to elicit a general cortical stress response in human subjects [11], elevate cytokine expression and oxidative stress in different regions of the rat brain [12], contribute to neuroinflammation and potentially lead to a rise in early markers of neurodegenerative disease [13].
Evidence suggests that DE not only affects outcomes commonly associated with cognitive deficits, such as impaired recall memory and perceptual motor speed, but is also linked to balance impairment (indicating dysfunction of vestibular, cerebellar, and associated afferent and efferent pathways for postural control) [14]. Postural stability or control represents a complex motor skill that is characterized by the ability to balance and orient the body’s position in space, and can be adversely affected by injury or disease to the vestibular system and/or brain, including Parkinson’s disease [15]. Cognitive factors are thought to play a role in the control of stability during activities such as walking and standing [16]. Additionally, environmental and occupational exposures, such as those to ambient air manganese and lead, have been reported to provoke poor postural balance [17, 18].
Human studies to date have provided limited insight into the acute effects of exposure to DE on postural stability. We conducted a crossover experiment with adult subjects who were exposed to DE under controlled settings, and hypothesized that DE inhalation would result in decreased postural stability, as assessed by the Balance Error Scoring System [19], a brief and easily-administered test of static balance. The Effects of Air Pollution on Cognition (EAPOC) study marks one of the largest human controlled DE exposure studies.
Methods
Subject recruitment and screening
A total of 36 study subjects were recruited via posters in or near Vancouver, B.C. transportation hubs, online notices, and e-mail notifications to the local health authority list-serve.
An initial telephone screening assessed the suitability of potential subjects according to set of inclusion and exclusion criteria that included: (i) subjects between the ages of 19 and 49; (ii) healthy; (iii) nonsmoker; (iv) speaking and reading proficiency in English; (v) not pregnant or breast-feeding; and, (vi) absence of co-existing medical conditions or medications that could interfere with the study protocol. Subjects were also excluded based on having (vii) a moderate-to-high degree of claustrophobia, and (viii) the presence of implanted metal that could interfere with functional magnetic resonance imaging (fMRI), which were considered as part of a separate component of this study not covered in this article.
A secondary screening of subjects included an in-person medical/health questionnaire and brief physical exam by our study clinician. Each qualifying subject was then presented with a detailed outline and explanation of the study protocol, and written and informed consent was obtained. Consent forms were approved by the University of British Columbia Clinical Research Ethics Board (# H12–03025), Vancouver Coastal Health Ethics Board (# V12–03025), and Health Canada’s Research Ethics Board (# 2012–0040).
Environmental exposure procedure
Study participants were exposed to two conditions: filtered air (FA) and DE (300 μg PM2.5/m3, nominally) for 120 min, in a double-blinded, crossover study with randomized inhalation exposures separated by four weeks. Using a system previously reported [20], exposures were conducted in the Air Pollution Exposure Laboratory (APEL), which creates fresh DE, suitably aged (4 min) and diluted for human experimentation at realistic and safe concentrations. The DE dose reflects the short-term, high-ambient PM exposures commonly occurring in busy transport corridors of large cities [21], and certain occupational settings where diesel-powered machinery and generators are used [22]. The DE exposure is standardized to 300 μg PM2.5/m3, which aligns with the most common DE human health effects studies [20]. Blinding to exposure conditions was previously validated [23].
Peripheral blood was collected before and at three time points following the exposure (0-h; 3-h post-exposure; 24-h post-exposure) as part of a separate component of this study not covered in this article. Details of this blood collection procedure and serum/plasma analysis has been previously documented [24].
During the exposure session, participants alternated between rest (40 min/h) and cycling on a stationary bike (20 min/h) at a light effort with a load set to achieve a minute ventilation of approximately 15 L/min/m2 body surface area. Participants were closely monitored during the 120-min exposure and vitals, including heart rate, blood pressure and peripheral oxygen saturation, were measured at 20-min intervals (Table 1 and Fig. 1).
Postural stability assessment (balance error scoring system)
The BESS protocol is considered a useful test for assessing balance and one that is easy to administer [25]. A systematic review of the protocol concluded that BESS has construct validity and moderate to good reliability to assess static balance [26]. During the secondary screening and prior to data collection, each subject participated in a BESS orientation/learning session, and was also asked to maintain the same pre-test routine including the same mode of travel to the laboratory, pretest meal and caffeine intake, for each day of exposure. BESS assessments were conducted immediately prior to each randomized exposure and immediately following the exposure. Briefly, the BESS consists of a sequence of three stances (double leg stance, single leg stance, and tandem stance) performed on two surfaces (firm floor and medium density foam) [26] (Fig. 2). With hands on hips and eyes closed, each stance is held for 20 s. “Error” points are awarded for explicit deviations, including opening eyes, lifting hands off hips, abduction or flexion of the hip beyond 30 ̊, or stepping or stumbling. Higher score totals reflect worse performance on the test, and all tests were video recorded and scored by a blinded kinesiologist experienced in the administration of the BESS. This evaluation method has previously been shown to have an ICC of 0.88 [19].
Statistical analysis
Statistical analysis was conducted using R (https://www.r-project.org/). Pre- and post-exposure BESS “error” point total means for each of the six stances were reported. The scores of all stances were summed for each for each exposure condition, and the difference between baseline and post-exposure aggregate scores created a delta value. Normality and order effects were tested. A mixed effect model was performed to assess the significance of the association. P-values of <0.05 were considered significant.
Results
Subject characteristics
Of the 36 recruited candidates, 28 healthy adult participants (average age = 28 years (SD = 7.1; range = 21–49); and 40% female) completed the study’s two exposure condition sessions. Complete sets of before and after exposure BESS assessments were recorded for each of the 28 participants.
BESS assessment scores aggregated
Table 2 shows the average concentration of each BESS stance at baseline and post-exposure to DE or FA. The BESS scores for all six stances were summed, and delta values between the baseline and post-exposure timepoints were calculated for each exposure condition. Normality was assessed and the delta values were found to approximate a normal distribution. Order effects were tested by a paired t-test and found to be absent (p-value = 0.52).
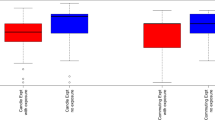
A mixed effect model assessed the significance of the association between exposure condition and the BESS delta value. In addition to the inclusion of a four-week washout period to counter potential carryover effects, our initial model evaluated the interaction between exposure and order and found no significant carryover effect (p-value = 0.66). Our data did demonstrate a trend of reduced postural stability in response to exposure to DE (Fig. 3). The mean change in BESS scores were greater following DE exposure (effect estimate: 1.50; 95% CI, 0.02 to 2.98), while the mean change following FA exposure was in a similar direction but was of lesser magnitude and not significant (effect estimate: 0.53; 95% CI, −0.94 to 2.01). In the mixed model, exposure did not significantly impact BESS delta score (p-value = 0.36), as suggested by the overlap in confidence intervals surrounding the estimates of effect for each exposure.
Discussion
To our knowledge, this is the first study of its kind to investigate the effects on postural stability, or static balance, following an acute DE exposure in healthy humans. As part of the greater EAPOC study – one of the largest known human controlled exposure studies of air pollution – we hypothesized that an exposure to DE approximating 300 mg/m3 PM2.5 would negatively disrupt postural stability as measured by the BESS protocol, relative to a sham exposure. Results from our study involving 28 healthy adult subjects suggest that acute exposure to DE, with PM levels approximating those intermittently present in highly polluted cities such as Beijing, China and New Delhi, India, does not cause a significant impact to an individual’s static balance. Trends in the direction of an adverse effect (i.e. worsening balance) following DE were observed, potentially motivating more extensive or detailed examination of the role of traffic-related air pollution on factors that control postural stability. It may be that BESS scores would be further compromised at a later timepoint, assuming neuroinflammation is delayed by slow penetration of particulate matter into the central nervous system, or in a more susceptible population, such as the elderly.
In a case-control study examining the effects of chronic DE exposure in 10 railroad workers and six electricians, Kilburn found balance impairments in the DE exposed group relative to the reference group of workers [14]. However, these results are difficult to interpret as participant selection was nonrandom, potential confounding variables were incompletely controlled, and contributions likely attributable to acute versus chronic exposures were difficult to distinguish.
A limitation of our study is that our acute 2-h exposure is inconsistent with the chronic PM exposure studies to date that have found adverse associations with cognitive endpoints. While this was intentional, as we wondered about the specific effects of short-term exposures, it may be that only prolonged exposures induces sufficient inflammation to induce change across the neuro-cognitive spectrum. Furthermore, if such changes indeed exist, the BESS assessment may not be sufficiently sensitive to pick up such signals following this acute exposure. Crossover experiments typically provide greater statistical power than parallel-group trials of similar size, and allow considerably smaller sample sizes for comparable type I and type II error risks [27, 28]. Regardless, the inconclusive, but suggestive, results may motivate further investigation using a similarly designed study with a greater sample size. As suggested earlier, another limitation to our study include the recruitment of healthy adults as opposed to alternative populations who are inherently more susceptible to the CNS effects of air pollution [4, 5, 29, 30].
Abbreviations
- APEL:
-
air pollution exposure laboratory
- BESS:
-
balance error scoring system
- CNS:
-
central nervous system
- CO:
-
carbon monoxide
- CO2:
-
carbon dioxide
- DE:
-
diesel exhaust
- EAPOC:
-
effects of air pollution on cognition
- FA:
-
filtered air
- FMRI:
-
functional magnetic resonance imaging
- ICC:
-
intraclass correlation coefficient
- NO:
-
nitrogen oxide
- NO2:
-
nitrogen dioxide
- PM:
-
particulate matter
- PM2.5:
-
particulate matter aerodynamic diameter less than 2.5 μm in aerodynamic diameter)
- Ppb:
-
parts per billion
- Ppm:
-
parts per million
- TRAP:
-
traffic-related air pollution
- TVOC:
-
total volatile organic compounds
References
Brook RD, Rajagopalan S, Pope CA, Brook JR, Bhatnagar A, Diez-Roux AV, et al. Particulate matter air pollution and cardiovascular disease: an update to the scientific statement from the American Heart Association. Circulation. 2010 Jun 1;121(21):2331–78.
Anderson HR, Favarato G, Atkinson RW. Long-term exposure to air pollution and the incidence of asthma: meta-analysis of cohort studies. Air Qual Atmos Health. 2013;6(1):47–56.
Chen J-C, Wang X, Wellenius GA, Serre ML, Driscoll I, Casanova R, et al. Ambient air pollution and neurotoxicity on brain structure: evidence from women's health initiative memory study. Ann Neurol. 2015 Sep;78(3):466–76.
Weuve J, Puett RC, Schwartz J, Yanosky JD, Laden F, Grodstein F. Exposure to particulate air pollution and cognitive decline in older women. Arch Intern Med. 2012;172(3):219–27.
Ranft U, Schikowski T, Sugiri D, Krutmann J, Krämer U. Long-term exposure to traffic-related particulate matter impairs cognitive function in the elderly. Environ Res. 2009 Nov;109(8):1004–11.
Calderon-Garciduenas L, Mora-Tiscareno A, Ontiveros E, Gomez-garza G, Barragan-Mejia G, Broadway J, et al. Air pollution, cognitive deficits and brain abnormalities: a pilot study with children and dogs. Brain Cogn. 2008 Nov;68(2):117–27.
Power MC, Adar SD, Yanosky JD, Weuve J. Exposure to air pollution as a potential contributor to cognitive function, cognitive decline, brain imaging, and dementia: a systematic review of epidemiologic research. Neurotoxicology. 2016 Sep;56:235–53.
Guxens M, Sunyer JA. Review of epidemiological studies on neuropsychological effects of air pollution. Swiss Med Wkly. 2012;141
Chen H, Kwong JC, Copes R, Tu K, Villeneuve PJ, van Donkelaar A, et al. Living near major roads and the incidence of dementia, Parkinson's disease, and multiple sclerosis: a population-based cohort study. Lancet. 2017 Feb 18;389(10070):718–26.
Volk HE, Hertz-Picciotto I, Delwiche L, Lurmann F, McConnell R. Residential proximity to freeways and autism in the CHARGE study. Environ Health Perspect. 2011;119(6):873–7.
Crüts B, van Etten L, Törnqvist H, Blomberg A. Exposure to diesel exhaust induces changes in EEG in human volunteers. Part Fibre …. 2008.
Gerlofs-Nijland ME, van Berlo D, Cassee FR, Schins RPF, Wang K, Campbell A. Effect of prolonged exposure to diesel engine exhaust on proinflammatory markers in different regions of the rat brain. Part Fibre Toxicol. 2010 May;7:12.
Levesque S, Surace MJ, McDonald J, Block ML. Air pollution & the brain: subchronic diesel exhaust exposure causes neuroinflammation and elevates early markers of neurodegenerative disease. J Neuroinflammation. 2011 Aug;8:105.
Kilburn K. Effects of diesel exhaust on neurobehavioral and pulmonary functions. 2000 Jan;55(1):11–7.
Allen NE, Sherrington C, Paul SS, Canning CG. Balance and falls in Parkinson's disease: a meta-analysis of the effect of exercise and motor training. Mov Disord. 2011 Aug 1;26(9):1605–15.
Woollacott M, Shumway-Cook A. Attention and the control of posture and gait: a review of an emerging area of research. Gait & Posture. 2002 Aug;16(1):1–14.
Rugless F, Bhattacharya A, Succop P, Dietrich KN, Cox C, Alden J, et al. Childhood exposure to manganese and postural instability in children living near a ferromanganese refinery in southeastern Ohio. Neurotoxicol Teratol. 2014 Jan;41:71–9.
Chia SE, Chua LH, Ng TP, Foo SC, Jeyaratnam J. Postural stability of workers exposed to lead. Occup Environ Med. 1994 Nov;51(11):768–71.
Iverson GL, Koehle MS. Normative data for the balance error scoring system in adults. Rehabil Res Pract. 2013;2013(3):846418–5.
Birger N, Gould T, Stewart J, Miller MR, Larson T, Carlsten C. The air pollution exposure laboratory (APEL) for controlled human exposure to diesel exhaust and other inhalants: characterization and comparison to existing facilities. Inhal Toxicol. 2011 Mar;23(4):219–25.
Cao J-J, Shen Z-X, Chow JC, Watson JG, Lee S-C, Tie X-X, et al. Winter and summer PM2.5 chemical compositions in fourteen Chinese cities. J Air Waste Manage Assoc. 2012 Oct;62(10):1214–26.
Pronk A, Coble J, Stewart PA. Occupational exposure to diesel engine exhaust: a literature review. J Expo Sci Environ Epidemiol. 2009 Jul;19(5):443–57.
Carlsten C, Oron AP, Curtiss H, Jarvis S, Daniell W, Kaufman JD. Symptoms in response to controlled diesel exhaust more closely reflect exposure perception than true exposure. Arjomandi M. PLoS One. 2013;8(12):e83573.
Cliff R, Curran J, Hirota JA, Brauer M, Feldman H, Carlsten C. Effect of diesel exhaust inhalation on blood markers of inflammation and neurotoxicity: a controlled, blinded crossover study. Inhal Toxicol Taylor & Francis. 2016;28(3):145–53.
Furman GR, Lin C-C, Bellanca JL, Marchetti GF, Collins MW, Whitney SL. Comparison of the balance accelerometer measure and balance error scoring system in adolescent concussions in sports. Am J Sports Med. 2013 Jun;41(6):1404–10.
Bell DR, Guskiewicz KM, Clark MA, Padua DA. Systematic review of the balance error scoring system. Sports health. Sage publications. Inc. 2011 May;3(3):287–95.
Coons SJ, Gwaltney CJ, Hays RD, Lundy JJ, Sloan JA, Revicki DA, et al. Recommendations on evidence needed to support measurement equivalence between electronic and paper-based patient-reported outcome (PRO) measures: ISPOR ePRO good research practices task force report. Value Health. 2009 Jun;12(4):419–29.
Wellek S, Blettner M. On the proper use of the crossover Design in Clinical Trials Part 18 of a series on evaluation of scientific publications. Dtsch Arztebl Int. 2012;109(15):276–81.
Block ML, Elder A, Auten RL, Bilbo SD, Chen H, Chen J-C, et al. The outdoor air pollution and brain health workshop. Neurotoxicology. 2012 Oct;33(5):972–84.
Power MC, Weisskopf MG, Alexeeff SE, Coull BA, Spiro A, Schwartz J. Traffic-related air pollution and cognitive function in a cohort of older men. Environ Health Perspect. 2011 May 1;119(5):682–7.
Acknowledgements
The authors would like to acknowledge Health Canada for their funding support, and Dr. Robin Shutt and Dr. Ling Liu for their collaborations throughout the study.
Funding
A Health Canada contract provided the funding for this study. The funding body participated in an advising role.
Availability of data and materials
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Contributions
JC conceptualized the study and the design, recruited subjects, supervised the exposures, and analyzed and interpreted the patient data. RC performed the balance assessments and supervised the exposures. MK advised and consulted on the use of the Balance Error Scoring System (BESS). NS reviewed and scored all Balance Error Scoring System (BESS) videos. CC was the study’s principal investigator and conceptualized the study and the design, as well as conducted the physical exams on subjects and supervised the general operations of the Air Pollution Exposure Lab (APEL). All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Consent forms were approved by the University of British Columbia Clinical Research Ethics Board (# H12–03025), Vancouver Coastal Health Ethics Board (# V12–03025), and Health Canada’s Research Ethics Board (# 2012–0040). All subjects participating in the study provided informed, written consent.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Curran, J., Cliff, R., Sinnen, N. et al. Acute diesel exhaust exposure and postural stability: a controlled crossover experiment. J Occup Med Toxicol 13, 2 (2018). https://doi.org/10.1186/s12995-017-0182-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12995-017-0182-5