Abstract
Background
The positive influences of glucagon-like peptide-1 (GLP-1) on blood glucose homeostasis, appetite sensations, and food intake provide a strong rationale for its therapeutic potential in the nutritional management of obesity and type 2 diabetes.
Aim
To summarize GLP-1 physiology and the nutritional modulation of its secretion in the context of obesity and type 2 diabetes management.
Findings
GLP-1 is mainly synthesized and secreted by enteroendocrine L-cells of the gastrointestinal tract. Its secretion is partly mediated by the direct nutrient sensing by G-protein coupled receptors which specifically bind to monosaccharides, peptides and amino-acids, monounsaturated and polyunsaturated fatty acids as well as to short chain fatty acids. Foods rich in these nutrients, such as high-fiber grain products, nuts, avocados and eggs also seem to influence GLP-1 secretion and may thus promote associated beneficial outcomes in healthy individuals as well as individuals with type 2 diabetes or with other metabolic disturbances.
Conclusion
The stimulation of endogenous GLP-1 secretion by manipulating the composition of the diet may be a relevant strategy for obesity and type 2 diabetes management. A better understanding of the dose-dependent effects as well as the synergistic effects of nutrients and whole foods is needed in order to develop recommendations to appropriately modify the diet to enhance GLP-1 beneficial effects.
Similar content being viewed by others
Background
Type 2 diabetes (T2D) is a major public health concern due to its pandemic occurrence and its association with several physical and psychological comorbidities [1, 2], as well as with a decreased quality of life [3]. It is widely accepted that obesity, especially excessive fat accumulation in the abdominal area, is the most important predictor of T2D development. Indeed, it is estimated that 60 to 90% of T2D incidence is related to excessive weight gain and obesity [4]. After the onset of T2D, obesity also contributes to increasing the risks of comorbidities and overall mortality rates [5]. The global prevalence of overweight and obesity is expected to reach 57.8% by 2030 [6]. Following a similar trend, the worldwide prevalence of T2D has been projected to double between 2000 (2.2%) and 2030 (4.4%) [7].
Nutrition, along with physical activity and behavioural changes, are cornerstones of obesity and T2D management. Weight loss is induced by a state of sustained negative energy balance, which almost inevitably involves a reduction of energy intake [8]. From a purely thermodynamic perspective, a decrease in energy intake results in weight loss regardless of the type of dietary modifications through which it is achieved. Nonetheless, weight maintenance and improvements in cardiometabolic biomarkers are significantly impacted by the composition of the diet (i.e. macro- and micronutrient contents) [8]. One potential mechanism by which diet composition can influence these outcomes is through its influence on the secretion of gastrointestinal (GI) peptide hormones, including that of glucagon-like peptide-1 (GLP-1) [9]. The latter has been associated with a reduction of appetite and food intake, and appears to positively influence blood glucose homeostasis, by acting on the pancreas and the central nervous system [10].
The secretion of GLP-1 is partly mediated by the binding of nutrients to G-protein coupled receptors (GPCRs) expressed by enteroendocrine GI cells [11]. Therefore, manipulating the diet in a way to promote interactions with these receptors could increase GLP-1 secretion and enhance its beneficial effects [11]. This review focuses on GLP-1 physiology and the nutritional modulation of its secretion from enteroendocrine GI cells in the context of obesity and T2D management. It presents recent evidence on possible mechanisms by which specific foods, as well as nutrients and their by-products, could increase GLP-1 secretion, and subsequently influence appetite, food intake, and blood glucose control.
Glucagon-like peptide-1 in the gut-brain-pancreas axis
Synthesis, secretion, and metabolism
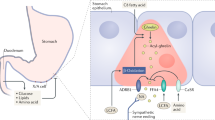
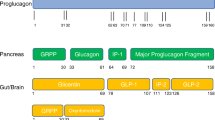
The GI tract accomplishes several functions, namely the digestion of food, the absorption of nutrients, and the secretion of digestive juices, mucus, and peptide hormones. The epithelium of the GI wall is composed of several cell types, including enteroendocrine cells which are a key component of the gut-brain-pancreas axis [12]. On their apical surface, enteroendocrine cells possess microvilli expressing several GPCRs that are binding to nutrients and other substrates present in the GI lumen [12]. Enteroendocrine cells can be divided into several subcategories depending on their distribution throughout the GI tract, their GPCRs’ expression and their secretory profile. GLP-1 is synthesized and secreted by enteroendocrine L-cells which are expressed over a large portion of the GI tract, starting in the proximal small intestine and progressively increasing in density down to the distal part of the colon. GLP-1 is stored in secretory granules of L-cells until its secretion is triggered, and then uses endocrine and neuronal routes to exert its functions in the pancreas and central nervous system [10]. In addition to L-cells, GLP-1 is synthesized to a lesser extent by neurons of the nucleus tractus solitarius (NTS) of the brainstem [13, 14].
GLP-1 is produced in two major active forms, namely GLP-1 (7-36 amide) and GLP-1 (7-37 amide), and is resulting from the differential processing of its precursor proglucagon [10]. Its synthesis appears to be attributed to tissue-specific expression of pro-hormone convertase 1 and 3 which cleave proglucagon [15]. Proglucagon is a 160-amino acid inactive precursor of several peptide hormones, including glucagon, oxyntomodulin and GLP-1 [16]. Proglucagon encoding gene is expressed in the intestine, the pancreas, as well as the central nervous system [16]. Several studies have confirmed that this gene produces identical messenger ribonucleic acid (mRNA) transcripts in these major expression sites, but is translated and processed differently, thus producing different bioactive peptides depending on the expressing tissue [17–19].
In humans, blood concentrations of GLP-1 generally range between 5 pmol/L and 15 pmol/L in fasting state and increase two- to four-folds after food ingestion [10]. More specifically, GLP-1 blood concentrations rise within 15 minutes after food ingestion and reach a pick after approximately 60 minutes [10]. In the second hour, GLP-1 concentrations start to decrease gradually until the next prandial episode [10]. Postprandial GLP-1 secretion is influenced by both neuroendocrine and nutritional factors, and exhibits a two-phase release profile that is in fact very similar to that of insulin. The initial phase of its secretion, which is detectable 10 to 15 minutes after food ingestion, is thought to be mostly influenced by neuroendocrine factors, and, to a lesser extent, by the interaction of nutrients with L-cells in the proximal small intestine. Conversely, the second phase of GLP-1 secretion, which occurs 30 to 60 minutes postprandially, is mostly influenced by the arrival of nutrients in the distal part of the small intestine and the colon [20]. Nutrients and their by-products bind to GPCRs and activate intracellular pathways that ultimately trigger GLP-1 exocytosis from L-cells’ secretory granules [10]. When secreted, GLP-1 can activate vagal afferent neural fibres, as well as diffuse into nearby capillaries and then reach the systemic circulation through the portal vein [10]. In the bloodstream, GLP-1 is highly susceptible to the catalytic activity of the enzyme dipeptidyl-peptidase IV (DPP-IV). The latter cleaves two NH2-terminal amino acids of the active forms of GLP-1 (i.e. 7-36 amide, 7-37 amide) leading to the production of their biologically inactive forms (i.e. 9-36 amide, 9-37 amide) [21–23]. As a result, GLP-1’s half-life is very short – about 1 to 2 min [22, 23]. Animal and in vitro studies have shown that less than 25% of the newly secreted bioactive GLP-1 reaches the liver intact [22, 23]. Further catalytic activities take place in the liver, and consequently, only approximately 10-15% of the newly secreted GLP-1 reaches the systemic circulation in its active forms [22, 23].
Action in the pancreas
One of the best-known and probably most important effects of GLP-1 is its ability to stimulate insulin secretion in response to carbohydrate consumption [24–27]. In pancreatic β-cells, GLP-1 activates intracellular pathways that increase intracellular calcium concentrations and subsequently leads to insulin exocytosis from secretory granules [24, 25]. GLP-1 has also been shown to promote insulin gene transcription and biosynthesis [28]. GLP-1 seems to be responsible for nearly half of the total postprandial insulin secretion [29]. This process, known under the name of “incretin effect”, is defined as the differential augmentation in insulin secretion observed after oral glucose intake, compared to intravenous glucose administration resulting in the same blood glucose concentrations [30].
GLP-1 is partly fulfilling its actions in pancreatic β-cells through an endocrine pathway by directly interacting with its receptors (GLP-1Rs). Indeed, competitive inhibition of GLP-1Rs of pancreatic β-cells in rodents and humans resulted in a significant reduction of insulin secretion, as well as in impaired glucose tolerance [26, 27]. Nonetheless, the glucose-lowering effects of GLP-1 are not solely related to its insulinotropic effect, but also to its strong inhibition of glucagon secretion [10, 31]. This effect seems to be partly mediated by the increased insulin and somatostatin secretion from pancreatic islets occurring during the postprandial period, as well as by GLP-1 direct interaction with its receptors on pancreatic α-cells [32–34]. In rodents, prolonged administration of GLP-1 also appears to promote pancreatic β-cell growth by increasing their proliferation and decreasing apoptosis [35–38].
Action in the central nervous system
In addition to its action in the pancreas, GLP-1 plays a role in both homeostatic and non-homeostatic regulations of food intake, which occur in distinct areas of the central nervous system [39, 40]. The homeostatic regulation of food intake, related to short- and long-term energy status, is mainly taking place in the hypothalamus and NTS, areas which convey and integrate numerous peripheral signals [39, 40]. The hypothalamus contains several interconnected nuclei, including the arcuate nucleus (ARC), the paraventricular nucleus (PVN), as well as the dorso-medial nucleus (DMN) [39, 40]. Due to its anatomical position, the ARC plays a critical role in transmitting peripheral information related to energy and nutrient status to other central structures. Indeed, the ARC is situated in the medio-basal area of the hypothalamus, where the blood-brain barrier is highly permeable, and thus likely has a greater exposition to circulating factors [39]. The ARC contains two distinct populations of neurons: 1) the orexigenic neuropeptide Y (NPY) and agouti-related peptide (AgRP) expressing neurons, and 2) the anorexigenic pro-opiomelanocortine (POMC), as well as cocaine and amphetamine-regulated transcript (CART) expressing neurons [39, 40]. Additionally, POMC is a precursor of the α-melanocyte stimulating hormone (α-MSH), a neuropeptide with potent anorexigenic effects [39, 40]. Upon release, POMC and α-MSH activate neurons of the PVN, which inhibits food intake [39, 40]. Conversely, by inhibiting PVN neurons’ activation, NPY and AgRP stimulate food intake [39, 40]. Opposite to the PVN, DMN neurons are activated by NPY and AgRP, promoting food intake, and are inhibited by POMC and α-MSH, reducing food intake [39, 40].
GLP-1Rs are widely expressed in the hypothalamus, with the highest expression being described to be in POMC/CART neurons of the ARC, followed by DMN neurons [13, 14, 41–44]. A small quantity of GLP-1 appears to diffuse through the blood-brain barrier and directly bind to its receptors on POMC/CART and NPY/AgRP neurons [45]. In rodents, the peripheral administration of a GLP-1 analogue has been shown to directly activate neurotransmission in POMC/CART neurons, while exerting an inhibitory action in NPY/AgRP neurons [46, 47]. Nonetheless, in reason of its short half-life, endogenous GLP-1 released from L-cells is likely mostly acting on the central nervous system by indirectly stimulating neurons of the NTS and ARC via the activation of vagal afferent neurons [48]. Indeed, GLP-1Rs have been identified on neurons of the nodose ganglion of the vagus nerve [43, 49], and the importance of this pathway in the regulation of food intake has been confirmed in rats, where vagal deafferentation decreased the effects of intraperitoneally administered GLP-1 [50, 51]. While the exact mechanisms and their relative contributions to the observed effects remain to be fully elucidated, the GLP-1’s implication in the homeostatic regulation of food intake is well established. In rodents, acute intraperitoneal, subcutaneous or intravenous administration of GLP-1 and GLP-1 analogues have constantly been shown to reduced meal size, as well as cumulative food and energy intakes [51–56]. Similarly, in humans, acute intravenous administration of GLP-1 and GLP-1 analogues decreased appetite, hunger and food intake, and increased fullness and satiety sensations [57–62].
In addition to physiological energy needs, food intake is driven by non-homeostatic central pathways involved in reward processing and reward-motivated behaviours [63, 64]. The palatability of food is a crucial determinant of the decision to eat. As a result, highly palatable food, typically high in energy, lipids, and simple carbohydrates, can trigger food intake in the absence of physiological energy needs [63, 64]. Several central structures, such as the orbitofrontal cortex, insula, amygdala, and striatum play an important role in the processing and evaluation of food cues [65]. Increased activation of these areas of the central nervous system in response to visual and olfactory food cues (referred to as “anticipatory food reward”) is associated with increased craving for highly palatable foods [66, 67]. Furthermore, upon food ingestion, dopaminergic neurons send projections from the ventral tegmental area to the nucleus accumbens and other forebrain areas [63, 64]. Dopamine release in these areas of the brain is well correlated with meal pleasantness in healthy individuals with a normal body mass index [68]. Activation of these areas in response to food consumption is referred to as “consummatory food reward”. Reduced consummatory food reward is associated with compensatory overeating [65–67, 69, 70]. GLP-1Rs have been identified in areas of the brain involved in anticipatory and consummatory food reward processing, and neurons of the NTS also share dense neuronal connections with these areas [71, 72]. Recent pre-clinical and clinical studies suggest a role of GLP-1 in the modulation of food reward processing. More specifically, the exogenous administration of GLP-1 and GLP-1 analogues appears to influence dopamine neurotransmission in several central areas, and has been associated with decreased anticipatory food reward, increased consummatory food reward and decreased intake of hyperpalatable foods [65, 69, 70, 73–77].
Glucagon-like peptide-1 as a target for obesity and type 2 diabetes management
The positive influences of GLP-1 on many metabolic disturbances associated with T2D, as well as key determinants of weight loss and weight maintenance, make it a therapeutic target with good potential. GLP-1 has been studied in relation to obesity and T2D pathophysiology and treatment. While few studies have shown contradictory results, it is generally well accepted that obesity and metabolic changes occurring with the development of T2D are associated with a decline in the postprandial secretion of GLP-1 from L-cells [78–83]. Overtime, this change in GLP-1’s secretion can contribute to further weight gain and adversely impact T2D progression. As a potential strategy to enhance GLP-1actions, several researchers have investigated the metabolic effects of intravenous administration of GLP-1 analogues. Interestingly, when receiving the same dose of a GLP-1 analogue, individuals with obesity and T2D exhibited metabolic and appetite responses that were very similar to their healthy counterparts [57–59, 62, 84, 85]. This finding suggests that sensibility to GLP-1’s action is maintained, which is contrary to several other peptide hormones involved in energy and blood glucose homeostasis [86, 87]. Consequently, targeting GLP-1Rs’ activation with GLP-1 analogues and increasing GLP-1 half-life in the bloodstream became an area of interest for the management of obesity and T2D. This led to the development of two classes of pharmacologic agents, namely GLP-1Rs agonists and DDP-IV inhibitors [88, 89]. GLP-1Rs agonists are widely used for blood glucose management in individuals with T2D and have recently been approved to use for weight management in the United States [88–90]. Similarly, DDP-IV inhibitors are used alone or in combination with other pharmaceutical agents to inhibit the catabolic deactivation of endogenous GLP-1 [88, 89]. Compared to other commonly used antihyperglycemic drugs such as sulfonylureas and thiazolidinedione, pharmaceutical agents targeting GLP-1’s action are associated with lower risks of hypoglycaemia, and have the advantage of promoting weight loss as well as potentially preventing, or at least delaying the progressive decrease in pancreatic β-cell function which generally requires increasing drug dosage [35–38, 89, 91]. Most frequent side effects reported with the use of these agents include GI discomfort, nausea and vomiting [88, 89].
Another relevant, yet less studied, strategy to increase GLP-1’s action is to promote its endogenous secretion from L-cells. This could potentially be achieved through pharmacological and dietary approaches. The main critic of dietary approaches is that dietary changes may not lead to a sufficient increase in GLP-1 blood concentration to promote beneficial physiological actions such as improvements in insulin secretion and blood glucose concentrations. Indeed, GLP-1Rs agonist mimic supraphysiological blood concentrations of GLP-1 and have a far longer half-life, varying between 1.5 hours and 5 days depending on the agent [89]. Nonetheless, GLP-1Rs agonists only exert their effects through an endocrine route, while endogenous GLP-1 employs both endocrine and neuronal routes, and therefore such high concentrations may not be needed. In fact, changes in GLP-1 blood concentrations after bariatric surgery suggest that a rise within physiologically normal blood concentrations may be sufficient to promote beneficial metabolic effects. Several studies have shown that Roux-en-Y gastric bypass increases GLP-1 blood concentrations up to fourfold 6 months to 1 year after the procedure [92, 93]. This increase in GLP-1 concentrations is associated with improvements in dietary choices and intake, further excess weight loss, improved glycaemic control, and often T2D resolution [92, 94]. Le Roux et al. [92] showed that individuals who underwent Roux-en-Y gastric bypass within 36 months had significantly higher GLP-1 blood concentrations 30 minutes following a meal ingestion (47.4 ± 11.4 pmol/L) compared to subjects with morbid obesity (13.5 ± 6.9 pmol/L). Postprandial GLP-1 concentrations observed in subjects who underwent Roux-en-Y gastric bypass were within physiologically normal GLP-1 concentration ranges, and therefore it is plausible that promoting a similar increase in GLP-1 postprandial secretion with dietary approaches would promote similar beneficial actions on food intake, glycaemic control and weight management over time.
Nutritional modulation of glucagon-like peptide-1 secretion
The nutrient composition of ingested food and meals varies considerably between individuals and within the same individual over the course of the day. In this regard, macronutrient composition of the diet has been shown to influence appetite and metabolic responses, including glycaemia and insulinemia [95, 96]. One possible mechanism to explain the differential effects of macronutrients on these parameters is the modulation of GLP-1 secretion by their catabolic by-products. As described above, L-cells’ GPCRs bind several products of food and macronutrients’ breakdown, including monosaccharides [97–102], short-chain fatty acids (SCFAs) [103–109], medium and long-chain fatty acids [110, 111] , as well as amino acids and peptides [112–116], leading to GLP-1 secretion.
Nutrients and single-nutrient foods
Monosaccharides
Upon ingestion, digestible carbohydrates undergo enzymatic degradation and are absorbed in the form of glucose, and to a lesser extent in the form of galactose and fructose. Glucose absorption by enterocytes as well as glucose-mediated GLP-1 secretion from L-cells appear to be mediated by the sodium glucose transporter-1 (SGLT-1), a membrane transport protein expressed in the small intestine [97–102]. Moriya and colleagues [98] investigated the mechanisms underlying glucose-stimulated GLP-1 secretion by administering glucose and Phloritzine, a competitive inhibitor of SGLT-1, in the small intestine of mice. While glucose administration alone acutely increased circulating GLP-1, co-administration of glucose and Phloritzine blocked first-phase glucose-induced GLP-1 secretion [98]. Similarly, compared to controls, SGLT-1 knockout mice had decreased first-phase GLP-1 secretion and developed glucose and galactose malabsorption [99]. This is explained by the fact that glucose binding to SGLT-1 induces the closure of ATP-sensitive potassium channels, leading to membrane depolarization and to a rise in intracellular calcium concentrations [117]. Collectively, this work outlines the importance of SGLT-1 in luminal monosaccharide absorption, as well as glucose-mediated GLP-1 secretion in the proximal small intestine. The characterization of GLP-1’s secretion as being bi-phasic led researchers to investigate the prolonged effect the pharmacological inhibition of SGLT-1 in rodents and humans [100–102]. While also confirming an inhibition of first-phase GLP-1 secretion and an increase in luminal glucose concentrations, Powell and al. [100] found an increase in second-phase GLP-1 secretion and a decrease in blood glucose excursions over a 6-hour postprandial period in mice. These results were confirmed in two clinical studies conducted in healthy adults, as well as in adults with T2D [101, 102]. The increase in second-phase GLP-1 secretion observed in these studies suggests that SGLT-1 inhibition enhances other mechanisms promoting GLP-1 secretion from the distal part of GI tract. As proposed by the authors, possible explanations include an enhanced opportunity for interactions with L-cells as carbohydrates move down the GI tract during digestion, as well as an increased fermentation of undigested carbohydrates in the colon which could raise the production of SCFAs [100–102]. Concordant with the latter hypothesis, Powell and al. [100] observed a decrease in the pH of the caecum – which could indeed be an indicator of increased bacterial fermentation – in SGLT-1 knockout mice as well as mice that received a SGLT-1 inhibitor.
Non-digestible carbohydrates and short-chain fatty acids
In the colon, non-digestible carbohydrates undergo fermentation, leading, depending on their type, to the production of various amounts of SCFAs [118–121]. SCFAs are carboxylic acids that contain fewer than 6 carbons, the most abundant ones being acetate, butyrate and propionate [122]. In humans, SCFAs concentrations range from ~130 mmol/L in the caecum to ~80 mmol/L in the descending colon [122, 123]. Acetate appears to be the most abundant SCFA in the colon, followed by propionate and butyrate, with an overall colonic molar ratio of 50-60:15-20:10-20, respectively [122, 123].
Fermentable dietary fiber and their metabolites, the SCFAs, seem to promote GLP-1 secretion from L-cells by interacting with the free fatty acid receptors 2 and 3 (FFAR2, FFAR3) [103–109]. An in vitro study showed that propionate was the most potent agonist of both FFAR2 and FFAR3, that acetate was more active and selective on FFAR2, and that butyrate was more active on FFAR3 than FFAR2 [105]. In colonic cell cultures, physiological concentrations of acetate, butyrate and propionate have been shown to stimulate GLP-1 secretion [124]. The role played by FFAR2 and FFAR3 in the SCFA-induced GLP-1 secretion was confirmed by demonstrating the loss of postprandial GLP-1 secretion in FFAR2 and FFAR3 knockout intestinal cells [124]. Similar results were found in vivo, where propionate-induced GLP-1 secretion was lost in FFAR2 knockout rodents [125]. Additionally, Chambers and colleagues [126] recently showed that acute targeted delivery of propionate in the colon through an inulin-propionate ester supplement acutely stimulated GLP-1 secretion following a standard breakfast meal and reduced energy intake at a buffet-style lunch in adults with overweight or obesity. Concordant with the acute effects of GLP-1 on appetite sensations and food intake, daily delivery of propionate in the colon over 6 months also reduced body weight, abdominal fat and hepatic lipid accumulation as assessed with magnetic resonance imagery and spectroscopy [126].
The effects of diets rich in fermentable fiber on GLP-1 secretion have also been investigated in animal models and humans [127, 128]. Indeed, the main way to increase colonic SCFA concentrations in humans is through non-digestible carbohydrate consumption. The results from six experimental studies assessing the impact of fiber on GLP-1 secretion in animal models and humans are summarized in Table 1. Compared to a standard control diet, the consumption of a diet enriched in fermentable fiber for 50 days increased GLP-1 concentrations in the proximal colon, which was associated with an increased expression of proglucagon in colonic L-cells [129]. Fermentable fiber also decreased food intake, weight gain, as well as blood triglyceride concentrations and triglyceride accumulation in the liver [129]. Similarly, while also increasing GLP-1 blood concentrations and proglucagon expression in the colon, consumption of a diet rich in resistant starch for 10 days increased peptide tyrosine-tyrosine (PYY) blood concentrations and colonic expression in rats [130]. This result is not surprising as the anorectic PYY is also synthesized and released from L-cells, and its secretion could be stimulated by similar mechanisms as that of GLP-1. In healthy humans, a crossover pilot study showed that the daily supplementation with 16 g of fermentable fiber for a two-week period increased postprandial satiety, and decreased hunger, prospective food consumption as well as energy intake over 24 hours following the intake of a standard breakfast meal [131]. As GLP-1 blood concentrations were not assessed in this study, it is not known whether these effects were mediated by an increase in its secretion or through other mechanisms [131]. The effects of fermentable fiber in rodent models of diabetes were comparable to those found in healthy animals. Indeed, consumption of a diet enriched with fermentable fiber for 4 to 6 weeks increased GLP-1 concentrations in the proximal colon and portal vein, as well proglucagon expression in the proximal colon [132, 133]. In streptozotocin-treated diabetic rats, the fiber-enriched diet also up-regulated pro-hormone convertase 1 expression in the proximal colon and increased pancreatic β-cell mass which potentially mediated the improvement in glucose tolerance and the observed increase in insulin and C-peptide blood concentrations [132]. In mice with high-fat diet-induced diabetes, these effects were diminished with the use of a GLP-1R antagonist and were completely lost in a GLP-1R knockout group of rats, therefore confirming the importance of GLP-1 interaction with its receptors for exerting its beneficial effects [133].
Overall, results from these studies suggest some pathways linking fermentable fiber to its positive effects on food intake, body weight gain and blood glucose homeostasis via an increased secretion of GLP-1. Fermentable fiber and SCFAs produced by their colonic fermentation appear to increase GLP-1 synthesis by up-regulating the expression of proglucagon [129, 132–134], as well as that of pro-hormone convertase 1 [132], which, as discussed above, is responsible for its cleavage [15]. Cani and colleagues [134] also found an increased proximal colon’s L-cell number with fermentable fiber consumption, which could be another possible explanation for the increased GLP-1 synthesis and secretion. The increase in L-cell number was also associated with an enhanced expression of transcription factors that are critical for the differentiation of intestinal stem cells into enteroendocrine cells, namely neuregenin-3 and neuro-D [134].
Free fatty acids
The majority of dietary lipids are triglycerides, which are composed of a glycerol molecule and three fatty acids [135]. Upon ingestion, triglycerides undergo emulsification by bile salts in the duodenum, hydrolysis by lipases, and are absorbed by enterocytes in the form of glycerol and free fatty acids [135].
Free fatty acids, such as unsaturated long-chain fatty acids, are potent stimulators of GLP-1 release through interactions with free fatty acid receptors 1 and 4 (FFAR1, FFAR4) [110, 111]. Substrate binding to FFAR1 and FFAR4 activate phospholipase C, leading to inositol triphosphate mediated calcium release from the endoplasmic reticulum into the cytosol [136]. The secretion of GLP-1 has been shown to be increased by unsaturated long-chain fatty acids. The main outcomes of nine experimental studies assessing the impact of lipid consumption on GLP-1 secretion and blood concentrations are summarized in Table 2. Thomsen and colleagues (1999, 2003) were the first ones to assess GLP-1 responses following a meal containing olive oil in healthy adults or adults with T2D [137, 138]. Compared with a meal containing butter, which is high in saturated fatty acids (SFAs), the ingestion of the olive oil containing meal resulted in higher postprandial GLP-1 blood concentrations [137, 138]. However, no significant acute difference in blood glucose, nor insulin blood concentrations was observed [137, 138]. In a subsequent study conducted in rodents, the prolonged consumption of an olive oil-enriched diet resulted in an increased GLP-1 secretion, which coincided with a higher glucose-stimulated insulin secretion, as well as an improved glucose tolerance at the 36th day of the intervention [139]. Comparable results were found in Streptozotocin-treated diabetic rats, where the intake of a diet rich in monounsaturated fatty acids (MUFAs) from olive oil for 50 days increased GLP-1 blood concentrations, decreased weight gain and improved insulin sensitivity [140]. In humans with abdominal obesity and insulin resistance, Paniagua and colleagues [141] showed that ingestion of a Mediterranean diet rich in olive oil for 28 days resulted in significantly higher postprandial GLP-1 blood concentrations. Compared to a diet high in SFAs, consumption of the Mediterranean diet also improved insulin sensitivity, an effect which could have mediated the observed decrease in insulin secretion as well as fasting and postprandial blood glucose concentrations [141].
In addition to the beneficial effects of MUFAs, some studies showed that the colonic administration of the polyunsaturated α-linolenic acid acutely increased GLP-1 and insulin blood concentrations, and decreased blood glucose concentrations in healthy and diabetic rat models [142]. Similarly, while also resulting in increased GLP-1 blood concentrations, long-term administration of α-linolenic acid has been shown to increase β-cell proliferation in rodents [143]. As GLP-1 has been shown to decrease apoptosis, as well as to increase neogenesis of pancreatic β-cells, the authors hypothesized that the increased β-cell proliferation was mediated by the increase in GLP-1 concentrations induced by α-linolenic acid ingestion [143]. A recent study showed that fish oil and flax seed oil, which are a source of α-linolenic acid, increased FFAR4 expression in rodents’ colon and decreased the expression of the pro-inflammatory tumour necrosis factor α (TNFα) [144].
Taken together, these studies suggest that, with similar energy contents, diets that are richer in MUFAs or omega-3 polyunsaturated fatty acids (PUFAs) than in SFAs, could increase GLP-1 secretion from L-cells, which may be a mediator for the increase in insulin secretion, insulin sensitivity, β-cell proliferation, as well as the improved glucose tolerance observed in animal models and in humans.
Peptides and amino acids
Dietary proteins are generally described as the most satiating nutrient, an effect which may be partly mediated by the stimulation of anorexigenic GI peptides secretion, including that of GLP-1 [145–147]. Upon ingestion, proteins are broken down by acid hydrolysis and proteases to produce peptones, tripeptides, dipeptides and single amino acids. Specifics mechanisms responsible for protein-induced GLP-1 secretion are relatively poorly understood, and the optimal profile of proteins’ breakdown products remains to be elucidated. Nonetheless, two pathways by which products of protein breakdown seem to stimulate GLP-1 secretion is through their binding to the calcium-sensing receptors (CaSR) and the class C, group 6, subtype A GPCR (GPCR-C6A) which are expressed on L-cells. CaSR binds to a variety of amino acids including phenylalanine, tryptophan, asparagine, arginine, glutamine and histidine, as well as to peptones, tri- and dipeptides [112–116]. In vitro, their binding to CaSR stimulated GLP-1 secretion, and this effect was abolished with the use of a CaSR inhibitor [112–116]. Oya and colleagues [148] have shown that GPCR-C6A binds to the basic amino acids arginine, lysine and ornithine. The stimulatory effect of these amino acids on GLP-1 secretion was abolished in GPCR-C6A knockout intestinal cells [148].
Mixed-nutrient foods
In contrast with single nutrients and single-nutrient foods (e.g. oil), more complex foods containing a mix of nutrients are more representative of what humans consume and could allow targeting a combination of enteroendocrine pathways that would synergistically enhance GLP-1 secretion. In regards to T2D management, clinical guidelines recommend carbohydrate intake from high-fiber foods such as vegetables, fruit, legumes and whole grains, while limiting SFAs intake and promoting that of MUFAs and omega-3 PUFAs [149, 150], all of which have been associated to different extents with an increased GLP-1 secretion.
Some experimental studies conducted with healthy individuals have examined the effects of several specific foods on glycemic response, subjective appetite sensations as well as energy intake at a subsequent meal [151–168]. The main findings of these studies are summarized in Table 3. Several researchers have investigated the effects of high-fiber grain products, which could enhance GLP-1 secretion by binding to SGLT-1, FFAR2 and FFAR3. In this regard, two recent studies compared appetite responses after isoenergetic breakfast meals containing ready-to-eat breakfast cereals (low-fiber) or oatmeal (high-fiber) among healthy young adults [151, 152]. Compared to ready-to-eat breakfast cereals, oatmeal (66.8 g) increased fullness, and reduced hunger, desire to eat, as well as subjective prospective food intake [151, 152]. Furthermore, oatmeal decreased energy intake at the following meal [152]. Likewise, in adults with insulin resistance, daily consumption of a high wheat-fiber breakfast cereal (test group, 24 g/day of fiber from the cereal) for one year, significantly increased colonic SCFAs production, as well as GLP-1 blood concentrations compared to low-fiber breakfast cereals (control group, 0.5 g/day of fiber from the cereal) [165]. More specifically, at 9 months into the intervention, acetate and butyrate plasma concentrations were already significantly higher in the test group [165]. Along the same lines, Nilsson and colleagues [166] showed that consumption of high-fiber barley kernel-based bread for 3 days was associated with increased fasting GLP-1 blood concentrations, as well as increased postprandial PYY blood concentrations in healthy adults. These changes in GI peptides concentrations were associated with improved insulin sensitivity and decreased postprandial blood glucose concentrations in healthy individuals [166]. The fact that the authors found an increase in breath hydrogen and SCFAs blood concentrations, suggests that the positive effects of the barley kernel-based bread were mediated by an increased SCFAs production triggered by the colonic of dietary fiber [166].
The addition of foods with a high protein, MUFAs and fiber content, such as almonds (30.0 to 90.0 g) or pistachios (28.0 to 85.0 g) to a high-carbohydrate meal has also been shown to improve postprandial glycemic responses in a dose-dependent manner [153–159, 167]. Jenkins and colleagues [156] also found a decrease in insulin secretion in healthy adults and in adults with hyperlipidemia following acute and prolonged almond consumption. Similarly, daily intake of 50.0 g of pistachios for 12 weeks decreased C-reactive protein blood concentrations, systolic blood pressure, and body mass index in adults with T2D [155]. In women with obesity, consumption of peanuts (42.5 g) or peanut butter (42.5 g) as part of a standard breakfast meal increased postprandial insulin blood concentrations and decreased desire to eat following a standard lunch meal [166]. Additionally, peanut butter significantly increased postprandial PYY blood concentrations and decreased postprandial blood glucose concentrations following the standard lunch meal [166]. Since GLP-1 and PYY are co-released from L-cells [12], it is plausible that peanut butter may also promote postprandial GLP-1 secretion. Only three of these studies assessed GLP-1 blood concentrations [154, 159, 168]. Mori et al. [159] found no significant differences in GLP-1 blood concentrations following the addition of 42.5 g of almonds to a carbohydrate-containing meal, which could be due to the small sample size of the study. Indeed, with a larger sample size, Kendall et al. [154] found higher GLP-1 and glucose insulinotropic peptide blood concentrations following the addition of 85.0 g of pistachios to a carbohydrate-containing meal in adults with metabolic syndrome. As Reis et al. [168] showed no statistically significant difference in GLP-1 blood concentrations with the addition of 42.5 g of peanuts or peanut butter to a carbohydrate-containing meal, it is also possible that higher quantities of nuts are necessary to induce a sufficient rise in GI hormone secretion.
Consumption of eggs (2 to 3), which are high in protein and also contain MUFAs, for breakfast or lunch, has been shown to improve subjective postprandial appetite sensations [160–163]. Furthermore, when compared to a bagel breakfast, consumption of a breakfast containing eggs (3) in adult men was associated with a lower postprandial blood glucose concentrations, decreased hunger, and reduced energy intake in the next 24 hours [160]. Men also reported higher subjective satisfaction after eating eggs [160]. While GLP-1 blood concentrations were not measured in these studies, Li and al. [163] found a significant increase in postprandial blood concentrations of PYY in adolescents following ingestion of an egg-containing breakfast compared to an isocaloric bagel breakfast. One study also showed that adding avocado (50 to 90 g), which is high in fiber and MUFAs, to a high-carbohydrate isocaloric meal improved subjective satiety sensation and satisfaction [164].
Discussion and Conclusion
In light of this literature review, it is evident that manipulating the composition of the diet in order to promote GLP-1 secretion represents a promising lifestyle strategy for obesity and T2D management. The therapeutic potential of GLP-1 has already been established as several pharmaceutical agents promoting its effects are successfully used for blood glucose management in individuals with T2D, and for body weight management in individuals with obesity [87, 89]. In comparison with the use of GLP-1Rs agonists and DPP-IV inhibitors, targeting endogenous pathways through dietary modifications has the added benefits of potentially stimulating the release of other anorexigenic gut peptides (e.g. PYY), promoting beneficial changes in several markers of cardiometabolic health such as helping normalize blood lipid profile and blood pressure, as well as avoiding side effects associated with medication. Furthermore, as DPP-IV pharmacological action depends on the bioavailability of endogenous GLP-1 into the blood-stream, combining the use of this drug type with a diet promoting GLP-1 secretion could promote DPP-IV inhibitors’ action and potentially allow the use of lower doses. Therefore, it would be worthwhile to investigate the relationship between diet composition and DPP-IV inhibitors’ efficacy in future studies.
When summarizing existing studies on the modulation of endogenous GLP-1 secretion from enteroendocrine L-cells by single nutrients and their by-products (e.g. non digestible carbohydrates and SCFAs), single-nutrient foods (e.g. olive oil, fish oil) as well as mixed-nutrient foods, it appears that the nutritional modulation of GLP-1 secretion involves several GPCRs expressed on L-cells. Furthermore, specific mechanisms for the increase in GLP-1 synthesis and secretion in L-cells include the up-regulation of proglucagon expression [129, 132–134], as well as that of post-transcriptional factors (e.g. pro-hormone convertase 1) [132]. The synthesis and secretion of GLP-1 may also be enhanced by promoting the differentiation of intestinal stem cells into L-cells [134].
In comparison with single nutrients, mixed-nutrient foods theoretically constitute a more promising and practical treatment option as they can allow targeting several enteroendocrine pathways. The main limitation of existing studies assessing the effects of specific foods is that it is impossible to distinguish the specific impact of macronutrient types (e.g. MUFAs versus SFAs) from the impact of the amount of the intake per se because none of the experimental meals were matched for macronutrient content. Furthermore, several studies [154, 156, 161, 167, 168] did not use isocaloric test and control meals. In these studies, the test meal was contained more calories which leads to question whether the observed effects is to be attributed to the tested foods or the energy content of the meal. These limitations call for more rigorous experimental designs to confirm the effects of these foods on GLP-1’s and other gut hormone’s secretion. Future studies should also aim to gain a better understanding of dose-specific effects of single nutrients, mixed nutrients, specific foods and food combinations in humans. There is also a need to compare the effects of food ingestion on GLP-1 secretion in healthy adults versus adults with metabolic disturbances such as metabolic syndrome, impaired glucose tolerance, and T2D. Well-designed randomised control trials should be planned to evaluate the impact of foods promoting GLP-1 secretion on the progression from impaired glucose tolerance to frank T2D. Lastly, efforts should be made to identify ranges of postprandial GLP-1 concentrations that are associated with beneficial metabolic effects acutely and in the long-term. Such knowledge advances could help individuals modify their dietary habits in a way that obesity and T2D could be better managed and maybe prevented.
Abbreviations
- AgRP:
-
Agouti-related peptide
- ARC:
-
Arcuate nucleus
- CART:
-
Cocaine and amphetamine-regulated transcript
- CaSR:
-
Calcium-sensing receptor
- DMN:
-
Dorso-medial nucleus
- DPP-IV:
-
Dipeptidyl-peptidase IV
- FFAR1-4:
-
Free fatty acid receptor 1-4
- GI:
-
Gastrointestinal
- GLP-1:
-
Glucagon-like peptide-1
- GLP-1R(s):
-
Glucagon-like peptide-1 receptor(s)
- GPCR(s):
-
G-protein coupled receptor(s)
- GPCR-C6A:
-
Class C group 6, subtype A G-protein coupled receptor
- mRNA:
-
Messenger ribonucleic acid
- MUFA(s):
-
Monounsaturated fatty acid(s)
- NPY:
-
Neuropeptide Y
- NTS:
-
Nucleus tractus solitarius
- POMC:
-
Pro-opiomelanocortine
- PUFA(s):
-
Polyunsaturated fatty acid(s)
- PVN:
-
Paraventricular nucleus
- PYY:
-
Peptide tyrosine-tyrosine
- SFA(s):
-
Saturated fatty acid(s)
- SGLT-1:
-
Sodium glucose transporter-1
- T2D:
-
Type 2 diabetes
- TNFα:
-
Tumour necrosis factor α
- α-MSH:
-
α-melanocyte stimulating hormone
References
Stettler C, Allemann S, Jüni P, Cull CA, Holman RR, Egger M, Krähenbühl S, Diem P. Glycemic control and macrovascular disease in types 1 and 2 diabetes mellitus: Meta-analysis of randomized trials. Am Heart J. 2006;152:27–38.
Nouwen A, Winkley K, Twisk J, Lloyd CE, Peyrot M, Ismail K, Pouwer F. Type 2 diabetes mellitus as a risk factor for the onset of depression: a systematic review and meta-analysis. Diabetologia. 2010;53:2480–6.
Goldney RD, Phillips PJ, Fisher LJ, Wilson DH. Diabetes, depression, and quality of life: a population study. Diabetes Care. 2004;27:1066–70.
Anderson JW, Kendall CWC, Jenkins DJA. Importance of weight management in Type 2 diabetes: review with meta-analysis of clinical studies. J Am Coll Nutr. 2003;22:331–9.
Whitlock G, Lewington S, Sherliker P, Clarke R, Emberson J, Halsey J, Qizilbash N, Collins R, Peto R. Body-mass index and cause-specific mortality in 900 000 adults: collaborative analyses of 57 prospective studies. Lancet. 2009;373:1083–96.
Kelly T, Yang W, Chen C-S, Reynolds K, He J. Global burden of obesity in 2005 and projections to 2030. Int J Obes (Lond). 2008;32:1431–7.
Wild S, Roglic G, Green A, Sicree R, King H. Global Prevalence of Diabetes: Estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27:1047–53.
Schoeller D a. The energy balance equation: looking back and looking forward are two very different views. Nutr Rev. 2009;67:249–54.
Doucet É, Cameron J. Appetite control after weight loss: what is the role of bloodborne peptides? Appl Physiol Nutr Metab. 2007;32:523–32.
Holst JJ. The physiology of glucagon-like peptide 1. Physiol Rev. 2007;87:1409–39.
Reimann F, Tolhurst G, Gribble FM. G-protein-coupled receptors in intestinal chemosensation. Cell Metab. 2012;15:421–31.
Gunawardene AR, Corfe BM, Staton CA. Classification and functions of enteroendocrine cells of the lower gastrointestinal tract. Int J Exp Pathol. 2011;92:219–31.
Han VK, Hynes MA, Jin C, Towle AC, Lauder JM, Lund PK. Cellular localization of proglucagon/glucagon-like peptide I messenger RNAs in rat brain. J Neurosci Res. 1986;16:97–107.
Larsen P, Tang-Christensen M, Holst J, Ørskov C. Distribution of glucagon-like peptide-1 and other preproglucagon-derived peptides in the rat hypothalamus and brainstem. Neuroscience. 1997;77:257–70.
Dhanvantari S, Seidah NG, Brubaker PL. Role of prohormone convertases in the tissue-specific processing of proglucagon. Mol Endocrinol. 1996;10:342–55.
Conlon J. Proglucagon-derived peptides: nomenclature, biosynthetic relationships and physiological roles. Diabetologia. 1988;31:563–6.
Mojsov S, Heinrich G, Wilson IB, Ravazzola M, Orci L, Habener JF. Preproglucagon gene expression in pancreas and intestine diversifies at the level of post-translational processing. J Biol Chem. 1986;261:11880–9.
Novak U, Wilks A, Buell G, McEwen S. Identical mRNA for preproglucagon in pancreas and gut. Eur J Biochem. 1987;164:553–8.
Tucker JD, Dhanvantari S, Brubaker PL. Proglucagon processing in islet and intestinal cell lines. Regul Pept. 1996;62:29–35.
Nauck MA, Siemsglüss J, Orskov C, Holst JJ. Release of glucagon-like peptide 1 (GLP-1 [7-36 amide]), gastric inhibitory polypeptide (GIP) and insulin in response to oral glucose after upper and lower intestinal resections. Z Gastroenterol. 1996;34:159–66.
Deacon CF, Johnsen AH, Holst JJ. Degradation of glucagon-like peptide-1 by human plasma in vitro yields an N-terminally truncated peptide that is a major endogenous metabolite in vivo. J Clin Endocrinol Metab. 1995;80:952–7.
Deacon CF, Pridal L, Klarskov L, Olesen M, Holst JJ. Glucagon-like peptide 1 undergoes differential tissue-specific metabolism in the anesthetized pig. Am J Physiol Endocrinol Metab. 1996;271:E458–464.
Hansen L, Deacon CF, Orskov C, Holst JJ. Glucagon-like peptide-1-(7-36)amide is transformed to glucagon-like peptide-1-(9-36)amide by dipeptidyl peptidase IV in the capillaries supplying the L cells of the porcine intestine. Endocrinology. 1999;140:5356–63.
Nauck MA, Homberger E, Siegel EG, Allen RC, Eaton RP, Ebert R, Creutzfeldt W. Incretin effects of increasing glucose loads in man calculated from venous insulin and C-peptide responses. J Clin Endocrinol Metab. 1986;63:492–8.
Orskov C, Wettergren A, Holst JJ. Secretion of the incretin hormones glucagon-like peptide-1 and gastric inhibitory polypeptide correlates with insulin secretion in normal man throughout the day. Scand J Gastroenterol. 1996;31:665–70.
Edwards CM, Todd JF, Mahmoudi M, Wang Z, Wang RM, Ghatei MA, Bloom SR. Glucagon-like peptide 1 has a physiological role in the control of postprandial glucose in humans: studies with the antagonist exendin 9-39. Diabetes. 1999;48:86–93.
Wang Z, Wang RM, Owji AA, Smith DM, Ghatei MA, Bloom SR. Glucagon-like peptide-1 is a physiological incretin in rat. J Clin Invest. 1995;95:417–21.
Drucker DJ, Philippe J, Mojsov S, Chick WL, Habener JF. Glucagon-like peptide I stimulates insulin gene expression and increases cyclic AMP levels in a rat islet cell line. Proc Natl Acad Sci U S A. 1987;84:3434–8.
Nauck M, Stöckmann F, Ebert R, Creutzfeldt W. Reduced incretin effect in Type 2 (non-insulin-dependent) diabetes. Diabetologia. 1986;29:46–52.
Madsbad S, Kehlet H, Hilsted J, Tronier B. Discrepancy between plasma C-peptide and insulin response to oral and intravenous glucose. Diabetes. 1983;32:436–8.
Holst JJ. Role of incretin hormones in the regulation of insulin secretion in diabetic and nondiabetic humans. AJP Endocrinol Metab. 2004;287:E199–206.
Kaneko K, Shirotani T, Araki E, Matsumoto K, Taguchi T, Motoshima H, Yoshizato K, Kishikawa H, Shichiri M. Insulin inhibits glucagon secretion by the activation of PI3-kinase in In-R1-G9 cells. Diabetes Res Clin Pract. 1999;44:83–92.
Maruyama H, Hisatomi A, Orci L, Grodsky GM, Unger RH. Insulin within islets is a physiologic glucagon release inhibitor. J Clin Invest. 1984;74:2296–9.
De Heer J, Rasmussen C, Coy DH, Holst JJ. Glucagon-like peptide-1, but not glucose-dependent insulinotropic peptide, inhibits glucagon secretion via somatostatin (receptor subtype 2) in the perfused rat pancreas. Diabetologia. 2008;51:2263–70.
Pick A, Clark J, Kubstrup C, Levisetti M, Pugh W, Bonner-Weir S, Polonsky KS. Role of apoptosis in failure of beta-cell mass compensation for insulin resistance and beta-cell defects in the male Zucker diabetic fatty rat. Diabetes. 1998;47:358–64.
Li Y, Hansotia T, Yusta B, Ris F, Halban PA, Drucker DJ. Glucagon-like peptide-1 receptor signaling modulates beta cell apoptosis. J Biol Chem. 2003;278:471–8.
Farilla L, Hui H, Bertolotto C, Kang E, Bulotta A, Di Mario U, Perfetti R. Glucagon-Like Peptide-1 Promotes Islet cell growth and inhibits apoptosis in Zucker diabetic Rats. Endocrinology. 2013;143:4397–408.
Perfetti R, Zhou J, Doyle ME, Egan JM. Glucagon-Like Peptide-1 induces cell proliferation and Pancreatic-Duodenum Homeobox-1 expression and increases endocrine cell mass in the pancreas of old, Glucose-Intolerant Rats. Endocrinology. 2013;141:4600–5.
Rui L. Brain regulation of energy balance and body weight. Rev Endocr Metab Disord. 2013;14:387–407.
Morton GJ, Meek TH, Schwartz MW. Neurobiology of food intake in health and disease. Nat Rev Neurosci. 2014;15:367–78.
Alvarez E, Roncero I, Chowen JA, Thorens B, Blázquez E. Expression of the glucagon-like peptide-1 receptor gene in rat brain. J Neurochem. 1996;66:920–7.
Merchenthaler I, Lane M, Shughrue P. Distribution of pre-pro-glucagon and glucagon-like peptide-1 receptor messenger RNAs in the rat central nervous system. J Comp Neurol. 1999;403:261–80.
Vahl TP, Tauchi M, Durler TS, Elfers EE, Fernandes TM, Bitner RD, Ellis KS, Woods SC, Seeley RJ, Herman JP, D’Alessio DA. Glucagon-like peptide-1 (GLP-1) receptors expressed on nerve terminals in the portal vein mediate the effects of endogenous GLP-1 on glucose tolerance in rats. Endocrinology. 2007;148:4965–73.
Göke R, Larsen PJ, Mikkelsen JD, Sheikh SP. Distribution of GLP-1 Binding sites in the Rat brain: evidence that Exendin-4 is a Ligand of Brain GLP-1 Binding Sites. Eur J Neurosci. 1995;7:2294–300.
Orskov C, Poulsen SS, Møller M, Holst JJ. Glucagon-like peptide I receptors in the subfornical organ and the area postrema are accessible to circulating glucagon-like peptide I. Diabetes. 1996;45:832–5.
Secher A, Jelsing J, Baquero AF, Hecksher-Sørensen J, Cowley MA, Dalbøge LS, Hansen G, Grove KL, Pyke C, Raun K, Schäffer L, Tang-Christensen M, Verma S, Witgen BM, Vrang N, Bjerre KL. The arcuate nucleus mediates GLP-1 receptor agonist liraglutide-dependent weight loss. J Clin Invest. 2014;124:4473–88.
Yang Y, Moghadam AA, Cordner ZA, Liang N-C, Moran TH. Long term exendin-4 treatment reduces food intake and body weight and alters expression of brain homeostatic and reward markers. Endocrinology. 2014;155:3473–83.
Dockray GJ. Enteroendocrine cell signalling via the vagus nerve. Curr Opin Pharmacol. 2013;13:954–8.
Nakagawa A, Satake H, Nakabayashi H, Nishizawa M, Furuya K, Nakano S, Kigoshi T, Nakayama K, Uchida K. Receptor gene expression of glucagon-like peptide-1, but not glucose-dependent insulinotropic polypeptide, in rat nodose ganglion cells. Auton Neurosci. 2004;110:36–43.
Rüttimann EB, Arnold M, Hillebrand JJ, Geary N, Langhans W. Intrameal hepatic portal and intraperitoneal infusions of glucagon-like peptide-1 reduce spontaneous meal size in the rat via different mechanisms. Endocrinology. 2009;150:1174–81.
Abbott CR, Monteiro M, Small CJ, Sajedi A, Smith KL, Parkinson JRC, Ghatei MA, Bloom SR. The inhibitory effects of peripheral administration of peptide YY(3-36) and glucagon-like peptide-1 on food intake are attenuated by ablation of the vagal-brainstem-hypothalamic pathway. Brain Res. 2005;1044:127–31.
Rodriquez de Fonseca F, Navarro M, Alvarez E, Roncero I, Chowen JA, Maestre O, Gómez R, Muñoz RM, Eng J, Blázquez E. Peripheral versus central effects of glucagon-like peptide-1 receptor agonists on satiety and body weight loss in Zucker obese rats. Metabolism. 2000;49:709–17.
Talsania T, Anini Y, Siu S, Drucker DJ, Brubaker PL. Peripheral exendin-4 and peptide YY(3-36) synergistically reduce food intake through different mechanisms in mice. Endocrinology. 2005;146:3748–56.
Chelikani PK, Haver AC, Reidelberger RD. Intravenous infusion of glucagon-like peptide-1 potently inhibits food intake, sham feeding, and gastric emptying in rats. Am J Physiol Regul Integr Comp Physiol. 2005;288:R1695–706.
Szayna M, Doyle ME, Betkey JA, Holloway HW, Spencer RG, Greig NH, Egan JM. Exendin-4 decelerates food intake, weight gain, and fat deposition in Zucker rats. Endocrinology. 2000;141:1936–41.
Williams KE, Washington MC, Johnson-Rouse T, Johnson RE, Freeman C, Reed C, Heath J, Sayegh AI. Exogenous glucagon-like peptide-1 acts in sites supplied by the cranial mesenteric artery to reduce meal size and prolong the intermeal interval in rats. Appetite. 2015;96:254–9.
Flint A, Raben A, Astrup A, Holst JJ. Glucagon-like peptide 1 promotes satiety and suppresses energy intake in humans. J Clin Invest. 1998;101:515–20.
Näslund E, Gutniak M, Skogar S, Rössner S, Hellström PM. Glucagon-like peptide 1 increases the period of postprandial satiety and slows gastric emptying in obese men. Am J Clin Nutr. 1998;68:525–30.
Näslund E, Barkeling B, King N, Gutniak M, Blundell JE, Holst JJ, Rössner S, Hellström PM. Energy intake and appetite are suppressed by glucagon-like peptide-1 (GLP-1) in obese men. Int J Obes Relat Metab Disord. 1999;23:304–11.
Gutzwiller JP, Drewe J, Göke B, Schmidt H, Rohrer B, Lareida J, Beglinger C. Glucagon-like peptide-1 promotes satiety and reduces food intake in patients with diabetes mellitus type 2. Am J Physiol. 1999;276(5 Pt 2):R1541–4.
Flint A, Raben A, Ersbøll AK, Holst JJ, Astrup A. The effect of physiological levels of glucagon-like peptide-1 on appetite, gastric emptying, energy and substrate metabolism in obesity. Int J Obes Relat Metab Disord. 2001;25:781–92.
Toft-Nielsen MB, Madsbad S, Holst JJ. Continuous subcutaneous infusion of glucagon-like peptide 1 lowers plasma glucose and reduces appetite in type 2 diabetic patients. Diabetes Care. 1999;22:1137–43.
Berthoud H-R, Lenard NR, Shin AC. Food reward, hyperphagia, and obesity. Am J Physiol Regul Integr Comp Physiol. 2011;300:R1266–77.
Volkow ND, Wang GJ, Fowler JS, Tomasi D, Baler R. Food and drug reward: overlapping circuits in human obesity and addiction. Curr Top Behav Neurosci. 2012;11:1–24.
Van Bloemendaal L, Veltman DJ, Ten Kulve JS, Groot PFC, Ruhé HG, Barkhof F, Sloan JH, Diamant M, Ijzerman RG. Brain reward-system activation in response to anticipation and consumption of palatable food is altered by glucagon-like peptide-1 receptor activation in humans. Diabetes Obes Metab. 2015;17:878–86.
Stice E, Spoor S, Ng J, Zald DH. Relation of obesity to consummatory and anticipatory food reward. Physiol Behav. 2009;97:551–60.
Stice E, Spoor S, Bohon C, Veldhuizen MG, Small DM. Relation of reward from food intake and anticipated food intake to obesity: a functional magnetic resonance imaging study. J Abnorm Psychol. 2008;117:924–35.
Small DM, Jones-Gotman M, Dagher A. Feeding-induced dopamine release in dorsal striatum correlates with meal pleasantness ratings in healthy human volunteers. Neuroimage. 2003;19:1709–15.
Van Bloemendaal L, Veltman DJ, ten Kulve JS, Drent ML, Barkhof F, Diamant M, IJzerman RG. Emotional eating is associated with increased brain responses to food-cues and reduced sensitivity to GLP-1 receptor activation. Obesity. 2015;23:2075–82.
Van Bloemendaal L, IJzerman RG, ten Kulve JS, Barkhof F, Konrad RJ, Drent ML, Veltman DJ, Diamant M. GLP-1 Receptor activation modulates appetite- and reward-related brain areas in humans. Diabetes. 2014;63:4186–96.
Dossat AM, Lilly N, Kay K, Williams DL. Glucagon-like peptide 1 receptors in nucleus accumbens affect food intake. J Neurosci. 2011;31:14453–7.
Alhadeff AL, Grill HJ. Hindbrain nucleus tractus solitarius glucagon-like peptide-1 receptor signaling reduces appetitive and motivational aspects of feeding. Am J Physiol Regul Integr Comp Physiol. 2014;307:R465–70.
Egecioglu E, Engel JA, Jerlhag E. The glucagon-like peptide 1 analogue, exendin-4, attenuates the rewarding properties of psychostimulant drugs in mice. PLoS One. 2013;8, e69010.
Ten Kulve JS, Veltman DJ, van Bloemendaal L, Barkhof F, Deacon CF, Holst JJ, Konrad RJ, Sloan JH, Drent ML, Diamant M, IJzerman RG. Endogenous GLP-1 mediates postprandial reductions in activation in central reward and satiety areas in patients with type 2 diabetes. Diabetologia. 2015.
Mietlicki-Baase EG, Ortinski PI, Rupprecht LE, Olivos DR, Alhadeff AL, Pierce RC, Hayes MR. The food intake-suppressive effects of glucagon-like peptide-1 receptor signaling in the ventral tegmental area are mediated by AMPA/kainate receptors. Am J Physiol Endocrinol Metab. 2013;305:E1367–74.
Wang X-F, Liu J-J, Xia J, Liu J, Mirabella V, Pang Correspondence ZP. Endogenous Glucagon-like Peptide-1 Suppresses High-Fat Food Intake by Reducing Synaptic Drive onto Mesolimbic Dopamine Neurons. Cell Reports. 2015;12:726–33.
Anderberg RH, Anefors C, Bergquist F, Nissbrandt H, Skibicka KP. Dopamine signaling in the amygdala, increased by food ingestion and GLP-1, regulates feeding behavior. Physiol Behav. 2014;136:135–44.
Fukase N, Igarashi M, Takahashi H, Manaka H, Yamatani K, Daimon M, Tominaga M, Sasaki H. Hypersecretion of Truncated Glucagon-like Peptide-1 and Gastric Inhibitory Polypeptide in Obese Patients. Diabet Med. 1993;10:44–9.
Fukase N, Manaka H, Sugiyama K, Takahashi H, Igarashi M, Daimon M, Yamatani K, Tominaga M, Sasaki H. Response of truncated glucagon-like peptide-1 and gastric inhibitory polypeptide to glucose ingestion in non-insulin dependent diabetes mellitus. Acta Diabetol. 1995;32:165–9.
Ahren B, Larsson H, Holst J. Reduced gastric inhibitory polypeptide but normal glucagon-like peptide 1 response to oral glucose in postmenopausal women with impaired glucose tolerance. Eur J Endocrinol. 1997;137:127–31.
Lugari R, Dei Cas A, Ugolotti D, Barilli AL, Camellini C, Ganzerla GC, Luciani A, Salerni B, Mittenperger F, Nodari S, Gnudi A, Zandomeneghi R. Glucagon-like peptide 1 (GLP-1) secretion and plasma dipeptidyl peptidase IV (DPP-IV) activity in morbidly obese patients undergoing biliopancreatic diversion. Horm Metab Res. 2004;36:111–5.
Toft-Nielsen MB, Madsbad S, Holst JJ. Determinants of the effectiveness of glucagon-like peptide-1 in type 2 diabetes. J Clin Endocrinol Metab. 2001;86:3853–60.
Toft-Nielsen MB, Damholt MB, Madsbad S, Hilsted LM, Hughes TE, Michelsen BK, Holst JJ. Determinants of the impaired secretion of glucagon-like peptide-1 in type 2 diabetic patients. J Clin Endocrinol Metab. 2001;86:3717–23.
Gutzwiller J-P, Drewe J, Goke B, Schmidt H, Rohrer B, Lareida J, Beglinger C. Glucagon-like peptide-1 promotes satiety and reduces food intake in patients with diabetes mellitus type 2. Am J Physiol Regul Integr Comp Physiol. 1999;276:R1541–1544.
Aulinger BA, Vahl TP, Wilson-Pérez HE, Prigeon RL, D’Alessio DA. β-Cell Sensitivity to GLP-1 in Healthy Humans Is Variable and Proportional to Insulin Sensitivity. J Clin Endocrinol Metab. 2015;100:2489–96.
Nauck MA, Heimesaat MM, Orskov C, Holst JJ, Ebert R, Creutzfeldt W. Preserved incretin activity of glucagon-like peptide 1 [7-36 amide] but not of synthetic human gastric inhibitory polypeptide in patients with type-2 diabetes mellitus. J Clin Invest. 1993;91:301–7.
Enriori PJ, Evans AE, Sinnayah P, Cowley MA. Leptin resistance and obesity. Obesity (Silver Spring). 2006;14 Suppl 5:254S–8S.
Drucker DJ, Nauck MA. The incretin system: glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet. 2006;368:1696–705.
Prasad-Reddy L, Isaacs D. A clinical review of GLP-1 receptor agonists: efficacy and safety in diabetes and beyond. Drugs Context. 2015;4:212283.
U.S. Food and Drug Administration. FDA approves weight-management drug Saxenda. 2014. Available at: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm427913.htm.
Cefalu WT, Buse JB, Del Prato S, Home PD, LeRoith D, Nauck MA, Raz I, Rosenstock J, Riddle MC. Beyond metformin: safety considerations in the decision-making process for selecting a second medication for type 2 diabetes management: reflections from a diabetes care editors’ expert forum. Diabetes Care. 2014;37:2647–59.
Le Roux CW, Aylwin SJB, Batterham RL, Borg CM, Coyle F, Prasad V, Shurey S, Ghatei MA, Patel AG, Bloom SR. Gut hormone profiles following bariatric surgery favor an anorectic state, facilitate weight loss, and improve metabolic parameters. Ann Surg. 2006;243:108–14.
Borg CM, le Roux CW, Ghatei MA, Bloom SR, Patel AG, Aylwin SJB. Progressive rise in gut hormone levels after Roux-en-Y gastric bypass suggests gut adaptation and explains altered satiety. Br J Surg. 2006;93:210–5.
Le Roux CW, Bueter M, Theis N, Werling M, Ashrafian H, Löwenstein C, Athanasiou T, Bloom SR, Spector AC, Olbers T, Lutz TA. Gastric bypass reduces fat intake and preference. Am J Physiol Regul Integr Comp Physiol. 2011;301:R1057–66.
Simpson RW, McDonald J, Wahlqvist ML, Atley L, Outch K. Macronutrients have different metabolic effects in nondiabetics and diabetics. Am J Clin Nutr. 1985;42:449–53.
Coulston AM, Hollenbeck CB, Liu GC, Williams RA, Starich GH, Mazzaferri EL, Reaven GM. Effect of source of dietary carbohydrate on plasma glucose, insulin, and gastric inhibitory polypeptide responses to test meals in subjects with noninsulin-dependent diabetes mellitus. Am J Clin Nutr. 1984;40:965–70.
Dashty M. A quick look at biochemistry: carbohydrate metabolism. Clin Biochem. 2013;46:1339–52.
Moriya R, Shirakura T, Ito J, Mashiko S, Seo T. Activation of sodium-glucose cotransporter 1 ameliorates hyperglycemia by mediating incretin secretion in mice. Am J Physiol Endocrinol Metab. 2009;297:E1358–65.
Gorboulev V, Schürmann A, Vallon V, Kipp H, Jaschke A, Klessen D, Friedrich A, Scherneck S, Rieg T, Cunard R, Veyhl-Wichmann M, Srinivasan A, Balen D, Breljak D, Rexhepaj R, Parker HE, Gribble FM, Reimann F, Lang F, Wiese S, Sabolic I, Sendtner M, Koepsell H. Na(+)-D-glucose cotransporter SGLT1 is pivotal for intestinal glucose absorption and glucose-dependent incretin secretion. Diabetes. 2012;61:187–96.
Powell DR, Smith M, Greer J, Harris A, Zhao S, DaCosta C, Mseeh F, Shadoan MK, Sands A, Zambrowicz B, Ding Z-M. LX4211 increases serum glucagon-like peptide 1 and peptide YY levels by reducing sodium/glucose cotransporter 1 (SGLT1)-mediated absorption of intestinal glucose. J Pharmacol Exp Ther. 2013;345:250–9.
Zambrowicz B, Ogbaa I, Frazier K, Banks P, Turnage A, Freiman J, Boehm KA, Ruff D, Powell D, Sands A. Effects of LX4211, a dual sodium-dependent glucose cotransporters 1 and 2 inhibitor, on postprandial glucose, insulin, glucagon-like peptide 1, and peptide tyrosine tyrosine in a dose-timing study in healthy subjects. Clin Ther. 2013;35:1162–73. e8.
Zambrowicz B, Ding Z-M, Ogbaa I, Frazier K, Banks P, Turnage A, Freiman J, Smith M, Ruff D, Sands A, Powell D. Effects of LX4211, a dual SGLT1/SGLT2 inhibitor, plus sitagliptin on postprandial active GLP-1 and glycemic control in type 2 diabetes. Clin Ther. 2013;35:273–85. e7.
Karaki S-I, Tazoe H, Hayashi H, Kashiwabara H, Tooyama K, Suzuki Y, Kuwahara A. Expression of the short-chain fatty acid receptor, GPR43, in the human colon. J Mol Histol. 2008;39:135–42.
Tazoe H, Otomo Y, Karaki S-I, Kato I, Fukami Y, Terasaki M, Kuwahara A. Expression of short-chain fatty acid receptor GPR41 in the human colon. Biomed Res. 2009;30:149–56.
Le Poul E, Loison C, Struyf S, Springael J-Y, Lannoy V, Decobecq M-E, Brezillon S, Dupriez V, Vassart G, Van Damme J, Parmentier M, Detheux M. Functional characterization of human receptors for short chain fatty acids and their role in polymorphonuclear cell activation. J Biol Chem. 2003;278:25481–9.
Brown AJ, Goldsworthy SM, Barnes AA, Eilert MM, Tcheang L, Daniels D, Muir AI, Wigglesworth MJ, Kinghorn I, Fraser NJ, Pike NB, Strum JC, Steplewski KM, Murdock PR, Holder JC, Marshall FH, Szekeres PG, Wilson S, Ignar DM, Foord SM, Wise A, Dowell SJ. The Orphan G protein-coupled receptors GPR41 and GPR43 are activated by propionate and other short chain carboxylic acids. J Biol Chem. 2003;278:11312–9.
Nilsson NE, Kotarsky K, Owman C, Olde B. Identification of a free fatty acid receptor, FFA2R, expressed on leukocytes and activated by short-chain fatty acids. Biochem Biophys Res Commun. 2003;303:1047–52.
Nøhr MK, Pedersen MH, Gille A, Egerod KL, Engelstoft MS, Husted AS, Sichlau RM, Grunddal KV, Poulsen SS, Han S, Jones RM, Offermanns S, Schwartz TW. GPR41/FFAR3 and GPR43/FFAR2 as cosensors for short-chain fatty acids in enteroendocrine cells vs FFAR3 in enteric neurons and FFAR2 in enteric leukocytes. Endocrinology. 2013;154:3552–64.
Akiba Y, Inoue T, Kaji I, Higashiyama M, Narimatsu K, Iwamoto K, Watanabe M, Guth PH, Engel E, Kuwahara A, Kaunitz JD. Short-chain fatty acid sensing in rat duodenum. J Physiol. 2015;593:585–99.
Edfalk S, Steneberg P, Edlund H. Gpr40 is expressed in enteroendocrine cells and mediates free fatty acid stimulation of incretin secretion. Diabetes. 2008;57:2280–7.
Hauge M, Vestmar MA, Husted AS, Ekberg JP, Wright MJ, Di Salvo J, Weinglass AB, Engelstoft MS, Madsen AN, Lückmann M, Miller MW, Trujillo ME, Frimurer TM, Holst B, Howard AD, Schwartz TW. GPR40 (FFAR1) - Combined Gs and Gq signaling in vitro is associated with robust incretin secretagogue action ex vivo and in vivo. Mol Metab. 2015;4:3–14.
Leech CA, Habener JF. Regulation of Glucagon-Like Peptide-1 Receptor and Calcium-Sensing Receptor Signaling by l-Histidine. Endocrinology. 2013;144:4851–8.
Mace OJ, Schindler M, Patel S. The regulation of K- and L-cell activity by GLUT2 and the calcium-sensing receptor CasR in rat small intestine. J Physiol. 2012;590(Pt 12):2917–36.
Diakogiannaki E, Pais R, Tolhurst G, Parker HE, Horscroft J, Rauscher B, Zietek T, Daniel H, Gribble FM, Reimann F. Oligopeptides stimulate glucagon-like peptide-1 secretion in mice through proton-coupled uptake and the calcium-sensing receptor. Diabetologia. 2013;56:2688–96.
Joshi S, Tough IR, Cox HM. Endogenous PYY and GLP-1 mediate l-glutamine responses in intestinal mucosa. Br J Pharmacol. 2013;170:1092–101.
Pais R, Gribble FM, Reimann F. Signalling pathways involved in the detection of peptones by murine small intestinal enteroendocrine L-cells. Peptides. 2015;77:9–15.
Gribble FM, Williams L, Simpson AK, Reimann F. A novel glucose-sensing mechanism contributing to Glucagon-Like Peptide-1 Secretion from the GLUTag cell line. Diabetes. 2003;52:1147–54.
Mortensen PB, Holtug K, Rasmussen HS. Short-chain fatty acid production from mono- and disaccharides in a fecal incubation system: implications for colonic fermentation of dietary fiber in humans. J Nutr. 1988;118(April):321–5.
Titgemeyer E, Bourquin L, Fahey GCJ, Garleb K. Fermentability of various fiber sources by human fecal bacteria in vitro. Am J Clin Nutr. 1991;53:1418–24.
Bourquin LD, Titgemeyer EC, Fahey GC. Fermentation of various dietary fiber sources by human fecal bacteria. Nutr Res. 1996;16:1119–31.
Velázquez M, Davies C, Marett R, Slavin JL, Feirtag JM. Effect of Oligosaccharides and fibre substitutes on short-chain fatty acid production by Human Faecal Microflora. Anaerobe. 2000;6:87–92.
Cummings JH, Pomare EW, Branch WJ, Naylor CP, Macfarlane GT. Short chain fatty acids in human large intestine, portal, hepatic and venous blood. Gut. 1987;28:1221–7.
Weaver GA, Krause JA, Miller TL, Wolin MJ. Short chain fatty acid distributions of enema samples from a sigmoidoscopy population: an association of high acetate and low butyrate ratios with adenomatous polyps and colon cancer. Gut. 1988;29:1539–43.
Tolhurst G, Heffron H, Lam YS, Parker HE, Habib AM, Diakogiannaki E, Cameron J, Grosse J, Reimann F, Gribble FM. Short-chain fatty acids stimulate glucagon-like peptide-1 secretion via the G-protein-coupled receptor FFAR2. Diabetes. 2012;61:364–71.
Psichas A, Sleeth ML, Murphy KG, Brooks L, Bewick GA, Hanyaloglu AC, Ghatei MA, Bloom SR, Frost G. The short chain fatty acid propionate stimulates GLP-1 and PYY secretion via free fatty acid receptor 2 in rodents. Int J Obes (Lond). 2015;39:424–9.
Chambers ES, Viardot A, Psichas A, Morrison DJ, Murphy KG, Zac-Varghese SEK, MacDougall K, Preston T, Tedford C, Finlayson GS, Blundell JE, Bell JD, Thomas EL, Mt-Isa S, Ashby D, Gibson GR, Kolida S, Dhillo WS, Bloom SR, Morley W, Clegg S, Frost G. Effects of targeted delivery of propionate to the human colon on appetite regulation, body weight maintenance and adiposity in overweight adults. Gut. 2015;64:1744–54.
Berggren AM, Björck IME, Nyman EMGL, Eggum BO. Short-chain fatty acid content and pH in caecum of rats given various sources of carbohydrates. J Sci Food Agric. 1993;63:397–406.
Jenkins DJ, Vuksan V, Kendall CW, Würsch P, Jeffcoat R, Waring S, Mehling CC, Vidgen E, Augustin LS, Wong E. Physiological effects of resistant starches on fecal bulk, short chain fatty acids, blood lipids and glycemic index. J Am Coll Nutr. 1998;17:609–16.
Cani PD, Neyrinck AM, Maton N, Delzenne NM. Oligofructose promotes satiety in rats fed a high-fat diet: involvement of glucagon-like Peptide-1. Obes Res. 2005;13:1000–7.
Zhou J, Martin RJ, Tulley RT, Raggio AM, McCutcheon KL, Shen L, Danna SC, Tripathy S, Hegsted M, Keenan MJ. Dietary resistant starch upregulates total GLP-1 and PYY in a sustained day-long manner through fermentation in rodents. Am J Physiol Endocrinol Metab. 2008;295:E1160–6.
Cani PD, Joly E, Horsmans Y, Delzenne NM. Oligofructose promotes satiety in healthy human: a pilot study. Eur J Clin Nutr. 2005;60:567–72.
Cani PD, Daubioul CA, Reusens B, Remacle C, Catillon G, Delzenne NM. Involvement of endogenous glucagon-like peptide-1(7-36) amide on glycaemia-lowering effect of oligofructose in streptozotocin-treated rats. J Endocrinol. 2005;185:457–65.
Cani PD, Knauf C, Iglesias MA, Drucker DJ, Delzenne NM, Burcelin R. Improvement of Glucose Tolerance and Hepatic Insulin Sensitivity by Oligofructose Requires a Functional Glucagon-Like Peptide 1 Receptor. Diabetes. 2006;55:1484–90.
Cani PD, Hoste S, Guiot Y, Delzenne NM. Dietary non-digestible carbohydrates promote L-cell differentiation in the proximal colon of rats. Br J Nutr. 2007;98:32–7.
Tvrzicka E, Kremmyda L-S, Stankova B, Zak A. Fatty acids as biocompounds: their role in human metabolism, health and disease--a review. Part 1: classification, dietary sources and biological functions. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2011;155:117–30.
Hirasawa A, Tsumaya K, Awaji T, Katsuma S, Adachi T, Yamada M, Sugimoto Y, Miyazaki S, Tsujimoto G. Free fatty acids regulate gut incretin glucagon-like peptide-1 secretion through GPR120. Nat Med. 2005;11:90–4.
Thomsen C, Rasmussen O, Lousen T, Holst JJ, Fenselau S, Schrezenmeir J, Hermansen K. Differential effects of saturated and monounsaturated fatty acids on postprandial lipemia and incretin responses in healthy subjects. Am J Clin Nutr. 1999;69:1135–43.
Thomsen C, Storm H, Holst JJ, Hermansen K. Differential effects of saturated and monounsaturated fats on postprandial lipemia and glucagon-like peptide 1 responses in patients with type 2 diabetes. Am J Clin Nutr. 2003;77:605–11.
Prieto PG, Cancelas J, Villanueva-Peñacarrillo ML, Valverde I, Malaisse WJ. Effects of an olive oil-enriched diet on plasma GLP-1 concentration and intestinal content, plasma insulin concentration, and glucose tolerance in normal rats. Endocrine. 2005;26:107–15.
Cancelas J, Prieto PG, Villanueva-Peñacarrillo ML, Valverde I, Malaisse WJ. Effects of an olive oil-enriched diet on glucagon-like peptide 1 release and intestinal content, plasma insulin concentration, glucose tolerance and pancreatic insulin content in an animal model of type 2 diabetes. Horm Metab Res. 2006;38:98–105.
Paniagua JA, de la Sacristana AG, Sánchez E, Romero I, Vidal-Puig A, Berral FJ, Escribano A, Moyano MJ, Peréz-Martinez P, López-Miranda J, Pérez-Jiménez F. A MUFA-rich diet improves posprandial glucose, lipid and GLP-1 responses in insulin-resistant subjects. J Am Coll Nutr. 2007;26:434–44.
Adachi T, Tanaka T, Takemoto K, Koshimizu T, Hirasawa A, Tsujimoto G. Free fatty acids administered into the colon promote the secretion of glucagon-like peptide-1 and insulin. Biochem Biophys Res Commun. 2006;340:332–7.
Tanaka T, Yano T, Adachi T, Koshimizu T, Hirasawa A, Tsujimoto G. Cloning and characterization of the rat free fatty acid receptor GPR120: in vivo effect of the natural ligand on GLP-1 secretion and proliferation of pancreatic beta cells. Naunyn Schmiedebergs Arch Pharmacol. 2008;377:515–22.
Cheshmehkani A, Senatorov IS, Kandi P, Singh M, Britt A, Hayslett R, Moniri NH. Fish oil and flax seed oil supplemented diets increase FFAR4 expression in the rat colon. Inflamm Res. 2015;64:809–15.
Veldhorst M, Smeets A, Soenen S, Hochstenbach-Waelen A, Hursel R, Diepvens K, Lejeune M, Luscombe-Marsh N, Westerterp-Plantenga M. Protein-induced satiety: effects and mechanisms of different proteins. Physiol Behav. 2008;94:300–7.
Lejeune MP, Westerterp KR, Adam TC, Luscombe-Marsh ND, Westerterp-Plantenga MS. Ghrelin and glucagon-like peptide 1 concentrations, 24-h satiety, and energy and substrate metabolism during a high-protein diet and measured in a respiration chamber. Am J Clin Nutr. 2006;83:89–94.
Smeets AJ, Soenen S, Luscombe-Marsh ND, Ueland O, Westerterp-Plantenga MS. Energy Expenditure, Satiety, and Plasma Ghrelin, Glucagon-Like Peptide 1, and Peptide Tyrosine-Tyrosine Concentrations following a Single High-Protein Lunch. J Nutr. 2008;138:698–702.
Oya M, Kitaguchi T, Pais R, Reimann F, Gribble F, Tsuboi T. The G protein-coupled receptor family C group 6 subtype A (GPRC6A) receptor is involved in amino acid-induced glucagon-like peptide-1 secretion from GLUTag cells. J Biol Chem. 2013;288:4513–21.
Dworatzek PD, Arcudi K, Gougeon R, Husein N, Sievenpiper JL, Williams SL. Nutrition therapy. Can J Diabetes. 2013;37 Suppl 1:S45–55.
American Diabetes Association. 3. Foundations of Care and Comprehensive Medical Evaluation. Diabetes Care. 2015;39(Supplement 1):S23–35.
Rebello CJ, Johnson WD, Martin CK, Xie W, O’Shea M, Kurilich A, Bordenave N, Andler S, van Klinken BJW, Chu Y-F, Greenway FL. Acute effect of oatmeal on subjective measures of appetite and satiety compared to a ready-to-eat breakfast cereal: a randomized crossover trial. J Am Coll Nutr. 2013;32:272–9.
Rebello CJ, Johnson WD, Martin CK, Han H, Chu Y-F, Bordenave N. Instant Oatmeal Increases Satiety and Reduces Energy Intake Compared to a Ready-to-Eat Oat-Based Breakfast Cereal: A Randomized Crossover Trial. J Am Coll Nutr. 2016;35:41–9.
Kendall CWC, Josse a R, Esfahani a, Jenkins DJ a. The impact of pistachio intake alone or in combination with high-carbohydrate foods on post-prandial glycemia. Eur J Clin Nutr. 2011;65:696–702.
Kendall CWC, West SG, Augustin LS, Esfahani a, Vidgen E, Bashyam B, Sauder K a, Campbell J, Chiavaroli L, Jenkins a L, Jenkins DJ. Acute effects of pistachio consumption on glucose and insulin, satiety hormones and endothelial function in the metabolic syndrome. Eur J Clin Nutr. 2014;68:370–5.
Parham M, Heidari S, Khorramirad A, Hozoori M, Hosseinzadeh F, Bakhtyari L, Vafaeimanesh J. Effects of Pistachio Nut Supplementation on Blood Glucose in Patients with Type 2 Diabetes: A Randomized Crossover Trial. Rev Diabet Stud. 2014;11:190–6.
Jenkins DJ a, Kendall CWC, Josse AR, Salvatore S, Brighenti F, Augustin LS a, Ellis PR, Vidgen E, Rao a V. Almonds decrease postprandial glycemia, insulinemia, and oxidative damage in healthy individuals. J Nutr. 2006;136:2987–92.
Jenkins DJ a, Kendall CWC, Marchie A, Josse AR, Nguyen TH, Faulkner D a, Lapsley KG, Singer W. Effect of almonds on insulin secretion and insulin resistance in nondiabetic hyperlipidemic subjects: a randomized controlled crossover trial. Metabolism. 2008;57:882–7.
Cohen AE, Johnston CS. Almond ingestion at mealtime reduces postprandial glycemia and chronic ingestion reduces hemoglobin A1c in individuals with well-controlled type 2 diabetes mellitus. Metabolism. 2011;60:1312–7.
Mori AM, Considine RV, Mattes RD. Acute and second-meal effects of almond form in impaired glucose tolerant adults: a randomized crossover trial. Nutr Metab (Lond). 2011;8:6.
Ratliff J, Leite JO, de Ogburn R, Puglisi MJ, VanHeest J, Fernandez ML. Consuming eggs for breakfast influences plasma glucose and ghrelin, while reducing energy intake during the next 24 hours in adult men. Nutr Res. 2010;30:96–103.
Vander Wal JS, Marth JM, Khosla P, Jen K-LC, Dhurandhar NV. Short-term effect of eggs on satiety in overweight and obese subjects. J Am Coll Nutr. 2005;24:510–5.
Pombo-Rodrigues S, Calame W, Re R. The effects of consuming eggs for lunch on satiety and subsequent food intake. Int J Food Sci Nutr. 2011;62:593–9.
Liu a G, Puyau RS, Han H, Johnson WD, Greenway FL, Dhurandhar NV. The Effect of an Egg Breakfast on Satiety in Children and Adolescents: A Randomized Crossover Trial. J Am Coll Nutr. 2015;34:1–6.
Wien M, Haddad E, Oda K, Sabaté J. A randomized 3 × 3 crossover study to evaluate the effect of Hass avocado intake on post-ingestive satiety, glucose and insulin levels, and subsequent energy intake in overweight adults. Nutr J. 2013;12:155.
Freeland KR, Wilson C, Wolever TMS. Adaptation of colonic fermentation and glucagon-like peptide-1 secretion with increased wheat fibre intake for 1 year in hyperinsulinaemic human subjects. Br J Nutr. 2010;103:82–90.
Nilsson AC, Johansson-Boll EV, Björck IME. Increased gut hormones and insulin sensitivity index following a 3-d intervention with a barley kernel-based product: a randomised cross-over study in healthy middle-aged subjects. Br J Nutr. 2015;114:899–907.
Josse AR, Kendall CWC, Augustin LSA, Ellis PR, Jenkins DJA. Almonds and postprandial glycemia-a dose-response study. Metabolism. 2007;56:400–4.
Reis CEG, Ribeiro DN, Costa NMB, Bressan J, Alfenas RCG, Mattes RD. Acute and second-meal effects of peanuts on glycaemic response and appetite in obese women with high type 2 diabetes risk: a randomised cross-over clinical trial. Br J Nutr. 2013;109:2015–23.
Acknowledgements
Not applicable.
Funding
This review was supported by a pilot study grant from the Montfort Hospital Research Institute to IG. AMB is supported by master scholarships from the Canadian Institute of Health Research and from the Montfort Hospital Research Institute. RB is supported by a doctoral scholarship from Fonds de Recherche en Santé du Québec.
Availability of data and material
Not applicable as no datasets were generated or analysed for this review article.
Authors’ contribution
AMB, DP and IG formulated the aim of this review. AMB conducted the bibliographical search, critically reviewed articles included in this paper and took primary responsibility in its redaction. DP, RB and IG participated in the critical review of selected articles and revised this paper for important intellectual content. All co-authors read and approved the final version of this manuscript.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
Not applicable.
Ethics approval and consent to participate
Not applicable.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Bodnaruc, A.M., Prud’homme, D., Blanchet, R. et al. Nutritional modulation of endogenous glucagon-like peptide-1 secretion: a review. Nutr Metab (Lond) 13, 92 (2016). https://doi.org/10.1186/s12986-016-0153-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12986-016-0153-3