Abstract
Porcine circovirus 3 (PCV3) is a newly emerging virus and has been found associated with porcine dermatitis and nephropathy syndrome in pigs. Compared with PCV2, research into PCV3 cap gene sequencing is deficient. To investigate the prevalence and genotype distribution of PCV3, we collected 1291 samples from 211 pig farms throughout 15 provinces and municipalities. 312 out of 1291 samples were tested positive by PCR. We further sequenced and analyzed 164 PCR-positive samples. The majority (61.8%) of isolates we sequenced belong to genotype PCV3c. PCV3c is also the dominant genotype in Hubei, Hunan, Hebei province and Chongqing city. We found 3 sites under positive selection and located in predicted epitope peptide, revealing that the pig’s immunity may be a reason those sites are undergoing highly positive selection.
Similar content being viewed by others
Introduction
Porcine circovirus 3 (PCV3) is the third circovirus identified in pigs which was first reported in 2016 in the United States using metagenomics [1].
PCV3 was first detected in pigs with cardiac and multi-systemic inflammation and was subsequently proven to have a relationship with Porcine Dermatitis and Nephropathy Syndrome (PDNS), as well as reproductive failure [2]. By challenged with infectious clone of PCV3, specific pathogen-free (SPF) pigs displayed PDNS-like clinical symptoms and subsequently suffered from lung, kidney, and lymph system damage [3]. Another experiment showed that PCV3-challenged mice presented symptoms of pneumonia [4]. PCV3 showed relatively high co-infection rates with porcine reproductive and respiratory syndrome virus (PRRSV), porcine parvovirus (PPV), and torque teno sus virus (TTSV) [5,6,7], which are associated with reproductive disorders.
Since its discovery, PCV3 has been detected in locations worldwide and has been found in samples tracing back to 1996 [8]. It has also been identified in wild boar, ticks, mosquitoes, and dogs [9,10,11]. These animals could act as reservoirs for PCV3, demonstrating this virus's complicated transmission circulation and origin. Therefore, PCV3 has already attracted a great deal of attention since it was discovered.
We previously conducted the first report in China on PCV3 [12], indicating the primary early infection information of PCV3 in the intensive pig farms, followed by whole genomic sequencing. The genome of PCV3 is circular, covalently closed, and consists of 2000 nucleotides. It has two major open reading frames: ORF1 encodes replication-associated proteins, while ORF2 encodes the Cap protein, which forms the capsid of the virus [2]. Compared with Porcine circovirus 2 (PCV2), research into PCV3 cap gene sequencing is deficient. To investigate the prevalence and genotype distribution of PCV3, we collected samples from 15 provinces and municipalities. We also sequenced 164 PCR-positive PCV3 samples and analyzed the epidemiological and genetic characteristics of those sequences.
Material and method
Sample collection and DNA extraction
1291 samples including hearts, spleens, kidneys, lungs, lymph nodes and anti-agglutinated blood were collected from 211 pig farms throughout 15 provinces and municipalities. Samples were resuspended in PBS (Phosphate-buffered saline) and homogenized by Qiagen TissueLyser II. Three Cycles of freezing and thawing were done to further rupture tissues and release viruses. After centrifuging under 13,400 × g for 10 min, 200 μL supernatant was used for DNA extraction by Tiangen.
Polymerase chain reaction (PCR) detection and sequencing
A pair of primers was used to amplify the complete ORF2 gene of PCV3: PCV3-F: TTACTTAGAGAACGGACTTGTAACG, PCV3-R: AAATGAGACACAGAGCTATATTCAG. The reaction conditions were as follows: pre-denaturation at 94 °C for 5 min, 35 cycles of denaturation at 94 °C for 30 s, annealing at 55 °C for 30 s, extension at 72 °C for 1 min and a final extension at 72 °C for 10 min. PCV3-FS: ACATGCGAGGGCGTTTACCTGTG and PCV3-FR: CGGAGCATCCATAATGGGATACCAC were used to amplify an 840 bp amplicon(annealing at 57 °C, with the same reaction conditions as above PCR) contain the complete cap CDS gene and send to Sangon shanghai for Sanger sequencing. If more than one sample were positive from a farm tested positive, we would pick one sample for sequencing.
Data analyses and prediction
Maxlikelyhood tree was constructed by IQTree2 [13]. Nucleotide and similarities were analyzed by MEGA [14]. Hyphy [15] was used to calculate selection pressure for each epitope site. Immune Epitope Database (IEDB) prediction tools [16] was used to predict potential epitope site.
Results and discussion
In this study, 312 out of 1291 samples were tested positive, and we further collected and sequenced 164 PCR-positive samples which were collected 15 provinces and municipalities across China from 2016 to 2020. The positive rate is 24.17%, compared with existing researches, 12.2% of 616 samples in 21 Provinces of China during 2015–2017 [17]. 5% of 4040 tonsil samples which were collected from 89 farms in 25 provinces [18]. 86.7% of 491 Lung tissue samples from 19 pig slaughterhouses across 11 cities throughout Shanxi Province [19]. 63.14% of 36 large-scale pig farms were collected from 17 provinces [20]. 13.3% of 472 samples from domestic pigs were collected in Northeast China from 2015 to 2018 [21]. 28.4% of 2125 porcine samples from 910 cases in the Midwest of the USA were collected during 2016–2018 [22]. 36.70% of 79 tissue and serum samples from commercial farms in Brazil [6]. 20.5% of 49 tissue samples from Swedish pig herds. 44.2% of 360 samples from 73 pig farms in Korea [23]. Because of the sample collection location and sample type, the positive rate in different researches are very different. Our results only represent the positive rate of the sample we used.
We further analyze the positive rate in pigs at different age, and the results show that the positive rate is higher compared with the healthy pig in the nursery and grow stage,.reveal that pcv3 should be tested when the nursery and grow pig have emaciation, dermatitis or respiratory symptoms. In 222 aborted fetus samples we tested, 34.7% tested positive. A closely positive rate of Aborted fetuses also occurred in another research which was detected in 18/53 (33.9%) reproductive failure cases [24]. PCV3 should be considered and have further study when dealing with the abortion problem. The positive rate in healthy sows is 47%, higher than in healthy nursery and healthy grow pigs. More details are in Table 1.
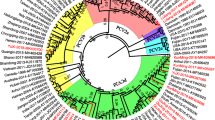
ORF2 sequence-based Maxlikelyhood tree was constructed by IQtree2, HKY + F + R2 were chosen as the models according to BIC, Testing tree branches by SH-like aLRT with 1000 replicates. Based on the phylogenetic analysis tree shown in Additional file 1: Fig. S1, all isolates can be divided into three branches [25], and the geographic distributions of the different genotypes are presented in Fig. 1. Results reveal the number of isolates belonging to subtype 3a, 3b and 3c are 33, 33, and 107 respectively. Thus, the majority (61.8%) of PCV3 isolates we sequenced belong to genotype PCV3c. Combined with geographical information, we could conclude that PCV3c is the dominant genotype in Hubei, Hunan, Hebei province and Chongqing city. Another research detected serum samples from 2,568 clinically healthy pigs from 36 large-scale pig farms were collected from 17 provinces (Hunan, Xinjiang, Jilin, Zhejiang, Jiangsu, Jiangxi, Guangxi, Hebei, Shandong, Shanxi, Anhui, Yunnan, Hubei, Inner Mongolia, Henan, Sichuan, and Guizhou) between 2019 and 2020 got the result of 86.96% (20/23) of PCV3 isolates were PCV3c strains[20].
Geographical distribution and phylogenetic tree of different genotypes. a Distribution of different genotypes of PCV3 in different provinces. Provinces in which we have sequenced PCV3 isolates were marked as green. b Phylogenetic tree of our isolates with reference isolates of different genotypes. The reference strains was marked with blue dot
Analysis of the nucleotide sequences of the ORF2 gene shows that the PCV3 strains we sequenced share 93.4–100% nucleotide similarities and 95–100% amino acid similarities. This indicates a relatively high mutation frequency in the PCV3 ORF2 gene.
Subsequently, 293 PCV3 ORF2 gene sequences were downloaded from the National Center for Biotechnology Information (NCBI) database. According to the results shown in Additional file 1: Table S2, sites 5, 24, and 137 were proved under positive selection by the four methods of single likelihood ancestor counting, mixed effects model of evolution, fast unconstrained Bayesian approximation, and fixed effects likelihood. Sites 3, 156, and 214 were shown to be positively selected by two methods. The sites that tested positive using more than three methods can be considered as robust positive selection sites. Site 24 was also detected under positive selection by the other researchers [26, 27]. Positive selection corresponds with increasing virus fitness and is one of the main evolution forces [28, 29]. The designs of PCR detection primers and epitope vaccines should consider the positive select effect of these specific sites because these sites may have associated with virus fitness and higher mutation rate.
Using the Immune Epitope Database (IEDB) prediction tools, we found seven potential peptides more likely to be epitopes (Additional file 1: Table S3). Combined with the positive selection information we obtained in the previous section, we determined that site 5 and site 24, which are positive selection sites, are also found in the second prediction epitope peptide, while site 137 is the fifth peptide. These results reveal that the immunity antibody may be the reason why those sites are undergoing highly positive selection, considering the high positive rates (56.6% in the USA, 52.6% in Zhejiang province) of the PCV3 antibody in serum in some publications[30, 31]. The sites under the high positive selective pressure may correspond with increasing virus fitness of hosts to make PCV3 more adaptive to the host immunity system. The biological significance and the role of the positively selected site should be paid more attention to in further studies. Considering that some clinical cases and animal infection experiments have confirmed that PDNS and the injury of lymph, which is the crucial immune organ, are significant damage, pcv3 may evolve with more damage when its more adaptive to the pig host immunity system.
So far, there is no commercial vaccine against PCV3. Since PCV3 has a relatively high positive rate and is under immune selective pressure, PCV3 may increase its immune evasion capacity through mutation. Therefore, vaccine and anti-viral drug development should be put on the agenda, and the site under positive selection should be considered when developing a vaccine.
Compared with PCV2, research into PCV3 cap gene sequencing is deficient. Our sequencing data enriches the PCV3 ORF2 data, and all the sequencing data have been uploaded to the NCBI GenBank database and can be used by other researchers for further analysis. However, a more detailed molecular characterization of PCV3 is required to monitor and study its evolution pattern to develop more specific vaccines like subunits, peptides, DNA, and RNA vaccines.
In this study, we tested and sequenced samples from 15 regions across China to gather the necessary data that can be used to indicate PCV3 epidemiology in China. This can help us to understand the worldwide distribution of this virus and provide information helping the development of sensitive and specific diagnostic tools and vaccines.
Biosecurity statement
All the virus detection procedure was performed in BSL-2 laboratory. The rest tissue sample and amplified product was heat-inactivated before disposal.
Availability of data and materials
The gene sequences sequenced during the current study are available in the GenBank database (accession numbers were in supplementary materials).
References
Phan TG, Giannitti F, Rossow S, Marthaler D, Knutson TP, Li L, Deng X, Resende T, Vannucci F, Delwart E. Detection of a novel circovirus PCV3 in pigs with cardiac and multi-systemic inflammation. Virol J. 2016;13:1–8.
Klaumann F, Correa-Fiz F, Franzo G, Sibila M, Núñez JI, Segalés J. Current knowledge on porcine circovirus 3 (PCV-3): a novel virus with a yet unknown impact on the swine industry. Front Vet Sci. 2018;5:315.
Jiang H, Wang D, Wang J, Zhu S, She R, Ren X, Tian J, Quan R, Hou L, Li Z. Induction of porcine dermatitis and nephropathy syndrome in piglets by infection with porcine circovirus type 3. J Virol. 2019;93:e02045-e12018.
Jiang Z, Wu J, Jiang M, Xie Y, Bu W, Liu C, Zhang G, Luo M. A novel technique for constructing infectious cloning of type 3 porcine circovirus. Front Microbiol. 2020;11:1067.
Zheng S, Wu X, Zhang L, Xin C, Liu Y, Shi J, Peng Z, Xu S, Fu F, Yu J. The occurrence of porcine circovirus 3 without clinical infection signs in Shandong Province. Transbound Emerg Dis. 2017;64:1337–41.
Dal Santo AC, Cezario KC, Bennemann PE, Machado SA, Martins M. Full-genome sequences of porcine circovirus 3 (PCV3) and high prevalence in mummified fetuses from commercial farms in Brazil. Microb Pathog. 2020;141:104027.
Ha Z, Xie CZ, Li JF, Wen SB, Zhang KL, Nan FL, Zhang H, Guo YC, Wang W, Lu HJ. Molecular detection and genomic characterization of porcine circovirus 3 in pigs from Northeast China. BMC Vet Res. 2018;14:1–7.
Sun J, Wei L, Lu Z, Mi S, Bao F, Guo H, Tu C, Zhu Y, Gong WJT, Diseases E. Retrospective study of porcine circovirus 3 infection in China. Transbound Emerg Dis. 2018;65:607–13.
Franzo G, Grassi L, Tucciarone CM, Drigo M, Martini M, Pasotto D, Mondin A, Menandro ML. A wild circulation: High presence of Porcine circovirus 3 in different mammalian wild hosts and ticks. Transbound Emerg Dis. 2019;66:1548–57.
Sun W, Wang W, Xin J, Cao L, Zhuang X, Zhang C, Zhu Y, Zhang H, Qin Y, Du Q. An epidemiological investigation of porcine circovirus 3 infection in dogs in the Guangxi Province from 2015 to 2017, China. Virus Res. 2019;270:197663.
Ha Z, Li J-F, Xie C-Z, Li C-H, Zhou H-N, Zhang Y, Hao P-F, Nan F-L, Zhang J-Y, Han J-C. First detection and genomic characterization of porcine circovirus 3 in mosquitoes from pig farms in China. Vet Microbiol. 2020;240:108522.
Ku X, Chen F, Li P, Wang Y, Yu X, Fan S, Qian P, Wu M, He Q. Identification and genetic characterization of porcine circovirus type 3 in China. Transbound Emerg Dis. 2017;64:703–8.
Minh BQ, Schmidt HA, Chernomor O, Schrempf D, Woodhams MD, Von Haeseler A, Lanfear R. IQ-TREE 2: new models and efficient methods for phylogenetic inference in the genomic era. Mol Biol Evolut. 2020;37:1530–4.
Kumar S, Stecher G, Li M, Knyaz C, Tamura K. MEGA X: molecular evolutionary genetics analysis across computing platforms. Mol Biol Evolut. 2018;35:1547.
Spielman SJ. phyphy: Python package for facilitating the execution and parsing of HyPhy standard analyses. J Open Source Softw. 2018;3:514.
Jespersen MC, Peters B, Nielsen M, Marcatili P. BepiPred-2.0: improving sequence-based B-cell epitope prediction using conformational epitopes. Nucleic Acids Res. 2017;45:W24–9.
Qi S, Su M, Guo D, Li C, Wei S, Feng L, Sun D. Molecular detection and phylogenetic analysis of porcine circovirus type 3 in 21 Provinces of China during 2015–2017. Transbound Emerg Dis. 2019;66:1004–15.
Liu Y, Zhang S, Song X, Hou B, Gu X, Zhao B, Yang L, Wang C, Zhou Z. The prevalence of novel porcine circovirus type 3 isolates in pig farms in China. Transbound Emerg Dis. 2019;66:2143–51.
Yue W, Liu Y, Zhang X, Ma H, He J. Molecular detection of porcine circovirus type 3 in Shanxi Province, China. Anim Dis. 2021;1:1–9.
Ge M, Ren J, Xie Y-L, Zhao D, Fan F-C, Song X-Q, Li M-X, Xiao C-T. Prevalence and genetic analysis of porcine circovirus 3 in China From 2019 to 2020. Front Vet Sci 2021;8.
Xia D, Huang L, Xie Y, Zhang X, Wei Y, Liu D, Zhu H, Bian H, Feng L, Liu C. The prevalence and genetic diversity of porcine circovirus types 2 and 3 in Northeast China from 2015 to 2018. Arch Virol. 2019;164:2435–49.
Wang Y, Noll L, Lu N, Porter E, Stoy C, Zheng W, Liu X, Peddireddi L, Niederwerder M, Bai J. Genetic diversity and prevalence of porcine circovirus type 3 (PCV3) and type 2 (PCV2) in the Midwest of the USA during 2016–2018. Transbound Emerg Dis. 2020;67:1284–94.
Kwon T, Yoo SJ, Park C-K, Lyoo YS. Prevalence of novel porcine circovirus 3 in Korean pig populations. Vet Microbiol. 2017;207:178–80.
Saporiti V, Valls L, Maldonado J, Perez M, Correa-Fiz F, Segalés J, Sibila M. Porcine circovirus 3 detection in aborted fetuses and stillborn piglets from swine reproductive failure cases. Viruses. 2021;13:264.
Tan CY, Lin CN, Ooi PT. What do we know about porcine circovirus 3 (PCV3) diagnosis so far?: A review. Transbound Emerg Dis. 2021;68:2915–35.
Chen Y, Xu Q, Chen H, Luo X, Wu Q, Tan C, Pan Q, Chen J-L. Evolution and genetic diversity of porcine circovirus 3 in china. Viruses. 2019;11:786.
Li G, He W, Zhu H, Bi Y, Wang R, Xing G, Zhang C, Zhou J, Yuen KY, Gao GF. Origin, genetic diversity, and evolutionary dynamics of novel porcine circovirus 3. Adv Sci. 2018;5:1800275.
Presti AL, Rezza G, Stefanelli P. Selective pressure on SARS-CoV-2 protein coding genes and glycosylation site prediction. Heliyon. 2020;6:e05001.
Forni D, Cagliani R, Sironi M. Recombination and positive selection differentially shaped the diversity of betacoronavirus subgenera. Viruses. 2020;12:1313.
Klaumann F, Franzo G, Sohrmann M, Correa-Fiz F, Drigo M, Núñez J, Sibila M, Segalés J. Retrospective detection of Porcine circovirus 3 (PCV-3) in pig serum samples from Spain. Transbound Emerg Dis. 2018;65:1290–6.
Geng S, Luo H, Liu Y, Chen C, Xu W, Chen Y, Li X, Fang W. Prevalence of porcine circovirus type 3 in pigs in the southeastern Chinese province of Zhejiang. BMC Vet Res. 2019;15:1–8.
Acknowledgements
The authors wish to thank for the staff in Animal disease diagnostic center of Huazhong Agricultural University for their assistance in sample collection. The research was financially supported by MOF and MARA of China.
Funding
This work was supported by China Agriculture Research System of MOF and MARA (No. CARS-35).
Author information
Authors and Affiliations
Contributions
XK conceptualized this research and finished data curation and the original drift. CZ finished data analyses and made contribute to writing the original drift. PL, XY, QS, FX made contribute to data analyses and data curation. PQ and QH managed the project and conceptualized this research with XK. All authors reviewed the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
All authors agree to publish the research data.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Table S1.
Isolate geographic information and GeneBank accession number. Table S2. The result of positive/diversifying selection analyze results by different method. Table S3. Potential B cell peptides predicted by IEDB tools. Fig. S1. Nucleotide sequence consistency pattern of our isolates.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Ku, X., Zhang, C., Li, P. et al. Epidemiological and genetic characteristics of porcine circovirus 3 in 15 provinces and municipalities of China between 2016 and 2020. Virol J 19, 187 (2022). https://doi.org/10.1186/s12985-022-01893-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12985-022-01893-0