Abstract
Background
The pandemic caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has resulted in millions of infections worldwide. While the search for an effective antiviral is still ongoing, experimental therapies based on repurposing of available antivirals is being attempted, of which HIV protease inhibitors (PIs) have gained considerable interest. Inhibition profiling of the PIs directly against the viral protease has never been attempted in vitro, and while few studies reported an efficacy of lopinavir and ritonavir in SARS-CoV-2 context, the mechanism of action of the drugs remains to be validated.
Methods
We carried out an in-depth analysis of the efficacy of HIV PIs against the main protease of SARS-CoV-2 (Mpro) in cell culture and in vitro enzymatic assays, using a methodology that enabled us to focus solely on any potential inhibitory effects of the inhibitors against the viral protease. For cell culture experiments a dark-to-bright GFP reporter substrate system was designed.
Results
Lopinavir, ritonavir, darunavir, saquinavir, and atazanavir were able to inhibit the viral protease in cell culture, albeit in concentrations much higher than their achievable plasma levels, given their current drug formulations. While inhibition by lopinavir was attributed to its cytotoxicity, ritonavir was the most effective of the panel, with IC50 of 13.7 µM. None of the inhibitors showed significant inhibition of SARS-CoV-2 Mpro in our in vitro enzymatic assays up to 100 µM concentration.
Conclusion
Targeting of SARS-CoV-2 Mpro by some of the HIV PIs might be of limited clinical potential, given the high concentration of the drugs required to achieve significant inhibition. Therefore, given their weak inhibition of the viral protease, any potential beneficial effect of the PIs in COVID-19 context might perhaps be attributed to acting on other molecular target(s), rather than SARS-CoV-2 Mpro.
Similar content being viewed by others
Background
In December 2019, a novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) was identified as the etiological agent of viral pneumonia cases that occurred in Wuhan, Hubei Province, China. As of October 29th 2020, the pandemic has resulted in more than 44 million infections, and 1 million deaths worldwide according to the World Health Organization.
There is currently no standardized treatment protocol, and there is no antiviral treatment of proven efficacy recommended for COVID-19. Clinical management of patients is mainly supportive, including supplementary oxygen and mechanical ventilation if needed. However, given the overwhelming burden of the pandemic on national healthcare systems and the global economy, experimental therapies have been attempted, which are predominantly based on the repurposing of FDA approved antivirals, antimalarials, arthritis drugs, and blood plasma derivatives [1].
The HIV protease inhibitors (PIs) lopinavir and ritonavir have gained particular interest, having shown documented in vitro activity against SARS-CoV and the Middle East respiratory syndrome coronavirus (MERS), however, these studies did not identify a molecular target for the drugs, since their efficacy was solely determined based on the inhibition of cytopathic effects or viral replication, respectively [2, 3]. Given the 94.4% identity in amino acid sequence between SARS-CoV and SARS-CoV-2 [4], studying the efficacy of HIV protease inhibitors against SARS-CoV-2 would be of major relevance.
The genome of SARS-CoV-2 encodes for two viral cysteine proteases; nsp3 (papain-like protease) and nsp5 (main protease) [5]. The main protease (Mpro) of SARS-CoV-2; also named chymotrypsin-like protease (3CLpr), plays a crucial role in the viral life cycle, cleaving the initial polyproteins translated from the viral RNA at at least 11 of its 14 cleavage sites. Mpro of SARS-CoV-2 shares 96% sequence identity to that of SARS-CoV. The enzyme consists of three domains; two domains (I and II) which consist of antiparallel β-barrels, and an α-helical domain (I), which is responsible for dimerization and enzymatic activity [6, 7]. Recent structure determination confirmed the similarities between the two enzymes [8].
One potential target for the HIV PIs is the Mpro. In silico screening identified nelfinavir as its potential inhibitor [9], while lopinavir and ritonavir were found to be potential inhibitors of the viral enzyme by molecular dynamics simulation [10].
It is important to note that the HIV protease is a C2-symmetric homodimeric aspartyl protease, composed of two identical subunits that are 99 amino acids each. The active site is located at the interface between the two monomers, and contains the catalytic Asp-Thr-Gly residues [11]. Mpro on the other hand, is a cysteine protease that can also potentially be targeted by peptide mimetics. Given the structural difference between the two proteases, the efficacy of HIV protease inhibitors against SARS-CoV and SARS-CoV-2 is questionable.
Previous studies reported that a combination of lopinavir/ritonavir and ribavirin was effective against SARS-associated coronavirus, with concentrations of 4 µg/ml and 50 µg/ml, respectively [12]. However, a recent clinical trial of 99 patients with laboratory-confirmed SARS-CoV-2 infection who were treated with lopinavir–ritonavir concluded that no significant benefit was observed in the treated group, compared to those who received standard care [13]. Recently, a short communication reported that lopinavir inhibited SARS-CoV-2 replication in Vero E6 cells with IC50 of 26.63 μM, ritonavir, however, showed no inhibition of viral replication [14].
Early in vitro reports from China showed that darunavir inhibited SARS-CoV-2 replication, although at a very high concentration (300 µM) [15]. Clinical trials are currently ongoing [16].
Our aim was to test the efficacy of a panel of HIV PIs against SARS-CoV-2 Mpro, using a cell culture-based model. In this study, we determined the IC50 of the PIs with the aid of a dark-to-bright GFP substrate system that had been developed and applied previously for the investigation of caspases [17]. Moreover, in vitro enzymatic inhibitory assays were also carried out using purified Mpro and an oligopeptide substrate representing the AVLQ*SGFR cleavage site of SARS-CoV-2 polyprotein 1 ab (PP1ab).
Materials and methods
Plasmids and inhibitors
Coding sequence of SARS-CoV-2 Mpro (GenBank: MT291835.2) was cloned into pcDNA3.1( +) mammalian expression plasmid using BamHI/EcoRI restriction sites to create the SARS-CoV-2 Mpro coding plasmid; thereafter referred to as CoV-2 Mpro. The coding sequence of a dark-to-bright GFP reporter substrate; thereafter referred to as PR-Sub, was also cloned into pcDNA3.1( +) plasmid. The PR-Sub was designed to contain a sequence representing the N-terminal autoproteolytic cleavage site of SARS-CoV-2 Mpro (TSAVLQ*SGFRKM); corresponding to the nsp4/nsp5 cleavage site, between the GFP and the Influenza A/M2 protein hydrophobic tail (CNDSSDPLVVAASIIGILHLILWILDRL). For in vitro expression of the protease, the coding sequence of His6-tagged Mpro was cloned into pET11a bacterial expression plasmid using NdeI and BamHI enzymes. The above mentioned expression constructs were obtained using the gene synthesis service of GenScript.
The protease inhibitors darunavir, saquinavir, lopinavir, tipranavir, indinavir sulfate, and atazanavir sulfate were obtained through the NIH AIDS Reagent Program, Division of AIDS, NIAID, NIH. Ritonavir was obtained from Abbott laboratories, nelfinavir from Agouron, and atazanavir from Bristol-Myers Squibb.
A synthetic oligopeptide used in our in vitro enzymatic assay representing the N-terminal autoproteolytic cleavage site of SARS-CoV-2 Mpro (AVLQ*SGFR) was obtained from a peptide synthesis service (BioBasic).
Analysis of transfection efficiency and proteolysis
293 T human embryonic kidney cells (HEK-293 T) (Invitrogen) were maintained in T-75 flask in 15 mL Dulbecco’s modified Eagle’s medium (DMEM) (Sigma-Aldrich) supplemented with 10% fetal bovine serum (FBS), 1% glutamine and 1% penicillin–streptomycin. Cells were transfected at 70% confluency with 5 µg of either PR-Sub, or CoV-2 Mpro plus PR-Sub plasmids using PEI method [18]. After 24 h incubation, GFP fluorescence was analyzed by flow cytometry using FACS Calibur (BD Biosciences).
Inhibition profiling in cell culture
On the day of transfection, HEK-293 T cells were split and transferred into a 48-well plates (30,000 cells/well) containing serial dilutions of the inhibitor ranging from 200 µM to 5 nM in a total volume of 200 μL DMEM/well, supplemented with 10% FBS, 1% glutamine and 1% penicillin–streptomycin. After 3 h incubation at 37 °C, cells were transfected with 300 ng of CoV-2 Mpro and PR-Sub plasmids using lipofectamine LTX reagent (Thermo Fisher Scientific), then the cells were incubated for 24 h. GFP fluorescence was then measured by flow cytometry using FACS Calibur. The results were analyzed by FlowJo Software Version 10 (Becton, Dickinson and Company; 2019). Calculations of IC50 were performed using GraphPad Prism 5.0 (GraphPad Software, Inc).
Cell viability assay
The day before the assay, HEK-293 T cells were split into a 96-well plates (20,000 cells/well) containing serial dilutions of the inhibitor ranging from 200 µM to 100 nM in a total volume of 200 μL DMEM/well, supplemented with 10% FBS, 1% glutamine and 1% penicillin–streptomycin. The next day, the medium was replaced with 100 μL of OPTI-MEM culture media supplemented with 10% FBS, and 10 µL of the 12 mM 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) stock solution was added to the cells. After 4 h incubation at 37 °C, 85 µL of supernatant was removed, and 50 µL of DMSO was added to the cells followed by incubation for 10 min at 37 °C. Absorbance was measured at 540 nm using Synergy H1 Hybrid Multi-Mode Reader (Agilent).
Expression and purification of SARS-CoV-2 Mpro
The heat-shock transformed BL21(DE3) cells containing the pET11a-His6-Mpro plasmid were incubated in 30 ml Luria–Bertani (LB) medium supplemented with ampicillin (100 µg/ml final concentration) at 37 °C for 16 h. The pre-cultured medium was inoculated into 470 ml LB (100 µg/ml ampicillin) and further incubated at 37 °C. Protein expression was induced by isopropyl β-D-1-thiogalactopyranoside (IPTG) (1 mM final concentration) when the OD600 reached 0.6–0.8. After 3 h incubation, cells were pelleted by centrifugation at 4 °C for 20 min at 5,000 × g (Sorvall Lynx 4000, Thermo Fisher Scientific), the cell pellet was resuspended in 10 ml buffer A (20 mM Tris, 150 mM NaCl, 10 mM imidazole, pH 7.5) and lysed by sonication on ice (Branson Sonifier 450). After a repeated centrifugation at 4 °C for 20 min at 10,000 × g, the pellet was discarded and His6-Mpro was purified from the supernatant by Ni-chelate affinity chromatography with the aid of His-Trap Column (GE Healthcare) using Äkta Prime instrument (Amersham Pharmacia Biotech). The column was equilibrated and washed with buffer A, the His6-Mpro protein was eluted under 20 column volume with a linear gradient of imidazole (0—500 mM imidazole) using buffer B (20 mM Tris, 150 mM NaCl, 500 mM imidazole, pH 7.5). Afterwards, the purification buffer was exchanged to buffer C (20 mM Tris, 50 mM NaCl, 2 mM CaCl2 pH 7.5) using Amicon Ultra centrifugal filters (10 K, Merck Millipore) and then the protein was incubated with Factor Xa (10 µg FXa/ mg protein, BCXA-1060, Haematologic Technologies) at 16 °C for 16 h to remove His6 fusion tag. Before the next purification step, the buffer was changed to buffer D (20 mM Tris, 1 mM DTT, pH 8.0) and the protein was further purified by ion-exchange chromatography using HiTrap Q FF column (GE Healthcare) equilibrated with buffer D, and eluted with buffer E (20 mM Tris, 1 M NaCl, 1 mM DTT, pH 8.0) under 20 column volume with a linear gradient. The high-purity fractions of the untagged Mpro were dialyzed against buffer F (20 mM HEPES, 120 mM NaCl, 0.4 mM EDTA, 4 mM DTT, 20% glycerol pH 6.5), and stored at -20 °C in a small-volume aliquots.
In vitro protease assay
The AVLQ*SGFR oligopeptide was dissolved in distilled water and was used as substrate in activity measurements to test the inhibitory potential of the PIs.
The cleavage reactions contained 10 µL reaction buffer (20 mM Tris, 100 mM NaCl, pH 7.8), 4.8 µL oligopeptide substrate (1.37 mM final concentration), and 0.2 µL DMSO (in control samples) or 0.2 µL of the inhibitor (diluted in DMSO). For inhibitor screening, inhibitors were applied in 100 µM final concentration. Reactions were initiated by the addition of 5 µL of Mpro in a final total protein concentration of 0.12 µM, and the mixtures were incubated at 37 °C for 10 min. The reactions were terminated by the addition of 180 µL 1% trifluoroacetic acid (TFA). The cleavage products were detected using high performance liquid chromatography (HPLC), utilizing a 0–100% water-acetonitrile gradient in the presence of TFA using Merck Hitachi instrument. Relative activity was determined at less than 20% substrate hydrolysis. Activity measured in the presence of DMSO was considered to be 100%. While no potent inhibitor of Mpro was available to perform active-site titration, 100% activity was assumed for the enzyme.
Modeling
Homology modeling of dark-to-bright GFP substrate was performed using Phyre2 web portal [19]. 97% of residues were modelled at > 90% confidence. Structural figures were prepared PyMol Molecular Graphics System (Version 1.3 Schrödinger, LLC).
Results
Inhibition profiling in cell culture
To measure Mpro activity in cell culture experiments, we applied a modified version of a dark-to-bright GFP reporter substrate [17] which was adapted in this study for SARS-CoV-2 Mpro. The recombinant substrate consists of an N-terminal GFP, followed by a natural proteolytic cleavage site of SARS-CoV-2 polyprotein, and a C-terminal hydrophobic tail. Proteolysis at the inserted cleavage site releases the tail that serves as a hydrophobic quencher of fluorescence and facilitates tetramerization of GFP, which prevents chromophore maturation; the fluorescence is restored upon proteolysis (Fig. 1).
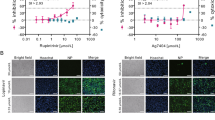
Firstly, we optimized the transfection of HEK-293 T cells for the use of the SARS-CoV-2 Mpro and the dark-to-bright GFP substrate. Transfection of cells with only the PR-Sub plasmid resulted in a maximum of 1% background fluorescence after 24 h incubation. When cells were transfected with both the PR-Sub and CoV-2 Mpro plasmids, GFP fluorescence ranged from 28–34%, indicating processing of the substrate and the activity of the protease (Fig. 2).
Optimization of HEK-293 T cell transfection with SARS-CoV-2-Mpro and the dark-to-bright GFP substrate. a Cells transfected with PR-Sub and CoV-2 Mpro under native microscopic light. b Visualization of cells transfected with PR-Sub under fluorescent microscope. c Visualization of cells transfected with PR-Sub and CoV-2 Mpro under fluorescent microscope. Co-transfection with both plasmids resulted in 28–34% GFP fluorescence
We then analyzed the inhibition efficacy of a panel of HIV PIs against SARS-CoV-2 Mpro. While none of the inhibitors was able inhibit the viral protease in nanomolar concentration; which is expected for effective transition state analogs, in micromolar range, ritonavir was the most effective (IC50 = 13.7 ± 1.1 µM). Saquinavir, darunavir, and atazanavir were also able to inhibit SARS-CoV-2 Mpro at higher concentrations (Table 1).
Although a combination of lopinavir and ritonavir resulted in better inhibition of the viral enzyme as compared to ritonavir alone (10.9 µM vs. 13.7 µM, respectively), when we carried out cell viability assays after treatment of the cells with the inhibitors, interestingly, inhibition by lopinavir was found to be a result of the high cytotoxicity observed at concentrations above 50 µM (90%) (Fig. 3).
In the case of ritonavir and saquinavir, cytotoxicity of > 50% was only observed at concentrations above 50 µM. High concentrations of darunavir and atazanavir on the other hand, were well tolerated by HEK-293 T cells.
Indinavir, nelfinavir and tipranavir however, failed to inhibit SARS-CoV-2 Mpro, even at 200 micromolar concentration of the inhibitors.
In vitro enzymatic assay
Following expression and purification of the untagged Mpro, we determined its catalytic activity after incubation with the AVLQ*SGFR oligopeptide substrate. Cleavage position within the substrate was determined using high-performance liquid chromatography coupled to electrospray ionization time-of-flight mass spectrometry (HPLC-ESI-TOF MS) (Additional file 1: Fig. 1). Inhibition profiling of the PIs was carried out after incubation of the inhibitors along with Mpro and the substrate, and the relative efficacies of PIs were compared. None of the inhibitors showed a significant inhibition of the Mpro in vitro (p values > 0.05) (Fig. 4).
Discussion
To our knowledge, direct determination of the inhibition efficacy of HIV PIs against SARS-CoV-2 Mpro in cell culture has not yet been published, although many in silico studies analyzing interaction between SARS-CoV-2 Mpro and potential inhibitors were published [20,21,22,23,24,25,26] (Additional file 1: Table 1). Antiviral assays using lopinavir and ritonavir in Calu-3 cells were previously carried out for MERS-CoV, and the IC50 for lopinavir, ritonavir and their combination was 11.6, 24.9, and 8.5 µM respectively [3]. In our analysis, we found that a combination of lopinavir plus ritonavir achieved the lowest IC50, this however was due to the high cytotoxicity of lopinavir, and not as a result of direct inhibition of SARS-CoV-2 Mpro. Ritonavir on the other hand was much more tolerable than lopinavir, and achieved the lowest IC50. This should be taken into consideration, given the current drug formulation of lopinavir which is in combination with ritonavir, where ritonavir is used as a pharmacokinetic enhancer due to its inhibition of the cytochrome P450 3A4 isoenzyme, thereby increasing the bioavailability of lopinavir [27]. As a result, administration of ritonavir in combination with other PIs was found to decrease its minimum blood plasma concentration level, as compared to the generic formulation of the drug [28]. Also, while our result regarding ritonavir was in direct contrast to what Choy et al. reported in their short communication [14], we believe that difference in methodologies is to blame for this discrepancy, as we examined the efficacy of ritonavir against the viral Mpro protease per se.
It is important to note that the IC50 of the inhibitors were in the micromolar range which is not considered optimal for the inhibition of the viral enzyme. Previous studies have reported that the minimum concentrations (Cmin) for lopinavir, darunavir, saquinavir, and atazanavir in patient’s serum under antiretroviral treatment was found to be 9.3, 3.3, 3.8, and < 1 µM, respectively [29,30,31,32]. It would indeed be challenging to achieve such high plasma levels of the inhibitors in order to block the viral replication, moreover, the cytotoxic effects of some of the inhibitors, in addition to the side effects commonly observed with PIs questions the use of anti-HIV PIs in the context of SARS-CoV-2.
Additionally, using an in vitro enzymatic assay, we were able to directly analyze any potential inhibition of Mpro by the HIV PIs. Our results show that none of the inhibitors was able to significantly inhibit Mpro in vitro.
A drawback of this study is that we were not able to assess the interaction between the PIs and the papain-like protease of SARS-CoV-2, as our methodology only enabled us to study the viral main protease. Whether or not these exert any inhibitory effect on the papain-like protease is a subject for future studies, although, a similar methodology may be adapted for SARS-CoV-2 papain-like protease, and other proteases as well. Also, while our cell culture assays were not performed in SARS-CoV-2 target cells, our methodology enabled us to directly examine any potential inhibition of the viral Mpro by the PIs, therefore, it is unlikely that different results will be obtained in target cells.
Conclusion
In conclusion, to our knowledge, thorough analysis of the efficacies of PIs against SARS-CoV-2 remains scarce, and the targets of the drugs are yet to be verified. While few studies examined the efficacy of some PIs against the replication of SARS-CoV-2, we set out to study whether or not the inhibitors exert a direct effect on the viral protease. In our experiments, even though some of the PIs developed for the treatment of HIV were able to inhibit SARS-CoV-2 Mpro, they were only able to do so at high concentrations. The combination of lopinavir plus ritonavir resulted in the lowest IC50 in cell culture, albeit at the cost of cellular viability. Although, darunavir and atazanavir required a much higher concentration to achieve the inhibition, cytotoxicity was not observed even at a concentration of 200 µM. It should be noted that there might be other molecular targets for the HIV PIs, as nelfinavir was recently shown to inhibit spike protein-mediated fusion of SARS-CoV-2 [33].
Taking everything into consideration, the use of HIV PIs in the context of COVID-19 might be of limited clinical potential, beneficial effects of which might perhaps be attributed to acting on other molecular target(s), rather than Mpro itself. Data from clinical trials will indeed shed more light on their clinical efficacy.
Availability of data and materials
All data generated during this study are included in this article and its supplementary information files.
Abbreviations
- SARS-CoV-2:
-
Severe acute respiratory syndrome coronavirus 2
- HIV:
-
Human immunodeficiency virus
- PI:
-
Protease inhibitor
- Mpro :
-
Main protease
- IC50 :
-
Half maximal inhibitory concentration
- COVID-19:
-
Coronavirus disease-2019
- WHO:
-
World Health Organization
- FDA:
-
Food and Drug Administration
- MERS:
-
Middle East respiratory syndrome
- PR:
-
Protease
- PEI:
-
Polyethylenimine
- GFP:
-
Green fluorescent protein
- FACS:
-
Fluorescence-activated cell sorting
- HEK-293 T:
-
Human embryonic kidney 293 cells
- DMSO:
-
Dimethyl sulfoxide
- MTT:
-
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- IPTG:
-
Isopropyl β-D-1-thiogalactopyranoside
- TFA:
-
Trifluoroacetic acid
- HPLC-ESI-TOF MS:
-
High-performance liquid chromatography coupled to electrospray ionization time-of-flight mass spectrometry
References
Li G, De Clercq E. Therapeutic options for the 2019 novel coronavirus (2019-nCoV). Nat Rev Drug Discovery. 2020;19(3):149–50. https://doi.org/10.1038/d41573-020-00016-0.
Chen F, Chan KH, Jiang Y, et al. In vitro susceptibility of 10 clinical isolates of SARS coronavirus to selected antiviral compounds. J Clin Virol. 2004;31(1):69–75. https://doi.org/10.1016/j.jcv.2004.03.003.
Sheahan TP, Sims AC, Leist SR, et al. Comparative therapeutic efficacy of remdesivir and combination lopinavir, ritonavir, and interferon beta against MERS-CoV. Nat Commun. 2020;11(1):222. https://doi.org/10.1038/s41467-019-13940-6.
Zhou P, Yang XL, Wang XG, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579(7798):270–3. https://doi.org/10.1038/s41586-020-2012-7.
Chan JF, Kok KH, Zhu Z, et al. Genomic characterization of the 2019 novel human-pathogenic coronavirus isolated from a patient with atypical pneumonia after visiting Wuhan. Emerg Microb Infect. 2020;9(1):221–36. https://doi.org/10.1080/22221751.2020.1719902.
Anand K, Ziebuhr J, Wadhwani P, et al. Coronavirus main proteinase (3CLpro) structure: basis for design of anti-SARS drugs. Science. 2003;300(5626):1763–7. https://doi.org/10.1126/science.1085658.
Gentile D, Patamia V, Scala A, et al. Putative inhibitors of SARS-CoV-2 main protease from a library of marine natural products: a virtual screening and molecular modeling study. Marine Drugs. 2020. https://doi.org/10.3390/md18040225.
Dai W, Zhang B, Su H, et al. Structure-based design of antiviral drug candidates targeting the SARS-CoV-2 main protease. Science. 2020. https://doi.org/10.1126/science.abb4489.
Mittal L, Kumari A, Srivastava M, et al. Identification of potential molecules against COVID-19 main protease through structure-guided virtual screening approach. J Biomol Struct Dyn. 2020. https://doi.org/10.1080/07391102.2020.1768151.
Nutho B, Mahalapbutr P, Hengphasatporn K, et al. Why are lopinavir and ritonavir effective against the newly emerged coronavirus 2019? atomistic insights into the inhibitory mechanisms. Biochemistry. 2020;59(18):1769–79. https://doi.org/10.1021/acs.biochem.0c00160.
Wlodawer A, Miller M, Jaskolski M, et al. Conserved folding in retroviral proteases: crystal structure of a synthetic HIV-1 protease. Science. 1989;245(4918):616–21. https://doi.org/10.1126/science.2548279.
Chu CM, Cheng VC, Hung IF, et al. Role of lopinavir/ritonavir in the treatment of SARS: initial virological and clinical findings. Thorax. 2004;59(3):252–6. https://doi.org/10.1136/thorax.2003.012658.
Cao B, Wang Y, Wen D, et al. A trial of lopinavir-ritonavir in adults hospitalized with severe Covid-19. N Engl J Med. 2020. https://doi.org/10.1056/NEJMoa2001282.
Choy KT, Wong AY, Kaewpreedee P, et al. Remdesivir, lopinavir, emetine, and homoharringtonine inhibit SARS-CoV-2 replication in vitro. Antiviral Res. 2020;178:104786. https://doi.org/10.1016/j.antiviral.2020.104786.
Dong L, Hu S, Gao J. Discovering drugs to treat coronavirus disease 2019 (COVID-19). Drug Discov Therapeut. 2020;14(1):58–60. https://doi.org/10.5582/ddt.2020.01012.
Lythgoe MP, Middleton P. Ongoing clinical trials for the management of the COVID-19 pandemic. Trends Pharmacol Sci. 2020. https://doi.org/10.1016/j.tips.2020.03.006.
Nicholls SB, Chu J, Abbruzzese G, et al. Mechanism of a genetically encoded dark-to-bright reporter for caspase activity. J Biol Chem. 2011;286(28):24977–86. https://doi.org/10.1074/jbc.M111.221648.
Baker A, Saltik M, Lehrmann H, et al. Polyethylenimine (PEI) is a simple, inexpensive and effective reagent for condensing and linking plasmid DNA to adenovirus for gene delivery. Gene Ther. 1997;4(8):773–82. https://doi.org/10.1038/sj.gt.3300471.
Kelley LA, Mezulis S, Yates CM, et al. The Phyre2 web portal for protein modeling, prediction and analysis. Nat Protoc. 2015;10(6):845–58. https://doi.org/10.1038/nprot.2015.053.
Shah B, Modi P, Sagar SR. In silico studies on therapeutic agents for COVID-19: drug repurposing approach. Life Sci. 2020;252:117652. https://doi.org/10.1016/j.lfs.2020.117652.
Sang P, Tian S-H, Meng Z-H, et al. Anti-HIV drug repurposing against SARS-CoV-2. RSC Adv. 2020;10(27):15775–83. https://doi.org/10.1039/D0RA01899F.
Pant S, Singh M, Ravichandiran V, et al. Peptide-like and small-molecule inhibitors against Covid-19. J Biomol Struct Dyn. 2020. https://doi.org/10.1080/07391102.2020.1757510.
Ortega JT, Serrano ML, Pujol FH, et al. Unrevealing sequence and structural features of novel coronavirus using in silico approaches: The main protease as molecular target. EXCLI J. 2020;19:400–9. https://doi.org/10.17179/excli2020-1189.
Calligari PB, Bobone S, Ricci G, Bocedi A. Molecular investigation of SARS–CoV-2 proteins and their interactions with antiviral drugs. Viruses. 2020;12:445. https://doi.org/10.3390/v12040445.
Beck BR, Shin B, Choi Y, et al. Predicting commercially available antiviral drugs that may act on the novel coronavirus (SARS-CoV-2) through a drug-target interaction deep learning model. Comput Struct Biotechnol J. 2020;18:784–90. https://doi.org/10.1016/j.csbj.2020.03.025.
Fischer A, Sellner M, Neranjan S, et al. Potential inhibitors for novel coronavirus protease identified by virtual screening of 606 million compounds. Int J Mol Sci. 2020. https://doi.org/10.3390/ijms21103626.
Hull MW, Montaner JS. Ritonavir-boosted protease inhibitors in HIV therapy. Ann Med. 2011;43(5):375–88. https://doi.org/10.3109/07853890.2011.572905.
van der Lugt J, Lange J, Avihingsanon A, et al. Plasma concentrations of generic lopinavir/ritonavir in HIV type-1-infected individuals. Antiviral Therapy. 2009;14(7):1001–4. https://doi.org/10.3851/IMP1410.
Lopez-Cortes LF, Ruiz-Valderas R, Sanchez-Rivas E, et al. Lopinavir plasma concentrations and virological outcome with lopinavir-ritonavir monotherapy in HIV-1-infected patients. Antimicrob Agents Chemother. 2013;57(8):3746–51. https://doi.org/10.1128/AAC.00315-13.
Gutierrez-Valencia A, Torres-Cornejo A, BenMarzouk-Hidalgo OJ, et al. Darunavir minimum plasma concentration and ritonavir-boosted darunavir monotherapy outcome in HIV-infected patients. Antiviral therapy. 2014;19(5):443–7. https://doi.org/10.3851/IMP2722.
Lotsch J, Harder S, Sturmer M, et al. Association of saquinavir plasma concentrations with side effects but not with antiretroviral outcome in patients infected with protease inhibitor-susceptible human immunodeficiency virus type 1. Antimicrob Agents Chemother. 2007;51(9):3264–72. https://doi.org/10.1128/AAC.00036-07.
Smith DE, Jeganathan S, Ray J. Atazanavir plasma concentrations vary significantly between patients and correlate with increased serum bilirubin concentrations. HIV Clin Trials. 2006;7(1):34–8. https://doi.org/10.1310/0KX0-H9VH-99EE-5D0L.
Musarrat F, Chouljenko V, Dahal A, et al. The anti-HIV drug nelfinavir mesylate (Viracept) is a potent inhibitor of cell fusion caused by the SARS-CoV-2 spike (S) glycoprotein warranting further evaluation as an antiviral against COVID-19 infections. J Med Virol. 2020. https://doi.org/10.1002/jmv.25985.
Acknowledgements
The authors would like to thank Szilvia Janics-Pető for her continuous help and technical assistance, and are grateful to the staff of the Laboratory of Retroviral Biochemistry. Authors are grateful to Tibor Nagy (Department of Applied Chemistry, Faculty of Science and Technology, University of Debrecen) for performing HPLC-ESI-TOF MS analysis.
Funding
This work was supported in part by the Higher Education Institutional Excellence Programme (NKFIH-1150–6/2019) of the Ministry of Innovation and Technology in Hungary, within the framework of the Biotechnology thematic programme of the University of Debrecen, and in part, by the Hungarian Scientific Research Fund (NKFI 125,238) to J.T. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Author information
Authors and Affiliations
Contributions
M. Mahdi, J.A.M., and J.T. designed the experiments and interpreted the results; M. Mahdi, and Zs. Sz. carried out cell culture experiments, J.A.M., M.G., and M. Miczi carried out in vitro experiments. All authors contributed to the writing of the manuscript, and approved the final draft.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Additional file 1
. Supplementary figure and table.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Mahdi, M., Mótyán, J.A., Szojka, Z.I. et al. Analysis of the efficacy of HIV protease inhibitors against SARS-CoV-2′s main protease. Virol J 17, 190 (2020). https://doi.org/10.1186/s12985-020-01457-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12985-020-01457-0