Abstract
Background
Astroviruses (AstVs) have been reported to infect and cause gastroenteritis in most animal species. Human AstVs were regarded the causative agent of viral diarrhea in children. In dogs, little is known about the epidemiology and clinical significance of AstV infection.
Findings
In this study, we collected and tested 253 rectal swabs from pet dogs; of which 64 samples (25.3%) tested positive for AstVs with diarrhea and 15 more samples (5.9%) also was identified as AstVs, however without any clinical signs. Phylogenetic analysis of 39 partial ORF1b sequences from these samples revealed that they are similar to AstVs, which can be subdivided into three lineages. Interestingly, out of the 39 isolates sequenced, 16 isolates are shown to be in the Mamastrovirus 5/canine astrovirus (CAstV) lineage and the remaining 23 isolates displayed higher similarities with known porcine astrovirus (PoAstV) 5 and 2. Further, analysis of 13 capsid sequences from these isolates showed that they are closely clustered with Chinese or Italy CAstV isolates.
Conclusions
The findings indicate that CAstVs commonly circulate in pet dogs, and our sequencing results have shown the genomic diversity of CAstVs leading to increasing number of clusters.
Similar content being viewed by others
Astroviruses (AstVs) are non-lipid enveloped, positive-sense single-stranded RNA icosahedral virus, containing three open reading frames (ORFs). ORF1a and ORF1b were located at the 5′ end of the genome, encoding non-structural proteins. ORF2 encoded a capsid protein, which located at the 3′-terminal end [1]. AstVs were first identified by electron microscopy in 1975 in the stools of infants hospitalized with diarrhea [2]. These star-like viruses can be subdivided into two groups; Mamastroviruses which generally infects mammals and Avastroviruses which infects avian species. Both of them are globally distributed. On that note, human AstVs is one of the major causative agent of acute gastroenteritis in young, elderly and immunocompromised patients [1]. AstVs also have been associated with extra intestinal diseases, such the encephalitis in human [1] and cattle [3], interstitial nephritis and growth retardation in chick [4], severe hepatitis in duck [5, 6], and shaking syndrome in mink [7]. Recently, AstVs were also discovered in Chinese bats and rodents [8, 9].
Canine astrovirus (CAstV) was first identified in the early 1980s and recently, it was characterized as a distinct Mamastrovirus species, which is the causative agent of gastroenteritis in pet dogs [10,11,12]. Evidently, CAstVs has spread widely in the dog population and produced higher genetic diverse, as shown Martella et al., where a novel CAstV was identified from dogs with gastroenteritis [13]. However, data on the clinical significance or association of astrovirus infection with other infectious diseases are limited. The aim of our study was to understand the prevalence, genetic diversity and evaluate the risk factors of co-infection with other infectious diseases from the samples collected.
A total of 253 fecal swabs of pet dogs; of which 64 with gastroenteritis and 15 without any syndrome were collected from pet hospitals in Guangxi from November 2015 to June 2016. Clinical signs were recorded in detail, including breed, age, gender, clinical signs. Canine Parvovirus (CPV), Canine Distemper Virus (CDV) and Canine Coronavirus (CCoV) were detected by commercial detection kit, not including the identification of bacterial and parasite pathogens in the stools of all of the pet dogs. The samples of fecal swabs diluted with PBS (200 U/ml penicillin, 200 mg/ml streptomycin and 100 μg/ml gentamicin) were vortexed for 1 min. Virus RNA was extracted from diluted rectal swab by using RNAiso Plus (TAKARA, Dalian), following the manufacturer’s instructions. Reverse transcription (RT) was carried out under standard conditions with stem-loop-2-like motif (S2 m) primer (5′-CCCTCGATCCTACTCGG) located in the 3′ non-coding region [14]. The RT-nested PCR methods developed by Chu et al. [8] were applied to amplify the partial RNA dependent-RNA polymerase (ORF1b). From the samples collected, 64/253(25.3%) and 15/253 (5.9%) resulted RT-PCR positive with gastroenteritis and without any illness, respectively (Table 1). This was higher when compared to recent reports 12% [12], 9.7% [15], 2.1% [16], 6% [17], but it was similar with 24.5% [11] and 20.9% [18] in dogs with diarrhea symptoms. Some of the factors which may have contributed to the differences were the various ages of the dogs investigated, areas, feeding environment, dog population selected and maintenance condition of fecal samples and methods for detection. The prevalence of CAstVs without any symptoms suggest that the persistent replication of CAstVs in the gastrointestinal system and due to the lack of envelop protein, it enables them to have exceptional durability to adapt the harsh gastrointestinal tract and the environment as shown by Lizasoain et al. [19]. The age of dogs investigated in this study has a wide range from 13 days to 15 years old, which was divided into four phases; younger pups (≤6 months), young pups (6 months − 2 years old), adult dogs (2–7 years old) and old dogs (≥7 years old). From the total of 79 positive samples analyzed, we found that the younger puppies were more susceptible, their positive infection rate reached up to 18.6%, but only 5.9% and 3.1% detected in young and adult dogs. There was no detection in older dogs (Table 1). The vulnerability of younger puppies towards infection are dependent on its immune system, feeding environment or stress factor. Apart from that, this may be due also to strain-dependent variations or the synergistic infection of other infectious diseases (CPV-2, CCoV and CDV). Based on the clinical manifestation, out of 79 positive samples, the mixed infection of CPV-2 took up to 23.4% (15/64), CCoV, CDV and CPV-2 + CCoV appeared to be only 3.1% (2/64), 10.9% (7/64) and 3.1% (2/64), respectively (Table 1). A variety of infectious agents may cause enteric sign, so the real pathogenic role of CAstV should be identified by the experimental infections to show that it is the primary causative agent of gastroenteritis in dogs.
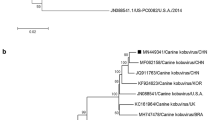
To confirm the specificity of RT-PCR, 39 of 79 positive samples were purified by Gel extraction Kit (OMEGA, USA) and sequenced. The nucleotide sequences of partial ORF1b genes and partial ORF2 were analyzed and aligned with the Megalign and Seqman program (DNASTAR, Madison, USA). Phylogenetic trees were generated by applying the neighbor-joining method of the Clustal W alignment algorithm from MEGA 7.0 software (http://www.megasoftware.net/), and bootstrap values of 1000 were used. Amino acid sequences were deduced from 3′-terminal conserved region of ORF1b gene segments and constructed phylogenetic tree (Fig. 1). Phylogenetic analysis of selected CAstV sequences revealed that there were three lineages (Group 1, 2 and 3). In the study, 39 isolates were characterized as the member of the genus Mamastrovirus, but displayed wide genetic diversity, shared 77.7–100% amino acid identity with each other. Among these CAstVs, 16 isolates were clustered into Mamastrovirus 5, which were described previously and more closely related to CAstVs, sharing with the identity of 76.0–100% at the amino acid level, compared with CAstVs originated from Italian and UK (Group 2). Notably, there was a small branch (GX914, GX74, GX771, GXK84 and GXL435), only sharing 76.0–86.8% amino acid identity with other CAstV lineage (Group 2a). It was found that these isolates have 16 amino acids changed in the C-terminal of ORF1b gene (Fig. 2), suggesting that it is a novel CAstV lineage. Importantly, except for the group of Mamastrovirus 5/CAstV, there was higher similarity with MAstV3 for PoAstV5 (87.6–98.3% amino acid identity) and PoAstV2 (84.3–100% amino acid identity), which belonged to novel genotypes circulating in pig population in China [20, 21]. This is the first report that showed CAstVs share higher homology with other lineage, suggesting that there may be potential recombination events in CAstVs infection. As previously reported, the ORF1b/ORF2 region was known as a crossover point [22,23,24]. There were several appearance of recombination event occurred in this region from different lineages or among same serotype [23,24,25,26]. Unfortunately, we failed to amplify the whole genome of CAstVs, as it was hard to evaluate the actual origin or recombination with other species.
Phylogenetic tree of the C-terminal amino acid sequences (117aa) of RNA-dependent RNA polymerase (RdRp gene). The trees were generated with the MEGA 7.0 program by using neighbor-joining method with the p-distance correction and branching order reliability was evaluated by 1000 replications of a bootstrap. Triangle indicates viruses were detected in our study
With the lack of sufficient rectal sample, only 13 partial ORF2 sequences of 79 isolates were successful to be amplified with specific primers (501F-5’CTAACAATC GTGGTCGCAAG, 1156R-5’TTGATTTGTGCATCCTTGTCC) targeting the conserved capsid gene and amplified a 634 bp fragment [18]. Comparing with all of partial ORF2 genes from China, Italy, UK, Brazil, and French, it was suggested that CAstVs may exist different genotype like porcine and human Astroviruses, some of them have association with the geographic location, like China [12], Italy [10, 11], French [18], but UK isolates and some France isolates owned a high level of genetic diversity, which were distributed in different groups [17, 18] (Fig. 3). Unlike the previous China isolates, 13 capsid strains in this study shared 93.4–100% at the amino acid level and 80.9–95.2% at the nucleotide level between strains and were grouped into two branches which were closed to China-like or Italy-like sublineage. In fact, the higher rate of evolution existed in all of AstVs, due to the increase of RNA recombination. So far, there are over 80 avian and mammalian host species detected in many species, which leads to its large diversity [27]. Generally, the majority of AstVs naturally infect only related host species, or more than one lineage could transmit in a single host [23, 28]. Whether this recombination event or cross-species transmission will emerge in CAstVs should raise corncens. Currently, the genetic diversity of the CAstV strain HUN/2012/8 with higher similarity of ORF1b and ORF2 to a mink astrovirus suggested the possible occurrence of inter-species transmission in dogs, posing a challenge for understanding the biological properties associated with viral fitness or virulence [29].
Taken together, the study not only showed that CAstVs commonly circulate in pet dog population of Guangxi, but also found the significant genetic diversity within the CAstVs isolates. To our knowledge, this is the first report that there were two genetically diverse groups identified by ORF1b gene of CAstVs which were closely related to MAstV3, suggesting potential recombination or due to inter species transmission. Therefore, the implementation of amplification of full genome or improving CAstVs isolation will be necessary to identify the emerging recombination of CAstVs or further explore the pathogenic role of CAstVs.
The partial RNA dependent RNA polymerase sequences have been submitted to GenBank. The accession numbers were from KY271968 to KY27200). The 13 partial capsid sequences have been deposited in GenBank. The accession numbers were from KY271968 to KY272006.
Abbreviations
- AstVs:
-
Astroviruses
- CAstVs:
-
Canine astroviruses
- CCoV:
-
Canine coronavirus
- CDV:
-
Canine distemper virus
- CPV:
-
Canine parvovirus
- PoAstVs:
-
Porcine astroviruses
- RT-PCR:
-
Reverse transcriptase-polymerase chain reaction
References
Martella V, Moschidou P, Buonavoglia C. Astroviruses in dogs. Vet Clin North Am Small Anim Pract. 2011;41:1087–95.
Madeley CR, Cosgrove BP. Letter: 28 nm particles in faeces in infantile gastroenteritis. Lancet. 1975;2:451–2.
Li L, Diab S, McGraw S, Barr B, Traslavina R, Higgins R, Talbot T, Blanchard P, Rimoldi G, Fahsbender E, et al. Divergent astrovirus associated with neurologic disease in cattle. Emerg Infect Dis. 2013;19:1385–92.
Imada T, Yamaguchi S, Mase M, Tsukamoto K, Kubo M, Morooka A. Avian nephritis virus (ANV) as a new member of the family Astroviridae and construction of infectious ANV cDNA. J Virol. 2000;74:8487–93.
Gough RE, Collins MS, Borland E, Keymer LF. Astrovirus-like particles associated with hepatitis in ducklings. Vet Rec. 1984;114:279.
Todd D, Smyth VJ, Ball NW, Donnelly BM, Wylie M, Knowles NJ, Adair BM. Identification of chicken enterovirus-like viruses, duck hepatitis virus type 2 and duck hepatitis virus type 3 as astroviruses. Avian Pathol. 2009;38:21–30.
Blomstrom AL, Widen F, Hammer AS, Belak S, Berg M. Detection of a novel astrovirus in brain tissue of mink suffering from shaking mink syndrome by use of viral metagenomics. J Clin Microbiol. 2010;48:4392–6.
Chu DK, Poon LL, Guan Y, Peiris JS. Novel astroviruses in insectivorous bats. J Virol. 2008;82:9107–14.
Hu B, Chmura AA, Li J, Zhu G, Desmond JS, Zhang Y, Zhang W, Epstein JH, Daszak P, Shi Z. Detection of diverse novel astroviruses from small mammals in China. J Gen Virol. 2014;95:2442–9.
Toffan A, Jonassen CM, De Battisti C, Schiavon E, Kofstad T, Capua I, Cattoli G. Genetic characterization of a new astrovirus detected in dogs suffering from diarrhoea. Vet Microbiol. 2009;139:147–52.
Martella V, Moschidou P, Lorusso E, Mari V, Camero M, Bellacicco A, Losurdo M, Pinto P, Desario C, Banyai K, et al. Detection and characterization of canine astroviruses. J Gen Virol. 2011;92:1880–7.
Zhu AL, Zhao W, Yin H, Shan TL, Zhu CX, Yang X, Hua XG, Cui L. Isolation and characterization of canine astrovirus in China. Arch Virol. 2011;156:1671–5.
Martella V, Moschidou P, Catella C, Larocca V, Pinto P, Losurdo M, Corrente M, Lorusso E, Banyai K, Decaro N, et al. Enteric disease in dogs naturally infected by a novel canine astrovirus. J Clin Microbiol. 2012;50:1066–9.
Jonassen CM, Jonassen TO, Grinde B. A common RNA motif in the 3′ end of the genomes of astroviruses, avian infectious bronchitis virus and an equine rhinovirus. J Gen Virol. 1998;79(Pt 4):715–8.
Takano T, Takashina M, Doki T, Hohdatsu T. Detection of canine astrovirus in dogs with diarrhea in Japan. Arch Virol. 2015;160:1549–53.
Choi S, Lim SI, Kim YK, Cho YY, Song JY, An DJ. Phylogenetic analysis of astrovirus and kobuvirus in Korean dogs. J Vet Med Sci. 2014;76:1141–5.
Caddy SL, Goodfellow I. Complete genome sequence of canine astrovirus with molecular and epidemiological characterisation of UK strains. Vet Microbiol. 2015;177:206–13.
Grellet A, De Battisti C, Feugier A, Pantile M, Marciano S, Grandjean D, Cattoli G. Prevalence and risk factors of astrovirus infection in puppies from French breeding kennels. Vet Microbiol. 2012;157:214–9.
Lizasoain A, Tort LF, Garcia M, Gomez MM, Leite JP, Miagostovich MP, Cristina J, Berois M, Colina R, Victoria M. Sewage surveillance reveals the presence of canine GVII norovirus and canine astrovirus in Uruguay. Arch Virol. 2015;160:2839–43.
Li JS, Li MZ, Zheng LS, Liu N, Li DD, Duan ZJ. Identification and genetic characterization of two porcine astroviruses from domestic piglets in China. Arch Virol. 2015;160:3079–84.
Shan T, Wang C, Tong W, Zheng H, Hua X, Yang S, Guo Y, Zhang W, Tong G. Complete genome of a novel porcine astrovirus. J Virol. 2012;86:13820–1.
Babkin IV, Tikunov AY, Zhirakovskaia EV, Netesov SV, Tikunova NV. High evolutionary rate of human astrovirus. Infect Genet Evol. 2012;12:435–42.
De Grazia S, Medici MC, Pinto P, Moschidou P, Tummolo F, Calderaro A, Bonura F, Banyai K, Giammanco GM, Martella V. Genetic heterogeneity and recombination in human type 2 astroviruses. J Clin Microbiol. 2012;50:3760–4.
Gabbay YB, Leite JP, Oliveira DS, Nakamura LS, Nunes MR, Mascarenhas JD, Heinemann MB, Linhares AC. Molecular epidemiology of astrovirus type 1 in Belem, Brazil, as an agent of infantile gastroenteritis, over a period of 18 years (1982-2000): identification of two possible new lineages. Virus Res. 2007;129:166–74.
Martella V, Medici MC, Terio V, Catella C, Bozzo G, Tummolo F, Calderaro A, Bonura F, Di Franco M, Banyai K, et al. Lineage diversification and recombination in type-4 human astroviruses. Infect Genet Evol. 2013;20:330–5.
Martella V, Pinto P, Tummolo F, De Grazia S, Giammanco GM, Medici MC, Ganesh B, L'Homme Y, Farkas T, Jakab F, Banyai K. Analysis of the ORF2 of human astroviruses reveals lineage diversification, recombination and rearrangement and provides the basis for a novel sub-classification system. Arch Virol. 2014;159:3185–96.
Mendenhall IH, Smith GJ, Vijaykrishna D. Ecological drivers of virus evolution: Astrovirus as a case study. J Virol. 2015;89:6978–81.
De Benedictis P, Schultz-Cherry S, Burnham A, Cattoli G. Astrovirus infections in humans and animals - molecular biology, genetic diversity, and interspecies transmissions. Infect Genet Evol. 2011;11:1529–44.
Mihalov-Kovacs E, Martella V, Lanave G, Bodnar L, Feher E, Marton S, Kemenesi G, Jakab F, Banyai K. Genome analysis of canine astroviruses reveals genetic heterogeneity and suggests possible inter-species transmission. Virus Res. 2017;232:162–70.
Acknowledgements
We thank the anonymous reviewer for critical comments and suggestions. We thank all the Animal Clinics of Guangxi.
Funding
The study was supported by the Guangxi Natural Science Foundation under Grant No. 2015GXNSFCA139002 and 2016GXNSFBA380219, Doctor Startup Funds of Guangxi University under Grant No. XBZ160123, University Scientific Research Project of education department in Guangxi under Grant No. YB2014005 and National Natural Science Foundation under Grant No. 31460671.
Availability of data and materials
The dataset supporting the conclusions of this article is available in the Genbank (https://www.ncbi.nlm.gov/genbank).
Author information
Authors and Affiliations
Contributions
YC, WJH, ZZW and KOY designed the experiments, YC and HBZ drafted the manuscript. HBZ collected the samples from different regions. RKL and LL carried out the test, YFQ and QLF helped finish the test. VRB and GJW editted this manuscript. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Zhou, H., Liu, L., Li, R. et al. Detection and genetic characterization of canine astroviruses in pet dogs in Guangxi, China. Virol J 14, 156 (2017). https://doi.org/10.1186/s12985-017-0823-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12985-017-0823-4