Abstract
Background
Human respiratory syncytial virus (HRSV) is a leading viral etiologic agent of pediatric lower respiratory infections, including bronchiolitis and pneumonia. Two antigenic subgroups, HRSV-A and B, each contain several genotypes. While viral load may vary among HRSV genotypes and affect the clinical course of disease, data are scarce regarding the actual differences among genotypes. Therefore, this study estimated and compared viral load among NA1 and ON1 genotypes of HRSV-A and BA9 of HRSV-B. ON1 is a newly emerged genotype with a 72-nucleotide duplication in the G gene as observed previously with BA genotypes in HRSV-B.
Findings
Children <5 years of age with an initial diagnosis of severe or very severe pneumonia at a hospital in the Philippines from September 2012 to December 2013 were enrolled. HRSV genotypes were determined and the viral load measured from nasopharyngeal swabs (NPS). The viral load of HRSV genotype NA1 were significantly higher than those of ON1 and BA9. Regression analysis showed that both genotype NA1 and younger age were significantly associated with high HRSV viral load.
Conclusions
The viral load of NA1 was higher than that of ON1 and BA9 in NPS samples. HRSV genotypes may be associated with HRSV viral load. The reasons and clinical impacts of these differences in viral load among HRSV genotypes require further evaluation.
Similar content being viewed by others
Findings
Human respiratory syncytial virus (HRSV) is a major etiologic agent of lower respiratory tract infections, including bronchiolitis and pneumonia, and a leading cause of hospitalizations among infants in both developing and developed countries [1]. Two subgroups, HRSV-A and HRSV-B, have been described. A total of 11 HRSV-A genotypes have been identified, including GA1 to GA7 [2], SAA1 [3], NA1 and NA2 [4], and, most recently, ON1, which has a 72-nucleotide duplication in the G gene [5]. A total of 19 HRSV-B genotypes have been reported, including GB1 to GB4 [2], SAB1 to SAB3 [3], and BA, with a 60-nucleotide duplication in the G gene, which is subdivided into BA1 to BA12 [6–9]. The newly-emerged BA genotypes have replaced the previous genotypes [10]; similarly, ON1, derived from NA1 of HRSV-A, has been recently reported in many regions worldwide [11–13]. Both BA and ON1 have nucleotide duplications in the hypervariable region of the G gene. However, the effect of these duplications on the spread of these genotypes remains unknown. Although, viral load is an important factor in HRSV disease and severity [14], to date, there are no data regarding differences in HRSV viral load among different genotypes. We therefore investigated HRSV viral load among three genotypes identified in hospitalized pediatric patients.
A case series study was conducted from September 2012 to August 2013 in the Biliran Provincial Hospital, a referral hospital on Biliran Island [15] in the Philippines. Children aged <5 years admitted with an initial diagnosis of severe or very severe pneumonia based on Integrated Management of Childhood Illness criteria (IMCI) [16] were asked participation into the study and written parental consent was obtained for each participant. Severe pneumonia were defined as cases with cough and/or difficulty of breathing accompanied by chest indrawing and/or one or more danger signs including inability to drink, convulsion or lethargic/asleep. Local medical staffs were trained with a standardized protocol for nasopharyngeal swab (NPS) samples through the study period. Samples were taken using EX-swab 002 (DENKA SEIKEN Tokyo, Japan), stirred in 3 ml viral transport medium, which consists of Hanks BSS (Balanced salt solution), 0.25 % gelatin, 0.035 % sodium bicarbonate, Streptomycin (500 μg/mL) and Penicillin (500 U/mL) and Amphotericin B (250 μg/mL) and kept at 0–4 °C. Viral RNA was extracted using the QIAamp MinElute Virus Spin Kit (QIAGEN, Hilden, Germany) and cDNA was synthesized using Moloney murine leukemia virus reverse transcriptase and random primers (Thermo Scientific, Boston, MA, USA).
The first real-time polymerase chain reaction (PCR) for screening of HRSV was performed using an Applied Biosystems StepOnePlus system (Applied Biosystems, Foster City, CA, USA) as previously described, with some modifications [17] in Research Institute for Tropical Medicine (RITM), Metro Manila. The region of the N gene targeted by the real-time PCR is highly conserved among subgroups and genotypes. The detection limit of the assay was 100 copies for all genotypes.
The positive cDNA samples were shipped from RITM to laboratory at Tohoku University and viral load was defined as viral RNA copy number estimated from mean threshold cycle (Ct) values in duplicated samples obtained from the second real-time PCR with synthesized viral genomic RNA standard samples for each genotype (N gene, 658 nucleotides). For subgrouping and genotyping, the C-terminus region of the G gene was amplified and sequenced as previously described [18].
Patient characteristics, clinical symptoms, elapsed time between illness onset and specimen collection, viral coinfection (influenza virus, human rhinovirus/enterovirus, human metapneumovirus, and human parainfluenza virus) and viral load (Log10 RNA copies/μL) were compared among HRSV genotypes using chi-square and Kruskal-Wallis tests and one-way analysis of variance (ANOVA). The beta coefficients for associations between viral load and genotypes or other factors were adjusted by generalized linear regression. Statistical analysis was performed using IBM SPSS Statistics for Windows, version 20 (IBM Corp, Armonk, NY, USA) and a P value of < 0.05 was considered statistically significant.
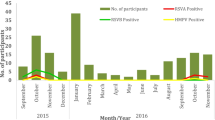
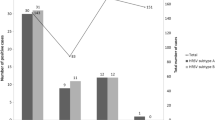
A total of 440 clinical samples were collected. Of these, 179 (40.7 %) were positive for HRSV by the first run of the real-time PCR. Viral load of the 33 samples were not included in the analysis due to not having enough volume for the replication (n = 29) or negative results in the second run (n = 4), therefore, duplicated Ct values were obtained from 146 (81.5 %) of HRSV positive samples. Moreover, six samples were excluded due to discordant results in two replicates (n = 4) and failure in subgrouping (n = 2). A total of 140 samples were genotyped and the numbers of NA1, ON1, and BA9 genotypes were 17 (12.1 %), 51 (36.4 %), and 71 (50.7 %), respectively. One sample (0.7 %) identified as genotype GB2 was excluded from further analysis. Genotype ON1 was first detected in December in the study population. Background characteristics and symptoms of the 139 HRSV positive patients included in the analyses are shown in Table 1. Their median age was 9 months old (interquartile range, 3–17 months) and 60.4 % were male. The mean interval between illness onset and specimen collection was 4 days. No congenital heart disease or chronic lung disease were reported.
Viral loads were higher in patients with genotype NA1 than in those with genotypes ON1 and BA9 (P = 0.0002, Table 1). Multiple regression analysis showed that younger age at admission and genotype NA1 were associated with higher HRSV viral load (Table 2). Because the NA1 viral load was also higher in infants less than 6 months of age (P = 0.032, Table 1), with possibly their first HRSV infections, the immunological effects of prior infection in this age group was minimal. Since genotype NA1 had been a major genotype [18] and ON1 newly introduced in this area during the study period, the effect of maternal immunity does not explain the low viral load of ON1. Therefore, the difference of viral loads among genotypes was not likely due to differences in preexisting immunity at enrollment. In addition, previous studies have shown that neutralizing antibody titer at disease onset is not associated with HRSV viral load [19]. HRSV viral load showed a strong positive correlation with younger age at admission. Young infants undergo significant anatomic and immunological changes during their development, which may affect HRSV replication patterns in different pediatric age groups.
Genetic differences among genotypes may explain the differences in viral load. ON1 has a 72-nucleotide insertion in the G gene [5]. This insertion is absent in the NA1 genotype and is the largest insertion described to date in this genus, which may affect the viral structure and pathogenesis. BA9, also characterized by an insertion in the G gene, also showed significantly lower viral load compared to NA1. The G protein is one of the major HRSV protective antigens and has significant roles in cell attachment [20], cell fusion [21], and immune response [22]. Insertions and duplications in the G gene may have affected the efficiency of HRSV replication in the newly emerged ON1 and BA genotypes. Detailed molecular analysis of the G protein gene sequence of several HRSV strains commonly reveal multiple short sequence repeats. The earlier report in HRSV-B of the 60-nucleotide duplication (the BA genotypes) had shown an exceptional example of repeated sequence in the G protein, which emerged and replaced previous genotypes within HRSV-B [10]. Further studies are needed to better understand why viral load differs among genotypes as well as the impact of new insertions in the G gene on HRSV pathogenesis.
Limitation of our observation is the small number of patients in NA1 group and NPS sampling by different study personnel, which may have had inter- sampling variation. Also viral load in NPS may not enhance the severity of lower respiratory infection. Others factors that may influence HRSV viral load such as, prematurity, breast feeding status and use of steroids or other immunosuppressive medication were not assessed in this study.
In the present study, age and viral genotypes were host and viral factors that independently predicted HRSV viral load. Age is a well-known predictor of HRSV disease severity [1] and is also associated with HRSV viral load. Although other studies using molecular techniques did not observe differences in viral load between HRSV subgroups [23, 24], differences in sample size, sample collection, or circulating genotypes during their study periods could explain this difference in findings. Our findings suggest the critical need for further studies to investigate potential differences in viral load among genotypes and their association with disease severity.
Abbreviations
Ct, cycle threshold; HRSV, human respiratory syncytial virus; NPS, nasopharyngeal swab; PCR, polymerase chain reaction
References
Borchers AT, Chang C, Gershwin ME, Gershwin LJ. Respiratory syncytial virus--a comprehensive review. Clin Rev Allergy Immunol. 2013;45:331–79.
Peret TC, Hall CB, Schnabel KC, Golub JA, Anderson LJ. Circulation patterns of genetically distinct group A and B strains of human respiratory syncytial virus in a community. J Gen Virol. 1998;79(Pt 9):2221–9.
Venter M, Madhi SA, Tiemessen CT, Schoub BD. Genetic diversity and molecular epidemiology of respiratory syncytial virus over four consecutive seasons in South Africa: identification of new subgroup A and B genotypes. J Gen Virol. 2001;82:2117–24.
Shobugawa Y, Saito R, Sano Y, Zaraket H, Suzuki Y, Kumaki A, Dapat I, Oguma T, Yamaguchi M, Suzuki H. Emerging genotypes of human respiratory syncytial virus subgroup A among patients in Japan. J Clin Microbiol. 2009;47:2475–82.
Eshaghi A, Duvvuri VR, Lai R, Nadarajah JT, Li A, Patel SN, Low DE, Gubbay JB. Genetic variability of human respiratory syncytial virus A strains circulating in Ontario: a novel genotype with a 72 nucleotide G gene duplication. PLoS One. 2012;7:e32807.
Khor CS, Sam IC, Hooi PS, Chan YF. Displacement of predominant respiratory syncytial virus genotypes in Malaysia between 1989 and 2011. Infect Genet Evol. 2013;14:357–60.
Trento A, Casas I, Calderón A, Garcia-Garcia ML, Calvo C, Perez-Breña P, Melero JA. Ten years of global evolution of the human respiratory syncytial virus BA genotype with a 60-nucleotide duplication in the G protein gene. J Virol. 2010;84:7500–12.
Trento A, Viegas M, Galiano M, Videla C, Carballal G, Mistchenko AS, Melero JA. Natural history of human respiratory syncytial virus inferred from phylogenetic analysis of the attachment (G) glycoprotein with a 60-nucleotide duplication. J Virol. 2006;80:975–84.
Tran DN, Pham TM, Ha MT, Tran TT, Dang TK, Yoshida LM, Okitsu S, Hayakawa S, Mizuguchi M, Ushijima H. Molecular epidemiology and disease severity of human respiratory syncytial virus in Vietnam. PLoS One. 2013;8:e45436.
Dapat IC, Shobugawa Y, Sano Y, Saito R, Sasaki A, Suzuki Y, Kumaki A, Zaraket H, Dapat C, Oguma T, et al. New genotypes within respiratory syncytial virus group B genotype BA in Niigata, Japan. J Clin Microbiol. 2010;48:3423–7.
Ren L, Xia Q, Xiao Q, Zhou L, Zang N, Long X, Xie X, Deng Y, Wang L, Fu Z, et al. The genetic variability of glycoproteins among respiratory syncytial virus subtype A in China between 2009 and 2013. Infect Genet Evol. 2014;27:339–47.
Pierangeli A, Trotta D, Scagnolari C, Ferreri ML, Nicolai A, Midulla F, Marinelli K, Antonelli G, Bagnarelli P: Rapid spread of the novel respiratory syncytial virus A ON1 genotype, central Italy, 2011 to 2013. Euro Surveill 2014;19:1–10.
Agoti CN, Otieno JR, Gitahi CW, Cane PA, Nokes DJ. Rapid spread and diversification of respiratory syncytial virus genotype ON1, Kenya. Emerg Infect Dis. 2014;20:950–9.
DeVincenzo JP, El Saleeby CM, Bush AJ. Respiratory syncytial virus load predicts disease severity in previously healthy infants. J Infect Dis. 2005;191:1861–8.
Kosai H, Tamaki R, Saito M, Tohma K, Alday PP, Tan AG, Inobaya MT, Suzuki A, Kamigaki T, Lupisan S, et al. Incidence and risk factors of childhood pneumonia-like episodes in biliran island, Philippines-a community-based study. PLoS One. 2015;10:e0125009.
UNICEF. WHODoCaAHaD: Handbook IMCI : integrated management of childhood illness / World Health Organization. Geneva: World Health Organization, Dept. of Child and Adolescent Health and Development; UNICEF.: World Health Organization, Dept. of Child and Adolescent Health and Development; UNICEF; 2005.
Bonroy C, Vankeerberghen A, Boel A, De Beenhouwer H. Use of a multiplex real-time PCR to study the incidence of human metapneumovirus and human respiratory syncytial virus infections during two winter seasons in a Belgian paediatric hospital. Clin Microbiol Infect. 2007;13:504–9.
Ohno A, Suzuki A, Lupisan S, Galang H, Sombrero L, Aniceto R, Okamoto M, Saito M, Fuji N, Otomaru H, et al. Genetic characterization of human respiratory syncytial virus detected in hospitalized children in the Philippines from 2008 to 2012. J Clin Virol. 2013;57:59–65.
Wright PF, Gruber WC, Peters M, Reed G, Zhu Y, Robinson F, Coleman-Dockery S, Graham BS. Illness severity, viral shedding, and antibody responses in infants hospitalized with bronchiolitis caused by respiratory syncytial virus. J Infect Dis. 2002;185:1011–8.
Hotard AL, Laikhter E, Brooks K, Hartert TV, Moore ML. Functional analysis of the 60-nucleotide duplication in the respiratory syncytial Virus Buenos Aires strain attachment glycoprotein. J Virol. 2015;89:8258–66.
Heminway BR, Yu Y, Tanaka Y, Perrine KG, Gustafson E, Bernstein JM, Galinski MS. Analysis of respiratory syncytial virus F, G, and SH proteins in cell fusion. Virology. 1994;200:801–5.
Tripp RA, Moore D, Jones L, Sullender W, Winter J, Anderson LJ. Respiratory syncytial virus G and/or SH protein alters Th1 cytokines, natural killer cells, and neutrophils responding to pulmonary infection in BALB/c mice. J Virol. 1999;73:7099–107.
Do LA, van Doorn HR, Bryant JE, Nghiem MN, Van Nguyen VC, Vo CK, Nguyen MD, Tran TH, Farrar J, de Jong MD. A sensitive real-time PCR for detection and subgrouping of human respiratory syncytial virus. J Virol Methods. 2012;179:250–5.
Kuypers J, Wright N, Morrow R. Evaluation of quantitative and type-specific real-time RT-PCR assays for detection of respiratory syncytial virus in respiratory specimens from children. J Clin Virol. 2004;31:123–9.
Acknowledgments
We are grateful to the staff of Biliran Provincial Hospital and Tohoku-RITM Collaborating Research Center on Emerging and Reemerging Diseases. We also thank R. Kojima, S. Abe, M. Kishi, and M. Estanislao for their technical assistance. This work was supported by a grant-in-aid from the Japan Initiative for Global Research Network on Infectious Diseases (J-GRID) from Japan Agency for Medical Research and Development (AMED), Japan, Science and Technology Research Partnership for Sustainable Development (SATREPS) from Japan International Cooperation Agency (JICA) and AMED.
Authors’ contributions
FK Study protocol development, performed experiments and manuscript drafting. MO Laboratory diagnosis and data quality assurance. YF Performed experiments and contributed to reviewing the manuscript. AS Study protocol development and hospital site operation. RT Hospital site operation and data analysis. IL Laboratory diagnosis and data analysis. CD Manuscript review. RM Molecular genetic studies. MSO Study coordination and laboratory data analysis. GR Hospital site coordination and clinical data management. VT Study planning, protocol development and data quality assurance. SL Study planning, protocol development, and study supervision. MS Data analysis and manuscript revision. HO Study planning, protocol development, and critical review of the revised manuscript. All authors read and approved the final manuscript.
Competing interests
None of the authors have any competing interests in the manuscript.
Ethics approval and consent to participate
This study was approved by the Ethics Committee of Tohoku University Graduate School of Medicine and Institutional Review Board of RITM.
Author information
Authors and Affiliations
Corresponding author
Appendix
Appendix
Quantitative PCR amplification of partial sequence (658 nucleotides) N genes of HRSV NA1, ON1 and BA9 genotypes. A dilution series of high-quality RNA copies was used to generate standard curves as describe in the methods. Reactions were performed in triplicate. HRSV genotypes Ct values stand for the cycle at which reporter fluorescence crosses the software-defined threshold. The linear range of threshold cycle vs RNA copies was (2 - 9) Log10 copies/μl. Solid line, linear regression of RNA copies vs Ct values. Dashed lines represent the 95 % confidence intervals for potential regression lines. The slopes and y-intercepts are -3.70, 44.35 for NA1, -3.77, 44.83 for ON1 and -3.74, 44.91 for BA9
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Kadji, F.M.N., Okamoto, M., Furuse, Y. et al. Differences in viral load among human respiratory syncytial virus genotypes in hospitalized children with severe acute respiratory infections in the Philippines. Virol J 13, 113 (2016). https://doi.org/10.1186/s12985-016-0565-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12985-016-0565-8